Combination of Morroniside and Diosgenin Prevents High Glucose-Induced Cardiomyocytes Apoptosis
Abstract
:1. Introduction
2. Results
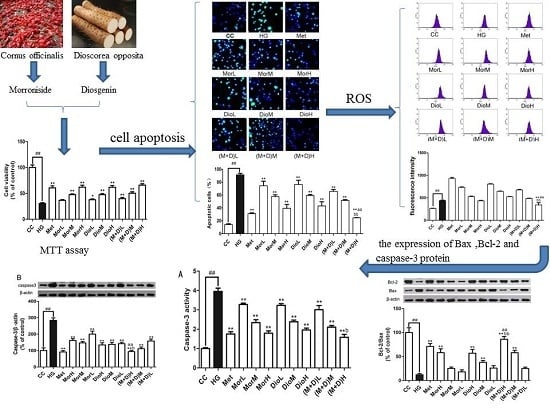
2.1. Effects of (M + D)H on Cell Viability
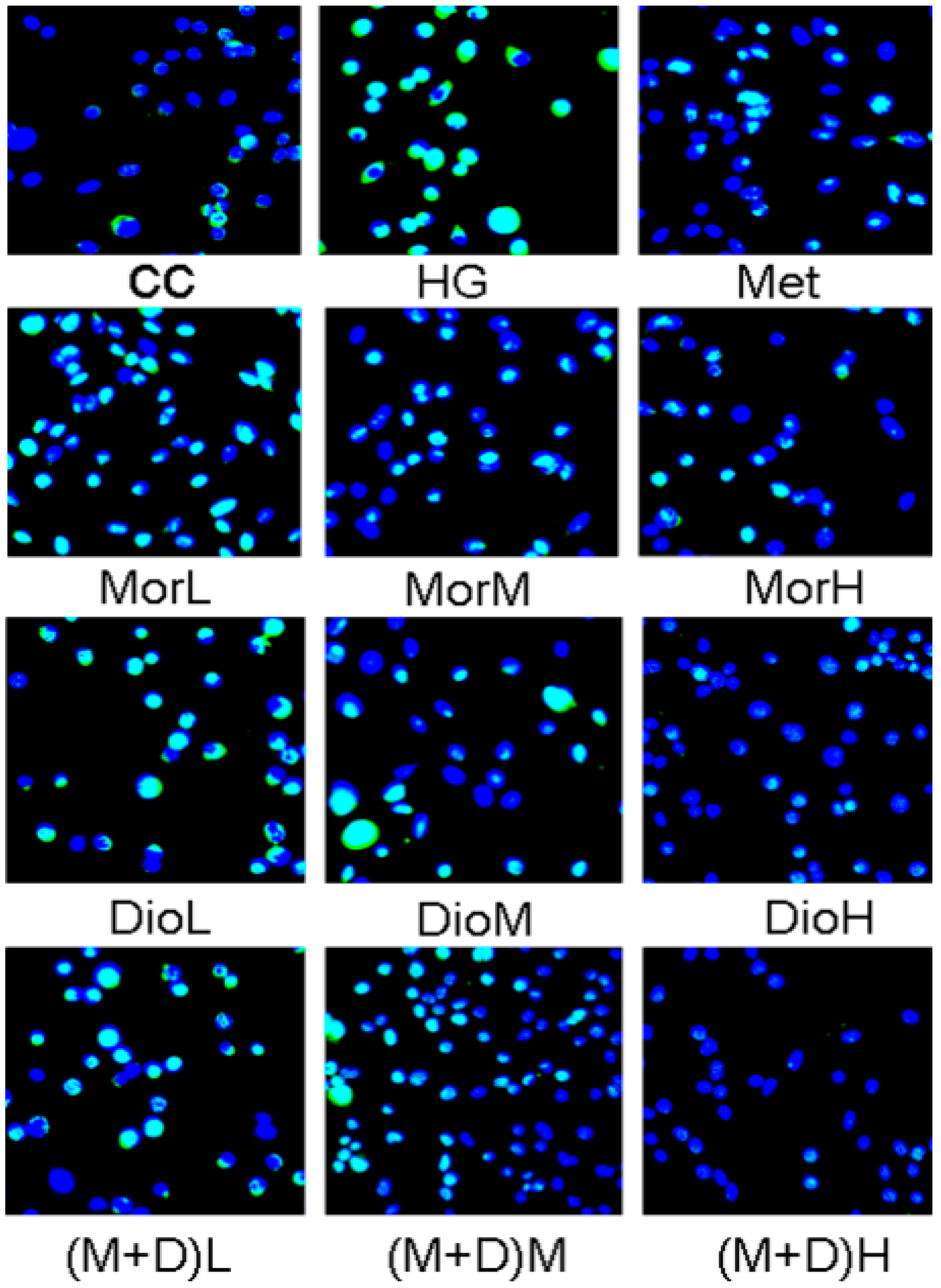
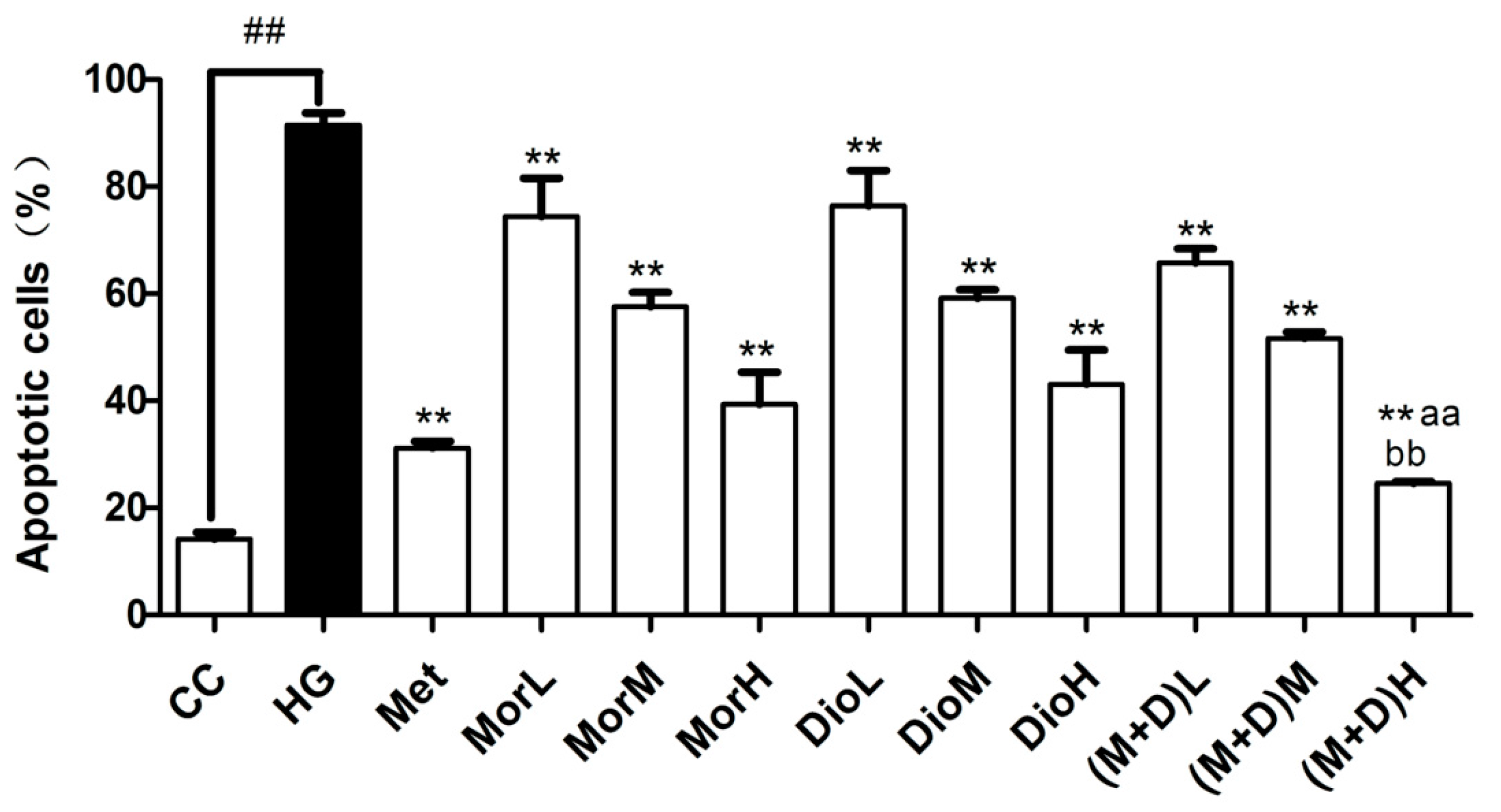
2.2. Effects of (M + D)H on Cell Apoptosis
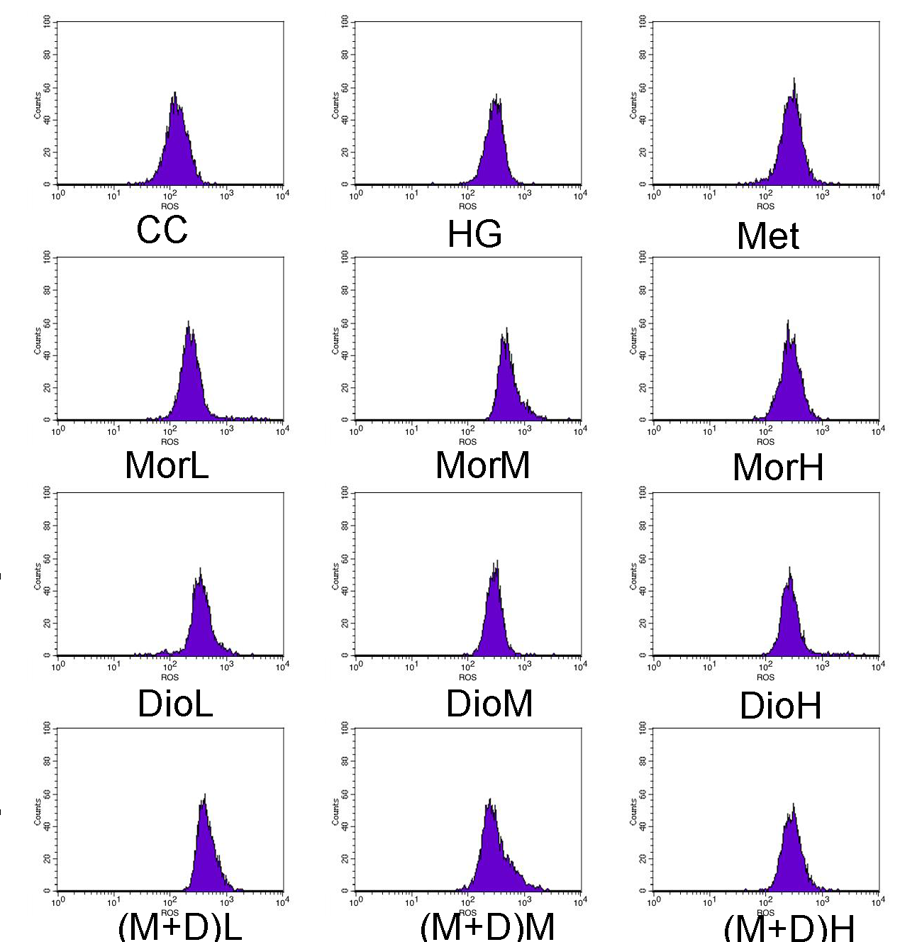
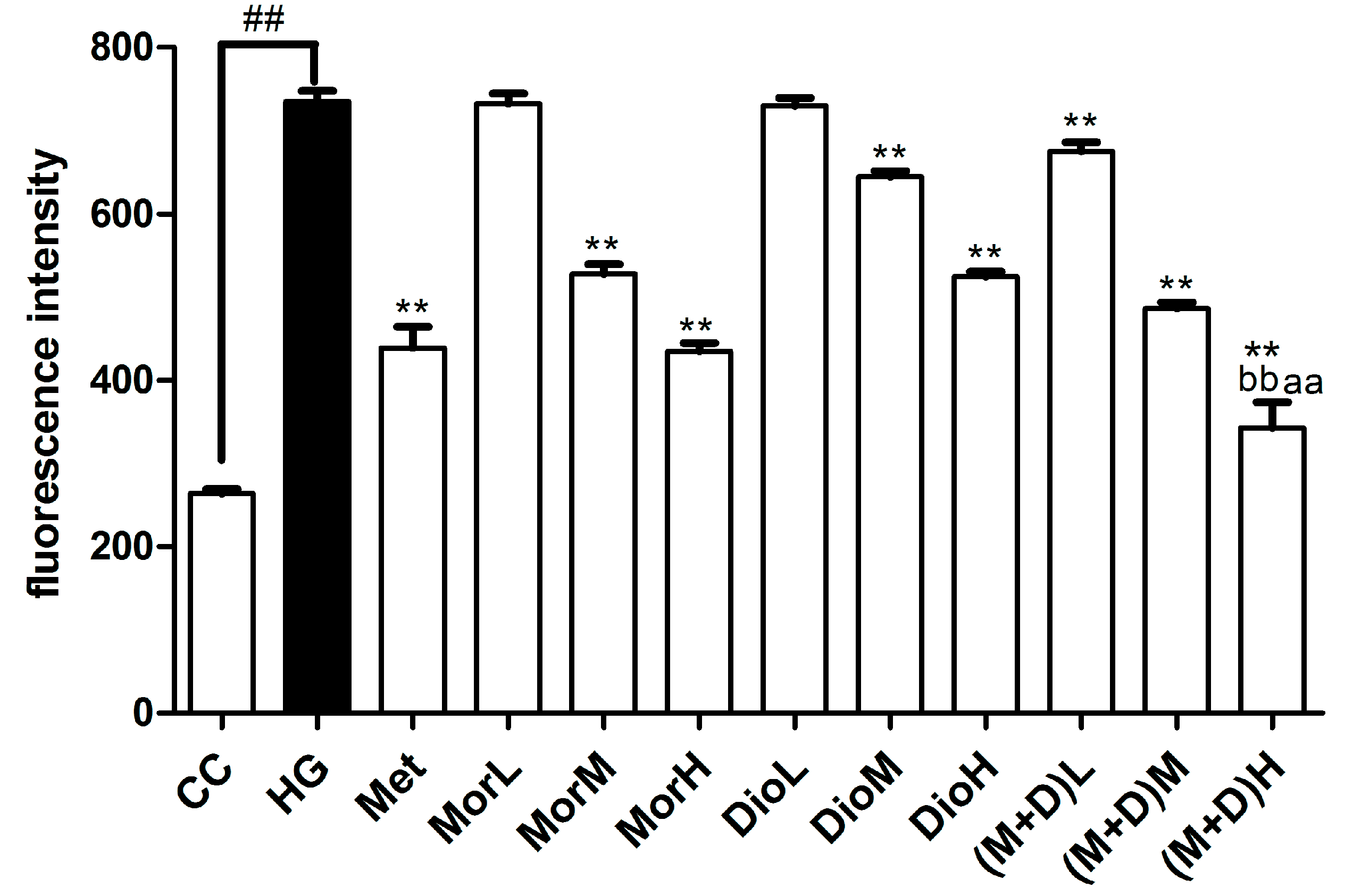
2.3. Effects of (M + D)H on Intracellular Reactive Oxygen Species (ROS)
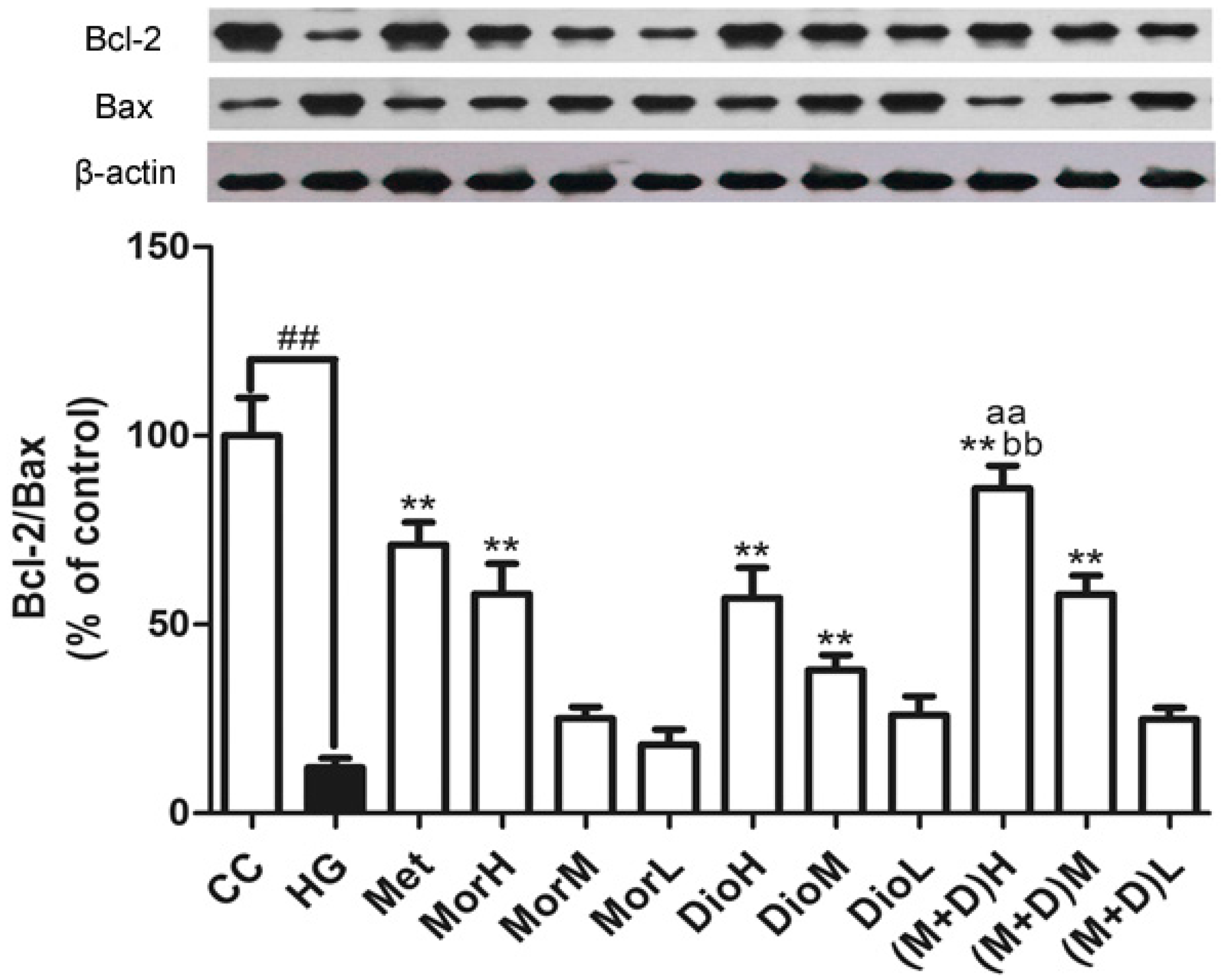
2.4. Effects of (M + D)H on the Expression Levels of Bax and Bcl-2 Protein
2.5. Effects of (M + D)H on the Expression Levels of Caspase-3 Protein
2.6. Effects of (M + D)H on Caspase-3 Activity
2.7. Discussion
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Primary Culture of Rat Cardiomyocytes
3.3. Grouping
3.4. Analysis of Cell Viability by MTT Assay
3.5. TUNEL Staining
3.6. Detection of Intracellular Reactive Oxygen Species
3.7. Western Blot Analysis
3.8. Caspase-3 Activity Assay
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DCM | diabetic cardiomyopathy |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
References
- Rubler, S.; Dlugash, J.; Yuceoglu, Y.Z.; Kumral, T.; Branwood, A.W.; Grishman, A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am. J. Cardiol. 1972, 30, 595–602. [Google Scholar] [CrossRef]
