Promising Inhibitory Effects of Anthraquinones, Naphthopyrone, and Naphthalene Glycosides, from Cassia obtusifolia on α-Glucosidase and Human Protein Tyrosine Phosphatases 1B
Abstract
:1. Introduction
2. Results
2.1. α-Glucosidase and PTP1B Inhibitory Activity of the MeOH Extract of C. obtusifolia and Its Solvent-Soluble Fractions
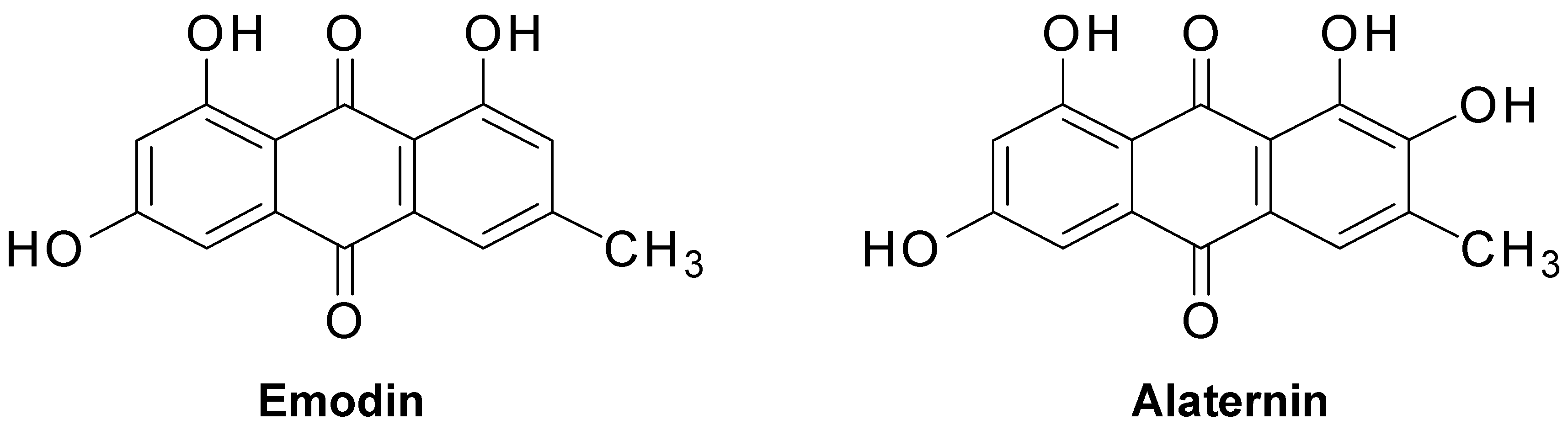
2.2. Inhibitory Activity of Anthraquinones, Naphthopyrone Glycosides, and a Naphthalene Glycoside from C. obtusifolia on PTP1B and α-Glucosidase
2.3. Kinetic Parameters of Alaternin
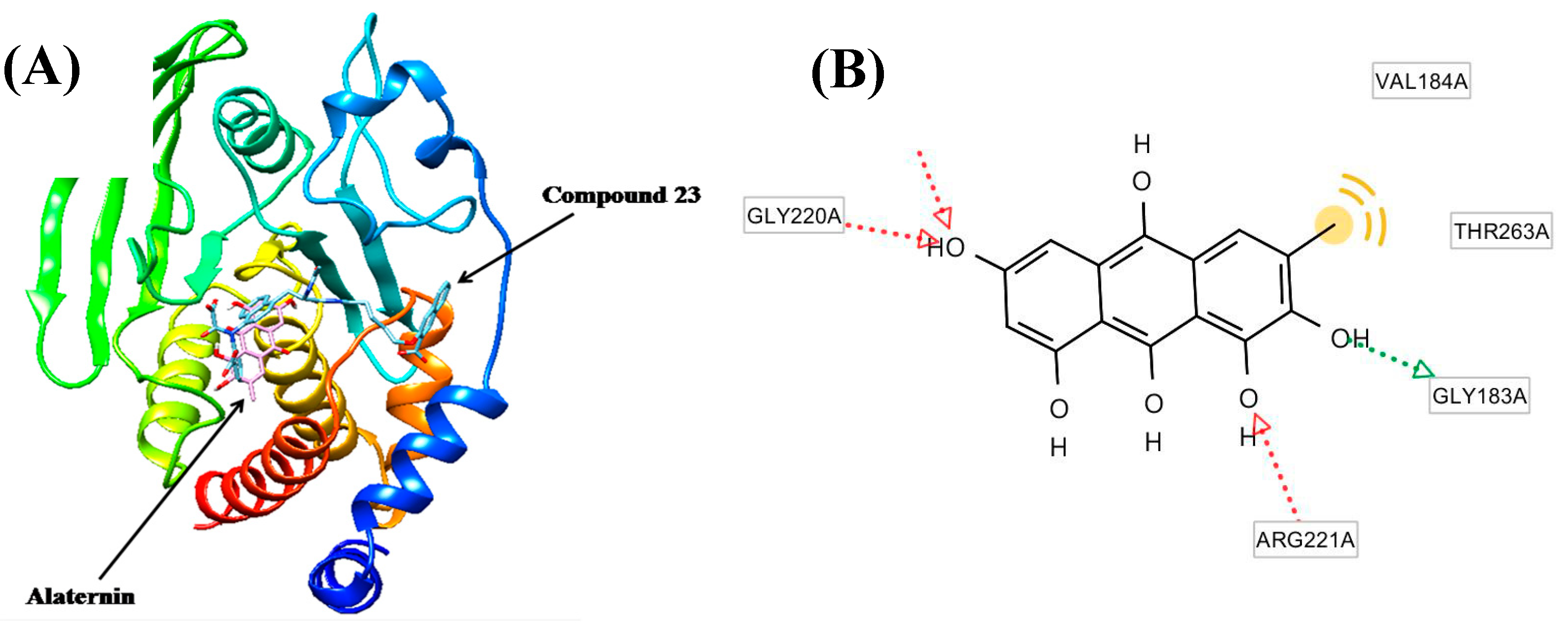
2.4. Molecular Docking Study of the Inhibitory Activity of Alaternin against PTP1B
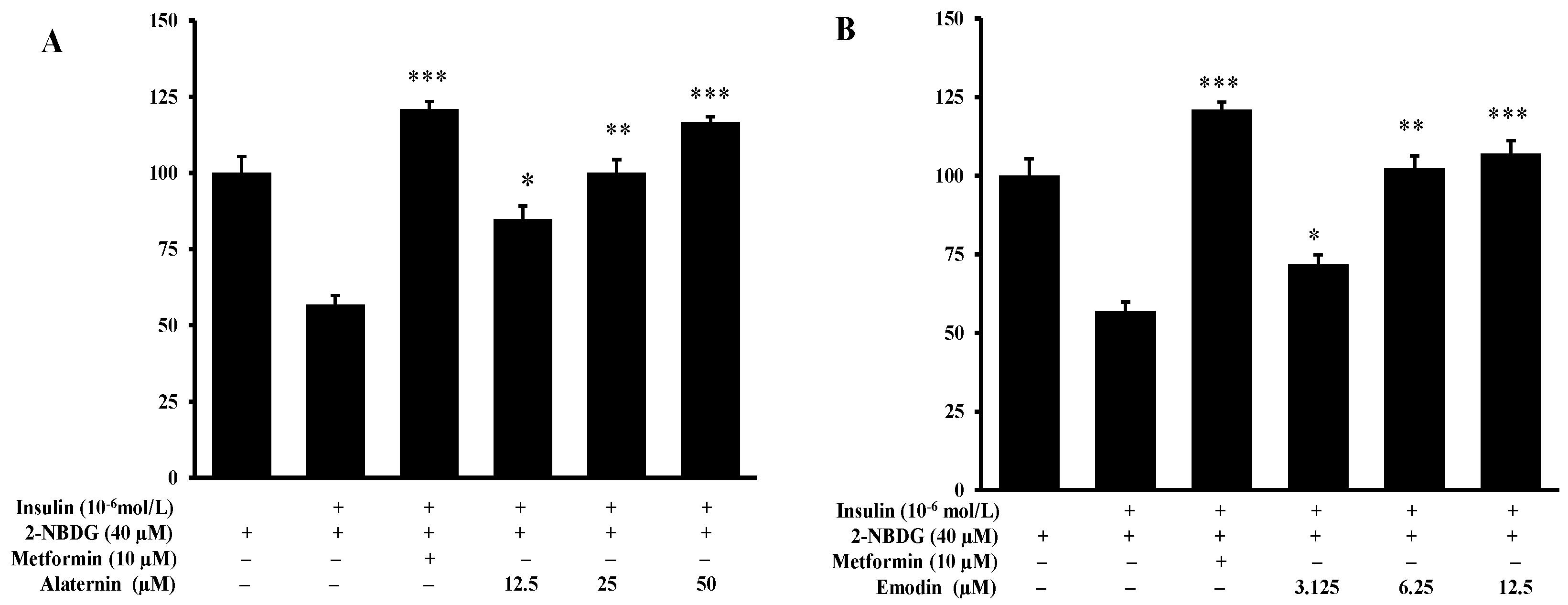
2.5. Effects of Alaternin and Emodin on Glucose Uptake
3. Discussion
4. Material and Methods
4.1. General Experimental Procedures
4.2. Chemicals and Reagents
4.3. Plant Material
4.4. Isolation of Compounds
4.5. α-Glucosidase Inhibitory Assay
4.6. Protein Tyrosine Phosphatase 1B (PTP1B) Inhibition Assay
4.7. Kinetic Parameters of Alaternin in Both PTP1B and α-Glucosidase Inhibitions
4.8. Molecular Docking Simulation in PTP1B Inhibition Using Autodock 4.2
4.9. 2-NBDG Glucose Uptake Assay
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hossain, P.; Kawar, B.; El Nahas, M. El Nahas M Obesity and diabetes in the developing world—A growing challenge. N. Engl. J. Med. 2007, 356, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.J.; Bitter-Kowalczyk, A.; White, M.F.; Harbeck, M.J. Tyrosine dephosphorylation and deactivation of insulin receptor substrate-1 by protein tyrosine phosphatase 1B. Possible facilitation by the formation of a ternary complex with the GRB2 adaptor protein. Biol. Chem. 2000, 275, 4283–4289. [Google Scholar] [CrossRef]
- Elchebly, M.; Payette, P.; Michaliszyn, E.; Cromlish, W.; Collins, S.; Loy, A.L.; Normandin, D.; Cheng, A.; Himms-Hagen, J.; Chan, C.C.; et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 1999, 283, 1544–1548. [Google Scholar] [CrossRef] [PubMed]
