13C-NMR Spectral Data of Alkaloids Isolated from Psychotria Species (Rubiaceae)
Abstract
:1. Introduction
2. Discussion
2.1. 13C-NMR Chemical Shifts of Monoterpene Indole Alkaloids Isolated from Psychotria Species
2.2. 13C-NMR Chemical Shifts of Pyrrolidinoindoline Alkaloids Isolated from Psychotria Species
2.3. 13C-NMR Chemical Shifts of Benzoquinolizidine Alkaloids Isolated from Psychotria Species
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Marques de Oliveira, A.; Lyra Lemos, R.P.; Conserva, L.M. β-Carboline alkaloids from Psychotria barbiflora DC. (Rubiaceae). Biochem. Syst. Ecol. 2013, 50, 339–341. [Google Scholar] [CrossRef]
- Takayama, H.; Mori, I.; Kitajima, M.; Aimi, N.; Lajis, N.H. New Type of Trimeric and Pentameric Indole Alkaloids from Psychotria rostrata. Org. Lett. 2004, 6, 2945–2948. [Google Scholar] [CrossRef] [PubMed]
- Benevides, P.J.C.; Young, M.C.M.; Bolzani, V.D.S. Biological Activities of Constituentsfrom Psychotria spectabilis. Pharm. Biol. 2004, 42, 565–569. [Google Scholar] [CrossRef]
- Amador, T.A.; Verotta, L.; Nunes, D.S.; Elisabetsky, E. Antinociceptive profile of hodgkinsine. Planta Med. 2000, 66, 770–772. [Google Scholar] [CrossRef] [PubMed]
- Both, F.L.; Kerber, V.A.; Henriques, A.T.; Elisabetsky, E. Analgesic Properties of Umbellatine from Psychotria umbellata. Pharm. Biol. 2002, 40, 336–341. [Google Scholar] [CrossRef]
- Matsuura, H.N.; Fett-Neto, A.G. The major indole alkaloid N,β-d-glucopyranosyl vincosamide from leaves of Psychotria leiocarpa Cham. & Schltdl. is not an antifeedant but shows broad antioxidant activity. Nat. Prod. Res. 2013, 27, 402–411. [Google Scholar] [PubMed]
- Muhammad, I.; Dunbar, D.C.; Khan, S.I.; Tekwani, B.L.; Bedir, E.; Takamatsu, S.; Ferreira, D.; Walker, L.A. Antiparasitic Alkaloids from Psychotria klugii. J. Nat. Prod. 2003, 66, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, Z.; Musa, M.; Ismail, N.; Lajis, N.H. Cytotoxic and Bacteriocidal Activities of Psychotria rostrata. Int. J. Pharmacogn. 1993, 31, 142–146. [Google Scholar] [CrossRef]
- Adjibade, Y.; Kuballa, B.; Cabalion, P.; Jung, M.L.; Beck, J.P.; Anton, R. Cytotoxicity on human leukemic and rat hepatoma cell lines of alkaloid extracts of Psychotria forsteriana. Planta Med. 1989, 55, 567–568. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.D. Medicinal Natural Products: A Biosynthetic Approach; Wiley: West Sussex, UK, 2002; p. 350. [Google Scholar]
- O’Connor, S.E.; Maresh, J.J. Chemistry and biology of monoterpene indole alkaloid biosynthesis. Nat. Prod. Rep. 2006, 23, 532–547. [Google Scholar] [CrossRef] [PubMed]
- Fasshuber, H. Strukturbestimmung sowie Untersuchung der Enzymkatalysierten Hydrolyse von Naturstoffen mit Glycosidstruktur aus der Pflanzengattung Psychotria L. Master’s Thesis, Universität Wien, Vienna, Austria, 2011; p. 96. [Google Scholar]