- Cai, L.; Li, W.; Wang, G.; Guo, L.; Jiang, Y.; Kang, Y.J. Hyperglycemia-induced apoptosis in mouse myocardium: Mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes 2002, 51, 1938–1948. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.H.; Xie, Z. Regulation of interplay between autophagy and apoptosis in the diabetic heart: New role of AMPK. Autophagy 2013, 9, 624–625. [Google Scholar] [CrossRef] [PubMed]
- Boudina, S.; Abel, E.D. Diabetic cardiomyopathy revisited. Circulation 2007, 115, 3213–3223. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Y.; Gan, X.; Liu, K.; Xing, L.V.; Shen, H.; Dai, G.; Xu, H. Iridoid glycoside from Cornus officinalis ameliorated diabetes mellitus-induced testicular damage in male rats: Involvement of suppression of the AGEs/RAGE/p38 MAPK signaling pathway. J. Ethnopharmacol. 2016, 194, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, H.; Zhang, D.; Liu, X.; Zhao, F.; Pang, X.; Wang, Q. Effect of advanced glycosylation end products on apoptosis in human adipose tissue-derived stem cells in vitro. Cell Biosci. 2015, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Mallidis, C.; Agbaje, I.; Rogers, D.; Glenn, J.; McCullough, S.; Atkinson, A.B.; Steger, K.; Stitt, A.; McClure, N. Distribution of the receptor for advanced glycation end products in the human male reproductive tract: Prevalence in men with diabetes mellitus. Hum. Reprod. 