- Klaman, L.D.; Boss, O.; Peroni, O.D.; Kim, J.K.; Martino, J.L.; Zabolotny, J.M.; Moghal, N.; Lubkin, M.; Kim, Y.B.; Sharpe, A.H.; et al. Increased energy expenditure, decreased adiposity, and tissue specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol. Cell. Biol. 2000, 20, 5479–5489. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cong, L.N.; Li, Y. A phosphotyrosyl mimetic peptide reverses impairment of insulin-stimulated translocation of GLUT4 caused by over expression of PTP1B in rat adipose cells. Biochemistry 1999, 38, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Venable, C.L.; Frevert, E.U.; Kim, Y.B. Overexpression of protein tyrosine phosphatase 1B in adipocytes inhibits insulin-stimulated phosphoinositide 3-kinase activity without altering glucose transport or Akt/protein kinase B activation. J. Biol. Chem. 2000, 275, 18318–18326. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, M.R.; Nilubon, J.A.; Gao, K.J. α-Glucosidase and α-amylase inhibitory activities of Nepalese medicinal herb Pakhanbhed (Bergenia ciliata Haw.). Food Chem. 2008, 106, 247–252. [Google Scholar] [CrossRef]
- Kao, Y.H.; Chang, H.H.; Lee, M.J.; Cheng, C.L. Tea, obesity and diabetes. Mol. Nutr. Food Res. 2006, 50, 188–210. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Ali, M.Y.; Jung, H.J.; Jeong, H.O.; Chung, H.Y.; Choi, J.S. Inhibitory activities of major anthraquinones and other constituents from Cassia obtusifolia against β-secretase and cholinesterases. J. Ethnopharmacol. 2016, 191, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Wang, Z.J.; Fu, M.H.; Yan, H.; Wei, H.W.; Lu, Q.H. A new anthraquinone glycoside from seeds of Cassia obtusifolia. Chin. Chem. Lett. 2008, 19, 1083–1085. [Google Scholar]
- Guo, H.; Chang, Z.; Yang, R.; Guo, D.; Zheng, J. Anthraquinones from hairy root cultures of Cassia obtusifolia. Phytochemistry 1998, 49, 1623–1625. [Google Scholar] [CrossRef]
- Hemen, D.; Lalita, L. A review on anthraquinones isolated from Cassia species and their applications. Indian J. Nat. Prod. Resour. 2012, 3, 291–319. [Google Scholar]
- Choi, J.S.; Chung, H.Y.; Jung, H.A.; Park, H.J.; Yokozawa, T. Comparative evaluation of antioxidant potential of alaternin (2-hydroxyemodin) and emodin. J. Agric. Food Chem. 2000, 48, 6347–6351. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Lee, H.J.; Park, K.Y.; Jung, G.O. In vitro antimutagenic effects of alaternin and isorubrofusarin gentiobioside from roasted Cassia tora. Nat. Prod. Sci. 1998, 4, 100–104. [Google Scholar]