- Patthy-luka, A.; Károlyházy, L.; Szabó, L.F.; Podányi, B. First Direct and Detailed Stereochemical Analysis of Strictosidine. J. Nat. Prod. 1997, 60, 69–75. [Google Scholar] [CrossRef]
- Achenbach, H.; Lottes, M.; Waibel, R.; Karikas, G.A.; Correa, M.D.; Gupta, M.P. Alkaloids and other compounds from Psychotria correae. Phytochemistry 1995, 38, 1537–1545. [Google Scholar] [CrossRef]
- Pimenta, A.T.A.; Braz-Filho, R.; Delprete, P.G.; de Souza, E.B.; Silveira, E.R.; Lima, M.A.S. Structure elucidation and NMR assignments of two unusual monoterpene indole alkaloids from Psychotria stachyoides. Magn. Reson. Chem. 2010, 48, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Farias, F.M.; Konrath, E.L.; Zuanazzi, J.A.S.; Henriques, A.T. Strictosamide from Psychotria nuda (Cham. et Schltdl) Wawra (Rubiaceae). Biochem. Syst. Ecol. 2008, 36, 919–920. [Google Scholar] [CrossRef]
- Paul, J.H.A.; Maxwell, A.R.; Reynolds, W.F. Novel Bis ( monoterpenoid ) Indole Alkaloids from Psychotria bahiensis. J. Nat. Prod. 2003, 66, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Faria, E.O.; Kato, L.; de Oliveira, C.M.A.; Carvalho, B.G.; Silva, C.C.; Sales, L.S.; Schuquel, I.T.A.; Silveira-Lacerda, E.P.; Delprete, P.G. Quaternary β-carboline alkaloids from Psychotria prunifolia (Kunth) Steyerm. Phytochem. Lett. 2010, 3, 113–116. [Google Scholar] [CrossRef]
- Van De Santos, L.; Fett-Neto, A.G.; Kerber, V.A.; Elisabetsky, E.; Quirion, J.-C.; Henriques, A.T. Indole monoterpene alkaloids from leaves of Psychotria suterella Müll. Arg. (Rubiaceae). Biochem. Syst. Ecol. 2001, 29, 1185–1187. [Google Scholar] [CrossRef]
- Kato, L.; De Oliveira, C.M.A.; Faria, E.O.; Ribeiro, L.C.; Carvalho, B.G.; Da Silva, C.C.; Schuquel, I.T.A.; Santin, S.M.O.; Nakamura, C.V.; Britta, E.A.; et al. Antiprotozoal Alkaloids from Psychotria prunifolia (Kunth) Steyerm. J. Braz. Chem. Soc. 2012, 23, 355–360. [Google Scholar]
- Kerber, V.A.; Gregianini, T.S.; Paranhos, T.; Farias, F.; Fett, J.P.; Fett-neto, A.G.; Zuanazzi, S.; Quirion, J.; Elizabetsky, E.; Henriques, T. Brachycerine, a Novel Monoterpene Indole Alkaloid from Psychotria brachyceras. J. Nat. Prod. 2001, 64, 677–679. [Google Scholar] [CrossRef] [PubMed]
- Kerber, V.A.; Passos, C.S.; Verli, H.; Quirion, J.P.; Henriques, A.T. Psychollatine, a Glucosidic Monoterpene Indole Alkaloid from Psychotria umbellata. J. Nat. Prod. 2008, 71, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Fasshuber, H.; Schinnerl, J.; Brecker, L.; Greger, H. Various types of tryptamine-iridoid alkaloids from Palicourea acuminata (=Psychotria acuminata, Rubiaceae). Phytochem. Lett. 2012, 5, 558–562. [Google Scholar] [CrossRef]