2007, 22, 2169–2177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, J.; Zhang, L.; Zhou, Y.; Xiao, J.; Li, S.; Chen, Y.; Qiao, Z.; Niu, J.; Gu, Y. Angiotensin-(1-7) attenuates damage to podocytes induced by preeclamptic serum through MAPK pathways. Int. J. Mol. Med. 2014, 34, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Normile, D. Asian medicine. The new face of traditional Chinese medicine. Science 2003, 299, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Roy, R. Studying traditional Chinese medicine. Science 2003, 300, 740–741. [Google Scholar] [CrossRef] [PubMed]
- Chinese Herbal Medicine Shanghai Science and Technology Press. Editorial Committee of Chinese Herbal Medicine; Chinese Herbal Medicine Shanghai Science and Technology Press: Shanghai, China, 1999; p. 4931. [Google Scholar]
- Park, C.H.; Noh, J.S.; Kim, J.H.; Tanaka, T.; Zhao, Q.; Matsumoto, K.; Shibahara, N.; Yokozawa, T. Evaluation of morroniside, iridoid glycoside from Corni Fructus, on diabetes-induced alterations such as oxidative stress, inflammation, and apoptosis in the liver of type 2 diabetic db/db mice. Biol. Pharm. Bull. 2011, 34, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Pi, W.; Cai, B.; Feng, X. Study on protective mechanism of loganin and Morroniside on high glucose induced myocardial injury. Chin. Tradit. Pat. Med. 2016, 38, 160–163. [Google Scholar]