- Choi, J.S.; Lee, H.J.; Kang, S.S. Alaternin, cassiaside and rubrofusarin gentiobioside, radical scavenging principles from the seeds of Cassia tora on 1,1-diphenyl-2-picryhydrazyl (DPPH) radical. Arch. Pharm. Res. 1994, 17, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.K.; Lee, H.; Kang, S.S.; Chung, H.Y.; Choi, J.S. Inhibitory activities of Cassia tora and its anthraquinone constituents on angiotensin-converting enzyme. Phytother. Res. 2009, 23, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.S.; Kim, H.G.; Choi, J.G.; Ryu, J.H.; Hur, J.; Kim, Y.J.; Oh, M.S. Cassiae semen, a seed of Cassia obtusifolia, has neuroprotective effects in Parkinson’s disease models. Food Chem. Toxicol. 2010, 48, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Patil, U.K.; Saraf, S.; Dixit, V.K. Hypolipidemic activity of seeds of Cassia tora Linn. J. Ethnopharmacol. 2004, 90, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, M.; Ignacimuthu, S.; Agastian, P. Potential hepatoprotective activity of ononitol monohydrate isolated from Cassia tora L. on carbon tetrachloride induced hepatotoxicity in Wistar rats. Phytomedicine 2009, 16, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, T.F.; Lu, H.J.; Liou, S.S.; Chang, C.J.; Liu, I.M. Cassia tora (Leguminosae) seed extract alleviates high-fat diet-induced nonalcoholic fatty liver. Food Chem. Toxicol. 2013, 51, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.J.; Kim, S.Y.; Lee, J.W. The effects of Korean wild vegetables on blood glucose levels and liver muscle metabolism of streptozotocin-induced diabetic rats. Korean J. Nutr. 1995, 28, 585–594. [Google Scholar]
- Lim, S.J.; Han, H.K. Hypoglycemic effect of fractions of Cassia tora extract in streptozotocin-induced diabetic rats. J. Korean Soc. Food Sci. Nutr. 1997, 13, 23–29. [Google Scholar]