- Farias, F.M.; Passos, C.S.; Arbo, M.D.; Barros, D.M.; Gottfried, C.; Steffen, V.M.; Henriques, A.T. Strictosidinic acid, isolated from Psychotria myriantha Mull. Arg. (Rubiaceae), decreases serotonin levels in rat hippocampus. Fitoterapia 2012, 83, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Simoes-Pires, C.A.; Farias, F.M.; Martston, A.; Queiroz, E.; Chaves, C.G.; Henriques, A.T.; Hostettmann, K. Indole monoterpenes with antichemotactic activity from Psychotria myriantha: Chemotaxonomic significance. Nat. Prod. Commun. 2006, 1, 1101–1106. [Google Scholar]
- Morita, H.; Ichihara, Y.; Takeya, K.; Watanabe, K.; Itokawa, H.; Motidome, M. A New Indole Alkaloid Glycoside from the Leaves of Palicourea marcgravii. Planta Med. 1989, 55, 288–289. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, F.; Messana, I.; Botta, B.; de Mello, J.F. Constituents of Guettarda Platypoda. J. Nat. Prod. 1986, 49, 1150–1151. [Google Scholar] [CrossRef]
- Henriques, A.T.; Lopes, S.O.; Paranhos, J.T.; Gregianini, T.S.; Von Poser, G.L.; Fett-Neto, A.G.; Schripsema, J. N,β-d-Glucopyranosyl vincosamide, a light regulated indole alkaloid from the shoots of Psychotria leiocarpa. Phytochemistry 2004, 65, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, A.T.A.; Braz-filho, R.; Silveira, E.R.; Lima, M.A.S. Alkaloid and other Chemical Constituents from Psychotria stachyoides Benth. J. Braz. Chem. Soc. 2011, 22, 2216–2219. [Google Scholar] [CrossRef]
- Liew, S.Y.; Mukhtar, M.R.; Hadi, A.H.A.; Awang, K.; Mustafa, M.R.; Zaima, K.; Morita, H.; Litaudon, M. Naucline, a new indole alkaloid from the bark of Nauclea officinalis. Molecules 2012, 17, 4028–4036. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, A.A.T.; Braz-Filho, R.; Delprete, P.G.; De Souza, E.B.; Silveira, E.R.; Lima, M.A.S. Unusual monoterpene indole alkaloids from Psychotria stachyoides Benth. Biochem. Syst. Ecol. 2010, 38, 846–849. [Google Scholar] [CrossRef]
- Kerber, V.A.; Passos, C.S.; Klein-Júnior, L.C.; Quirion, J.-C.; Pannecoucke, X.; Salliot-Maire, I.; Henriques, A.T. Three new monoterpene indole alkaloids from Psychotria umbellata Thonn. Tetrahedron Lett. 2014, 55, 4798–4800. [Google Scholar] [CrossRef]
- Passos, C.S.; Simões-Pires, C.A.; Nurisso, A.; Soldi, T.C.; Kato, L.; de Oliveira, C.M.A.; de Faria, E.O.; Marcourt, L.; Gottfried, C.; Carrupt, P.-A.; et al. Indole alkaloids of Psychotria as multifunctional cholinesterases and monoamine oxidases inhibitors. Phytochemistry 2013, 86, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Sacconnay, L.; Ryckewaert, L.; Passos, C.S.; Guerra, M.C.; Kato, L.; De Oliveira, C.M.A.; Henriques, A.; Carrupt, P.-A.; Simões-Pires, C.; Nurisso, A. Alkaloids from Psychotria Target Sirtuins: In Silico and In Vitro Interaction Studies. Planta Med. 2015, 81, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Abreu, P.; Pereira, A. A new indole alkaloid from Sarcocephalus latifolius. Heterocycles 1998, 48, 885–891. [Google Scholar] [CrossRef]