- Liu, Z.; Zhu, Z.; Zhang, H.; Tan, G.; Chen, X.; Chai, Y. Qualitative and quantitative analysis of Fructus Corni using ultrasound assisted microwave extraction and high performance liquid chromatography coupled with diode array UV detection and time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2011, 55, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, J.; Li, L.; Wang, P.; Ji, X.; Ai, H.; Zhang, L.; Li, L. Neuroprotective effect of morroniside on focal cerebral ischemia in rats. Brain Res. Bull. 2010, 83, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.J.; Lu, T.J.; Hwang, L.S. Isolation and identification of steroidal saponins in Taiwanese yam cultivar (Dioscorea pseudojaponica Yamamoto). J. Agric. Food Chem. 2003, 51, 6438–6444. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Su, Y.; Zhen, Z.; Huang, X.; Zhou, P. Study on the concerted application rules of Shanyao (Dioscorea opposita Thunb.) in formulae. China J. Tradit. Chin. Med. Pharm. 2013, 28, 328–330. [Google Scholar]
- Lin, J.T.; Liu, S.C.; Chen, S.L.; Chen, H.Y.; Yang, D.J. Effects of domestic processing on steroidal saponins in Taiwanese yam cultivar (Dioscorea pseudojaponica Yamamoto). J. Agric. Food Chem. 2006, 54, 9948–9954. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, G.; Ponmurugan, P.; Deepa, M.A.; Senthilkumar, B. Modulatory effects of diosgenin on attenuating the key enzymes activities of carbohydrate metabolism and glycogen content in streptozotocin-induced diabetic rats. Can. J. Diabetes 2014, 38, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Kalailingam, P.; Kannaian, B.; Tamilmani, E.; Kaliaperumal, R. Efficacy of