- Lee, G.Y.; Jang, D.S.; Lee, Y.M.; Kim, J.M.; Kim, J.S. Naphthopyrone glucosides from the seeds of Cassia tora with inhibitory activity on advanced glycation end products (AGEs) formation. Arch. Pharm. Res. 2006, 29, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Anselmino, M. Cardiovascular prevention in type 2 diabetes mellitus patients: The role of oral glucose-lowering agents. J. Diabetes Complicat. 2009, 23, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Scully, T. Diabetes in numbers. Nature 2012, 485, 2–3. [Google Scholar] [CrossRef]
- Israili, Z.H. Advances in the treatment of type 2 diabetes mellitus. Am. J. Ther. 2011, 18, 117–152. [Google Scholar] [CrossRef] [PubMed]
- Tonks, N.K.; Neal, B.G. Combinatorial control of the specificity of protein tyrosine phosphatases. Curr. Opin. Cell Biol. 2001, 13, 182–195. [Google Scholar] [CrossRef]
- Wu, X.; Hardy, V.E.; Joseph, J.I. Protein-tyrosine phosphatase activity in human adipocytes is strongly correlated with insulin-stimulated glucose uptake and is a target of insulin induced oxidative inhibition. Metabolism 2003, 52, 705–712. [Google Scholar] [CrossRef]
- Tiganis, T. PTP1B and TCPTP–non redundant phosphatases in insulin signaling and glucose homeostasis. FEBS J. 2013, 280, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Panzhinskiy, E.; Ren, J.; Nair, S. Pharmacological inhibition of protein tyrosine phosphatase 1B: A promising strategy for the treatment of obesity and type 2 diabetes mellitus. Curr. Med. Chem. 2013, 20, 2609–2625. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Azevedo, J.L.; Cortright, R.; Dohm, G.L.; Goldstein, B.J. Alterations in skeletal muscle protein-tyrosine phosphatase activity and expression in insulin-resistant human obesity and diabetes. J. Clin. Investig. 1997, 100, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.; Kusari, J.; Jansen, D.; Bandyopadhyay, D.; Kusari, A.; Bryer-Ash, M. Marked impairment of protein tyrosine phosphatase 1B activity in adipose tissue of obese subjects with and without type 2 diabetes mellitus. J. Lab. Clin. Med. 1999, 134, 115–123. [Google Scholar] [CrossRef]