- Passos, C.S.; Soldi, T.C.; Abib, R.T.; Apel, M.A.; Simões-Pires, C.; Marcourt, L.; Gottfried, C.; Henriques, A.T. Monoamine oxidase inhibition by monoterpene indole alkaloids and fractions obtained from Psychotria suterella and Psychotria laciniata. J. Enzyme Inhib. Med. Chem. 2013, 28, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Levesque, J.; Jacquesy, R. Alcoyl—Gluco-alcaloides: Nouveaux composes isoles de Pauridiantha lyalii Brem (Rubiacees). Tetrahedron 1982, 38, 1417–1434. [Google Scholar] [CrossRef]
- Paranhos, J.T.; Fragoso, V.; da Silveira, V.C.; Henriques, A.T.; Fett-Neto, A.G. Organ-specific and environmental control of accumulation of psychollatine, a major indole alkaloid glucoside from Psychotria umbellata. Biochem. Syst. Ecol. 2009, 37, 707–715. [Google Scholar] [CrossRef]
- Brandt, V.; Tits, M.; Geerlings, A.; Frédérich, M.; Penelle, J.; Delaude, C.; Verpoorte, R.; Angenot, L. β-Carboline glucoalkaloids from Strychnos mellodora. Phytochemistry 1999, 51, 1171–1176. [Google Scholar] [CrossRef]
- Naves, R.F. Estudo Fitoquímico das Folhas de Psychotria hoffmannseggiana Roem& Schult (Rubiaceae). Master’s Thesis, Universidade Federal de Goiás, Goiás, Brazil, 2014; pp. 1–212. [Google Scholar]
- Verotta, L.; Peterlongo, F.; Elisabetsky, E.; Amador, T.A.; Nunes, D. High-performance liquid chromatography-diode array detection-tandem mass spectrometry analyses of the alkaloid extracts of Amazon Psychotria species. J. Chromatogr. A 1999, 841, 165–176. [Google Scholar] [CrossRef]
- Verotta, L.; Pilati, T.; Tato, M.; Elisabetsky, E.; Amador, T.A. Pyrrolidinoindoline Alkaloids from Psychotria colorata. J. Nat. Prod. 1998, 61, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; He, H.; Wang, Y.; Hao, X. A New Dimeric Alkaloid from the Leaf of Psychotria calocarpa. Helv. Chim. Acta 2010, 93, 1650–1652. [Google Scholar] [CrossRef]
- Hart, N.; Johns, S.; Lamberton, J.; Summons, R. Psychotridine, a C55H62N10, Alkaloid from Psychotria beccarioides (Rubiaceae). Aust. J. Chem. 1974, 27, 639–646. [Google Scholar] [CrossRef]
- Libot, F.; Miet, C.; Kunesch, N.; Poisson, J.E. Rubiacées d’Océanie: Alcaloïdes de Psychotria oleoides de Nouvelle-Calédonie et de Calycodendron milnei du Vanuatu (Nouvelles-Hébrides). J. Nat. Prod. 1987, 50, 468–473. [Google Scholar] [CrossRef]