natural diosgenin on cardiovascular risk, insulin secretion, and beta cells in streptozotocin (STZ)-induced diabetic rats. Phytomedicine 2014, 21, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Kanthasamy, A.; Kitazawa, M.; Anantharam, V.; Kanthasamy, A.G. Caspase-3 dependent proteolytic activation of protein kinase C delta mediates and regulates 1-methyl-4-phenylpyridinium (MPP+)-induced apoptotic cell death in dopaminergic cells: Relevance to oxidative stress in dopaminergic degeneration. Eur. J. Neurosci. 2003, 18, 1387–1401. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zheng, D.; Fung, G.; Deng, H.; Chen, L.; Liang, J.; Jiang, Y.; Hu, Y. Mangiferin suppressed advanced glycation end products (AGEs) through NF-κB deactivation and displayed anti-inflammatory effects in streptozotocin and high fat diet-diabetic cardiomyopathy rats. Can. J. Physiol. Pharmacol. 2016, 94, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Khullar, M.; Al-Shudiefat, A.A.; Ludke, A.; Binepal, G.; Singal, P.K. Oxidative stress: A key contributor to diabetic cardiomyopathy. Can. J. Physiol. Pharmacol. 2010, 88, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Thandavarayan, R.A.; Harima, M.; Sari, F.R.; Gurusamy, N.; Veeraveedu, P.T.; Mito, S.; Arozal, W.; Sukumaran, V.; Laksmanan, A.P.; et al. Role of differential signaling pathways and oxidative stress in diabetic cardiomyopathy. Curr. Cardiol. Rev. 2010, 6, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, R.C.; Yang, Z.H.; Sun, G.B.; Wang, M.; Ma, X.J.; Yang, L.J.; Sun, X.B. Taxifolin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of oxidative stress and cell apoptosis. Food Chem. Toxicol. 2014, 63, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, R.A.; Burleson, K.O.; Kloner, R.A.; Babior, B.M.; Engler, R.L. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J. Clin. Investig. 1994, 94, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Bialik, S.; Geenen, D.L.; Sasson, I.E.; Cheng, R.; Horner, J.W.; Evans, S.M.; Lord, E.M.; Koch, C.J.; Kitsis, R.N. Myocyte apoptosis during acute myocardial infarction in the mouse localizes to hypoxic regions but occurs independently of p53. J. Clin. Investig. 1997, 100, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zuo, L.; Lv, Y.; Chen, C.; Yang, Y.; Xin, H.; Li, Y.; Qian, Y. Asiatic Acid Attenuates Myocardial Ischemia/Reperfusion Injury via Akt/GSK-3β/HIF-1α Signaling in Rat H9c2 Cardiomyocytes. Molecules 2016, 21, 1248. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zong, L.; Wang, X. TGF-beta improves myocardial function and prevents apoptosis induced by anoxia-reoxygenation, through the reduction of endoplasmic reticulum stress. Can. J. Physiol. Pharmacol. 2016, 94, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Hasenjager, A.; Gillissen, B.; Muller, A.; Normand, G.; Hemmati, P.G.; Schuler, M.; Dorken, B.; Daniel, P.T. Smac induces cytochrome c release and apoptosis independently from Bax/Bcl-x(L) in a strictly caspase-3-dependent manner in human carcinoma cells. Oncogene 2004, 23, 4523–4535. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, A.M.; Abuel-Ela, H.A.; El-Shourbagy, S.H. Increased caspase-3 and altered expression of apoptosis-associated proteins, Bcl-2 and Bax in lichen planus. Clin. Exp. Dermatol. 2009, 34, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Produit-Zengaffinen, N.; Pournaras, C.J.; Schorderet, D.F. Retinal ischemia-induced apoptosis is associated with alteration in Bax and Bcl-x(L) expression rather than modifications in Bak and Bcl-2. Mol. Vis. 2009, 15, 2101–2110. [Google Scholar] [PubMed]
- Hosseinzadeh, L.; Behravan, J.; Mosaffa, F.; Bahrami, G.; Bahrami, A.; Karimi, G. Curcumin potentiates doxorubicin-induced apoptosis in H9c2 cardiac muscle cells through generation of reactive oxygen species. Food Chem. Toxicol. 2011, 49, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huo, Z.; Yan, B.; Lin, X.; Zhou, Z.N.; Liang, X.; Zhu, W.; Liang, D.; Li, L.; Liu, Y.; et al. Prolyl hydroxylase 3 interacts with Bcl-2 to regulate doxorubicin-induced apoptosis in H9c2 cells. Biochem. Biophys. Res. Commun. 2010, 401, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; He, M.; Yi, B.; Guo, W.H.; Que, A.L.; Zhang, J.X. Pim-3 protects against cardiomyocyte apoptosis in anoxia/reoxygenation injury via p38-mediated signal