- Stull, A.J.; Wang, Z.Q.; Zhang, X.H.; Yu, Y.; Johnson, W.D.; Cefalu, W.T. Skeletal muscle protein tyrosine phosphatase 1B regulates insulin sensitivity in African Americans. Diabetes 2012, 61, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Gum, R.J.; Gaede, L.L.; Koterski, S.L.; Heindel, M.; Clampit, J.E.; Zinker, B.A.; Trevillyan, J.M.; Ulrich, R.G.; Jirousek, M.R.; Rondinone, C.M. Reduction of protein tyrosine phosphatase 1B increases insulin-dependent signaling in ob/ob mice. Diabetes 2003, 52, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Popov, D. Novel protein tyrosine phosphatase 1B inhibitors: Interaction requirements for improved intracellular efficacy in type 2 diabetes mellitus and obesity control. Biochem. Biophys. Res. Commun. 2011, 410, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Yu, H.; Wang, J. α-Glucosidase inhibitory compounds from seeds of Cassia obtusifolia. Chem. Nat. Compd. 2012, 48, 465–466. [Google Scholar] [CrossRef]
- Xu, Y.L.; Tang, L.Y.; Zhou, X.D.; Zhou, G.H.; Wang, Z.J. Five new anthraquinones from the seed of Cassia obtusifolia. Arch. Pharm. Res. 2015, 38, 1054–1058. [Google Scholar] [CrossRef] [PubMed]
- Na, M.; Jin, W.Y.; Min, B.S.; Ahn, J.S.; Bae, K. Protein tyrosine phosphatase 1B inhibitory activity of anthraquinones and stilbenes. Nat. Prod. Sci. 2008, 14, 143–146. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock 4 and AutoDockTools 4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Bustanji, Y.; Al-Masri, I.M.; Qasem, A.; Al-Bakri, A.G.; Taha, M.O. In silico screening for non-nucleoside HIV-1 reverse transcriptase inhibitors using physicochemical filters and high-throughput docking followed by in vitro evaluation. Chem. Biol. Drug Des. 2009, 74, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Cho, Y.S.; Oh, S.H.; Lee, S.H.; Min, B.S.; Moon, K.H.; Choi, J.S. Kinetics and molecular docking studies of pimarane-type diterpenes as protein tyrosine phosphatase (PTP1B) inhibitors from Aralia continentalis roots. Arch. Pharm. Res. 2013, 36, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Szczepankiewicz, B.G.; Liu, G.; Hajduk, P.J.; Abad-Zapatero, C.; Pei, Z.; Xin, Z.; Lubben, T.H.; Trevillyan, J.M.; Stashko, M.A.; Ballaron, S.J.; et al. Discovery of a potent, selective protein tyrosine phosphatase 1B inhibitor using a linked-fragment strategy. J. Am. Chem. Soc. 2003, 125, 4087–4096. [Google Scholar] [CrossRef] [PubMed]