- Gueritiz-voegelein, F.; Sévenet, T.; Pusset, J.; Adeline, M.-T.; Gillet, B.; Beloeil, J.-C.; Guénard, D.; Portier, P. Alkaloids from Psychotria Oleoides with Activity on Growth Hormone Release. J. Nat. Prod. 1992, 55, 923–930. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.-S.; Wang, X.-B.; Kong, L.-Y. Absolute configuration study of a new dimeric indole alkaloid from leaves and twigs of Psychotria henryi. J. Asian Nat. Prod. Res. 2014, 16, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.-S.; Wang, X.-B.; Kong, L.-Y. Two novel dimeric indole alkaloids from the leaves and twigs of Psychotria henryi. Fitoterapia 2013, 86, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Jannic, V.; Guéritte, F.; Lapre, Ol.; Serani, L.; Martin, M.-T.; Sévenet, T.; Potier, P. Pyrrolidinoindoline Alkaloids from Psychotria oleoides and Psychotria lyciiflora. J. Nat. Prod. 1999, 33, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Adjibade, Y.; Weniger, B.; Quirion, J.C.; Kuballa, B.; Cabalion, P.; Anton, R. Dimeric Alkaloids from Pychotria forsteriana. Phytochemistry 1992, 31, 317–319. [Google Scholar] [CrossRef]
- Solís, P.N.; Ravelo, A.G.; Palenzuela, J.A.; Gupta, M.P.; González, A.; Phillipson, J.D. Quinoline Alkaloids from Psychotria glomerulata. Phytochemistry 1997, 44, 963–969. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Cai, X.; Feng, T.; Liu, Y.; Li, Y.; Ren, J.; Zhu, H.-J.; Luo, X.-D. Psychotripine: A New Trimeric Pyrroloindoline Derivative from Psychotria pilifera. Org. Lett. 2011, 13, 5896–5899. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.; Kuballa, B.; Bounthanh, C.; Cabalion, P.; Sévenet, T.; Beck, J.P.; Anton, R. Cytotoxic Activity of Polyindoline Alkaloids of Psychotria forsteriana (Rubiaceae). Planta Med. 1986, 6, 450–453. [Google Scholar] [CrossRef]
- Rasolonjanahary, R.; Sévenet, T.; Voegelein, F.G.; Kordon, C. Psycholeine, a natural alkaloid extracted from Psychotria oleoides, acts as a weak antagonist of somatostatin. Eur. J. Pharmacol. 1995, 285, 19–23. [Google Scholar] [CrossRef]
- Roth, A.; Kuballa, B.; Cabalion, P.; Anton, R. Preliminary Study of the Alkaloids of Psychotria forsteriana. Planta Med. 1985, 51, 289. [Google Scholar] [CrossRef]
- Itoh, A.; Ikuta, Y.; Baba, Y.; Tanahashi, T.; Nagakura, N. Ipecac alkaloids from Cephaelis acuminata. Phytochemistry 1999, 52, 1169–1176. [Google Scholar] [CrossRef]
- Itoh, A.; Tanahashi, T.; Nagakura, N.; Nayeshiro, H. Tetrahydroisoquinoline-monoterpene glucosides from Alangium lamarckii and Cephaelis ipecacuanha. Phytochemistry 1994, 36, 383–387. [Google Scholar] [CrossRef]
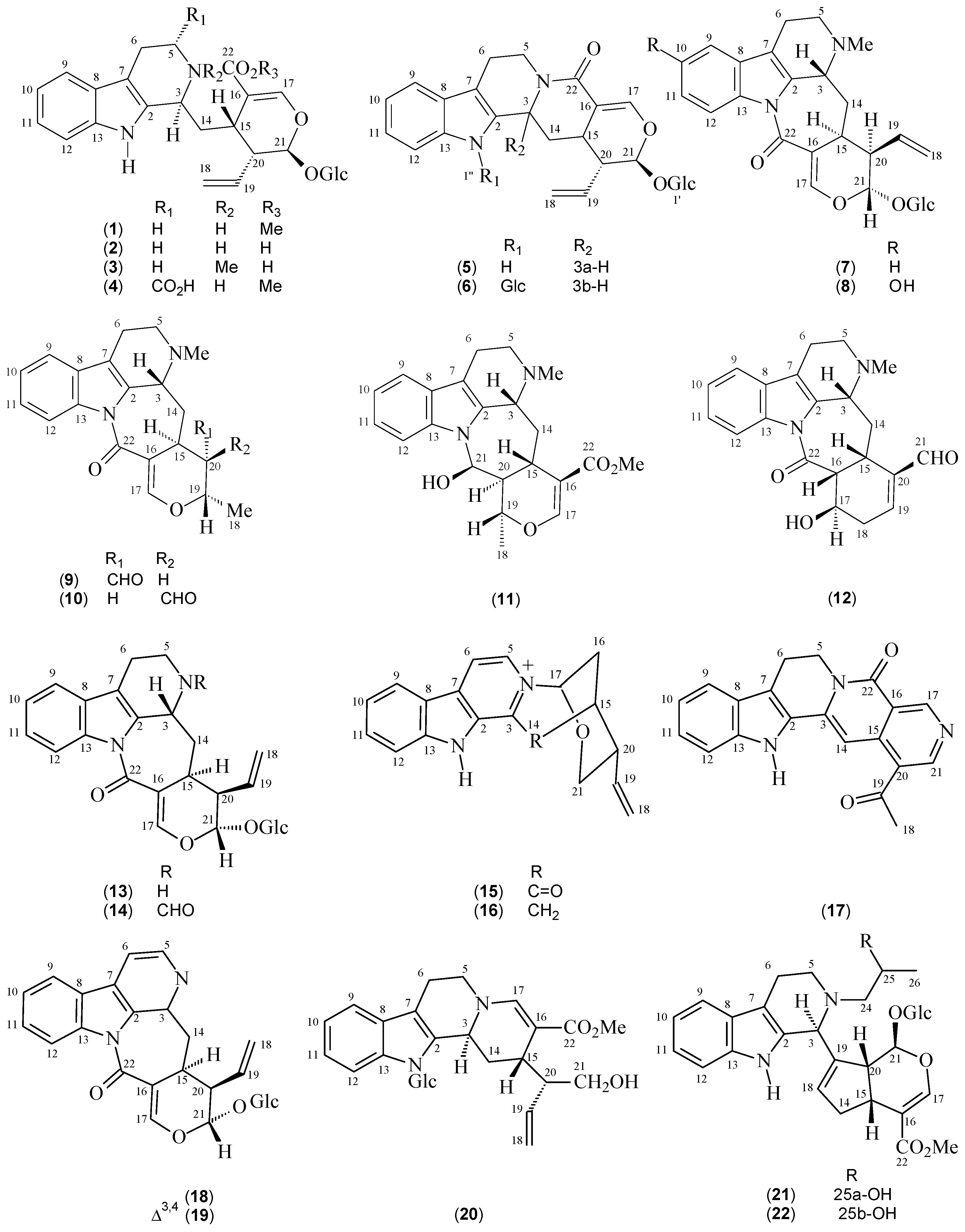





| Compounds | Species | References | 13C-NMR Data |
|---|---|---|---|
| Strictosidine (1) | P. elata | [12] | [13] |
| Strictosidinic acid (2) | P. acuminata P. barbiflora P. myriantha | [1,23,24,25] | [25] |
| Palicoside (3) | P. racemosa | [12] | [26] |
| 5α-Carboxystrictosidine (4) | P. acuminata P. bahiensis | [17,23] | [27] |