pathway. Int. J. Biochem. Cell Biol. 2009, 41, 2315–2322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pang, S.; Deng, B.; Qian, L.; Chen, J.; Zou, J.; Zheng, J.; Yang, L.; Zhang, C.; Chen, X.; et al. High glucose induces renal mesangial cell proliferation and fibronectin expression through JNK/NF-κB/NADPH oxidase/ROS pathway, which is inhibited by resveratrol. Int. J. Biochem. Cell Biol. 2012, 44, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds Morroniside and Diosgenin are available from the authors.


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pi, W.-X.; Feng, X.-P.; Ye, L.-H.; Cai, B.-C. Combination of Morroniside and Diosgenin Prevents High Glucose-Induced Cardiomyocytes Apoptosis. Molecules 2017, 22, 163. https://doi.org/10.3390/molecules22010163
Pi W-X, Feng X-P, Ye L-H, Cai B-C. Combination of Morroniside and Diosgenin Prevents High Glucose-Induced Cardiomyocytes Apoptosis. Molecules. 2017; 22(1):163. https://doi.org/10.3390/molecules22010163
Chicago/Turabian StylePi, Wen-Xia, Xiao-Peng Feng, Li-Hong Ye, and Bao-Chang Cai. 2017. "Combination of Morroniside and Diosgenin Prevents High Glucose-Induced Cardiomyocytes Apoptosis" Molecules 22, no. 1: 163. https://doi.org/10.3390/molecules22010163
APA StylePi, W.-X., Feng, X.-P., Ye, L.-H., & Cai, B.-C. (2017). Combination of Morroniside and Diosgenin Prevents High Glucose-Induced Cardiomyocytes Apoptosis. Molecules, 22(1), 163. https://doi.org/10.3390/molecules22010163