- Radhika, R.; Sudarsanam, D. Docking of Rheum emodi compounds against protein tyrosine phosphatase 1B. Int. J. Pharm. Biol. Sci. 2012, 3, 1150–1154. [Google Scholar]
- Rauwald, H.W.; Just, H.D. Isolierung und Charakterisierung der blaufluoreszierenden Leitstoffe aus der Rinde von Rhamnus catharticus L. Planta Med. 1981, 42, 108–109. [Google Scholar] [CrossRef] [PubMed]
- Park, T.H.; Kim, D.H.; Kim, C.H.; Jung, H.A.; Choi, J.S.; Lee, J.W.; Chung, H.Y. Peroxynitrite scavenging mode of alaternin isolated from Cassia tora. J. Pharm. Pharmacol. 2004, 56, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.Y.; Kim, D.H.; Hyun, S.K.; Jung, H.A.; Kim, J.M.; Park, S.J.; Kim, S.Y.; Cheong, J.H.; Choi, J.S.; Ryu, J.H. Alaternin attenuates delayed neuronal cell death induced transient cerebral hyperfusion in mice. Food Chem. Toxicol. 2010, 48, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Du, D.; Liu, F.; She, Z.; Lin, Y.; Zheng, Z. Secondary metabolites of a marine mangrove fungus Paecilomyces sp. Tree 1–7. Zhongshan Daxue Xuebao Ziran Kexueban 2007, 46, 125–127. [Google Scholar]
- Choi, J.S.; Lee, H.J.; Park, K.Y.; Ha, J.O.; Kang, S.S. In vitro antimutagenic effects of anthraquinone aglycons and naphthopyrone glycosides from Cassia tora. Planta Med. 1997, 63, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, X.D.; Song, Y.W.; Liu, J.W. A microplate-based screening method for α-glucosidase inhibitors. Chin. J. Clin. Pharmacol. Ther. 2005, 10, 1128–1134. [Google Scholar]
- Cui, L.; Na, M.; Oh, H.; Bae, E.Y.; Jeong, D.G.; Ryu, S.E.; Kim, S.; Kim, B.Y.; Oh, W.K.; Ahn, J.S. Protein tyrosine phosphatase 1B inhibitors from Morus root bark. Bioorg. Med. Chem. Lett. 2006, 16, 1426–1429. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Liu, T.; Chen, C.; Li, M.Y.; Wang, Z.Y.; Chen, R.S.; Wei, G.X.; Wang, X.Y.; Luo, D.Q. Fumosorinone, a novel PTP1B inhibitor, activates insulin signaling in insulin-resistance HepG2 cells and shows anti-diabetic effect in diabetic KKAy mice. Toxicol. Appl. Pharmacol. 2015, 285, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.


| Test Samples | PTP1B a | α-Glucosidase b |
|---|---|---|
| IC50 (Mean ± SEM) | IC50 (Mean ± SEM) | |
| MeOH extract | 14.79 ± 0.31 | 200.07 ± 7.90 |
| CH2Cl2 fraction | 85.31 ± 3.43 | 359.36 ± 10.81 |
| EtOAc fraction | 57.90 ± 0.92 | 74.50 ± 4.93 |
| n-BuOH fraction | 172.82 ± 4.87 | 372.12 ± 11.88 |
| H2O fraction | 214.52 ± 3.42 | 434.02 ± 12.61 |
| Ursolic acid c | 3.37 ± 0.18 | |
| Acarbose d | 123.54 ± 0.29 |
| Test Compounds | PTP1B a | α-Glucosidase b |
|---|---|---|
| IC50 (Mean ± SEM) | IC50 (Mean ± SEM) | |
| Anthraquinones | ||
| Physcion | 7.28 ± 0.49 | 2.38 ± 0.77 |
| Chrysophanol | 5.86 ± 0.99 | 46.81 ± 0.12 |
| Emodin | 3.51 ± 0.15 | 1.02 ± 0.01 |
| Alaternin | 1.22 ± 0.03 | 0.99 ± 0.02 |
| Obtusifolin | 35.27 ± 0.98 | 142.12 ± 0.77 |
| Obtusin | 6.44 ± 0.22 | 20.92 ± 0.41 |
| Questin | 5.69 ± 0.47 | 136.19 ± 0.01 |
| Chryso-obtusin | 14.88 ± 0.77 | 36.01 ± 0.89 |
| Aurantio-obtusin | 27.19 ± 0.31 | 41.20 ± 0.17 |
| 2-Hydroxyemodin-1 methylether | 5.22 ± 0.29 | 5.65 ± 0.20 |
| Gluco-obtusifolin | 53.35 ± 0.44 | 23.77 ± 0.72 |
| Gluco-aurantio obtusin | 31.30 ± 0.97 | 142.19 ± 1.22 |
| Chryso-obtusin-2-glucoside | 39.34 ± 1.07 | 178.85 ± 0.55 |
| Chrysophanol triglucoside | 80.17 ± 1.77 | 197.06 ± 1.09 |
| Chrysophanol tetraglucoside | 103.89 ± 1.22 | 228.79 ± 0.91 |
| Naphthopyrone glycosides | ||
| Cassiaside | 48.55 ± 1.27 | 129.23 ± 0.98 |
| Toralactone gentiobioside | 81.15 ± 0.15 | 37.60 ± 0.79 |
| Naphthalene glycoside | ||
| Cassitoroside | 103.89 ± 1.22 | 172.59 ± 0.74 |
| Aloe-emodin | 56.01 ± 0.76 | 1.40 ± 0.27 |
| Ursolic acid c | 3.37 ± 0.18 | |
| Acarbose d | 123.54 ± 0.29 | |
| Test Sample | PTP1B | α-Glucosidase |
|---|---|---|
| IC50 (µM) a | 1.61 | 1.31 |
| Ki b | 1.70 | 0.66 |
| Inhibition type c | Competitive | Mixed |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.A.; Ali, M.Y.; Choi, J.S. Promising Inhibitory Effects of Anthraquinones, Naphthopyrone, and Naphthalene Glycosides, from Cassia obtusifolia on α-Glucosidase and Human Protein Tyrosine Phosphatases 1B. Molecules 2017, 22, 28. https://doi.org/10.3390/molecules22010028
Jung HA, Ali MY, Choi JS. Promising Inhibitory Effects of Anthraquinones, Naphthopyrone, and Naphthalene Glycosides, from Cassia obtusifolia on α-Glucosidase and Human Protein Tyrosine Phosphatases 1B. Molecules. 2017; 22(1):28. https://doi.org/10.3390/molecules22010028
Chicago/Turabian StyleJung, Hyun Ah, Md Yousof Ali, and Jae Sue Choi. 2017. "Promising Inhibitory Effects of Anthraquinones, Naphthopyrone, and Naphthalene Glycosides, from Cassia obtusifolia on α-Glucosidase and Human Protein Tyrosine Phosphatases 1B" Molecules 22, no. 1: 28. https://doi.org/10.3390/molecules22010028
APA StyleJung, H. A., Ali, M. Y., & Choi, J. S. (2017). Promising Inhibitory Effects of Anthraquinones, Naphthopyrone, and Naphthalene Glycosides, from Cassia obtusifolia on α-Glucosidase and Human Protein Tyrosine Phosphatases 1B. Molecules, 22(1), 28. https://doi.org/10.3390/molecules22010028