| Strictosamide (5) | P. bahiensis P. nuda P. prunifolia P. suterella | [16,17,18,19] | [18] |
| N,β-D-Glucopyranosilvincosamide (6) | P. leiocarpa | [28] | [28] |
| Correantoside (7) | P. correae | [14] | [14] |
| 10-Hydroxycorreantoside (8) | P. correae | [14] | [14] |
| Correantine B (9) | P. correae | [14] | [14] |
| 20-epi-Correantine B (10) | P. correae | [14] | [14] |
| Correantine A (11) | P. correae | [14] | [14] |
| Correantine C (12) | P. correae | [14] | [14] |
| N-Desmethyl-correantoside (13) | P. stachyoides | [29] | [29] |
| Nor-Methyl-23-oxo-correantoside (14) | P. stachyoides | [15] | [15] |
| 14-Oxoprunifoleine (15) | P. prunifolia | [18,20] | [18] |
| 17-Vinyl-19-oxa-2-azonia-12-azapentacyclo[14.3.1.02,14.05,13.06,11]icosa-2(14),3,5(13),6(11),7,9-hexaene (16) | P. prunifolia | [18] | [18] |
| Naucletine (17) | P. suterella | [19] | [30] |
| Correantosine E (18) | P. stachyoides | [31] | [31] |
| Correantosine F (19) | P. stachyoides | [31] | [31] |
| Lagamboside (20) | P. acuminata | [23] | [23] |
| N4-[1-((R)-2-Hydroxypropyl)]-psychollatine (21) | P. umbellata | [32] | [32] |
| N4-[1-((S)-2-Hydroxypropyl)]-psychollatine (22) | P. umbellata | [32] | [32] |
| (E/Z)-Vallesiachotamine (23 + 24) | P. bahiensis P. laciniata | [17,33] | [34] |
| Isodolichantoside (25) | P. correae | [14] | [14] |
| Angustine (26) | P. bahiensis P. laciniata | [17,33] | [35] |
| 10-Hydroxy-iso-deppeaninol (27) | P. prunifolia | [20] | [20] |
| 10-Hydroxy-antirhine (28) | P. prunifolia | [20] | [20] |
| N-Oxide-10-hydroxyantirhine (29) | P. prunifolia | [20] | [20] |
| Stachyoside (30) | P. stachyoides | [15] | [15] |
| Lyaloside (31) | P. laciniata P. suterella | [19,36] | [37] |
| Myrianthosine (32) | P. myriantha | [25] | [25] |
| Brachycerine (33) | P. brachyceras | [21] | [21] |
| Psychollatine (34) | P. umbellata P. umbellata | [5,22,38] | [22] |
| 3,4-Dehydro-18,19-β-epoxy-psychollatine (35) | P. umbellata | [32] | [32] |
| Desoxycordifoline (36) | P. acuminata | [23] | [39] |
| Bahienoside B (37) | P. acuminata P. bahiensis | [17,23] | [17] |
| Bahienoside A (38) | P. bahiensis | [17] | [17] |
| Carbons | Compounds/δC (ppm) | |||||||||
| 1 I | 2 III | 3 III | 4 I | 5 I | 6 I | 7 I | 8 I | 9 II | 10 II | |
| C | ||||||||||
| 2 | 133.2 | 132.3 | 134.7 | 133.2 | 134.8 | 136.1 | 134.3 | 133.8 | 132.9 | 133.0 |
| 7 | 107.7 | 106.0 | 105.2 | 109.0 | 110.3 | 111.5 | 115.7 | 115.3 | 114.8 | 114.8 |
| 8 | 127.9 | 126.1 | 126.6 | 128.0 | 128.7 | 129.5 | 130.4 | 131.3 | 129.1 | 129.1 |
| 13 | 137.9 | 135.8 | 135.8 | 138.4 | 137.8 | 137.7 | 137.3 | 131.4 | 136.0 | 136.0 |
| 22 | 170.6 | 170.0 | 168.4 | 170.9 | 167.1 | 166.3 | 168.2 | 167.8 | 166.2 | 166.2 |
| 16 | 109.9 | 113.4 | 112.5 | 109.9 | 109.2 | 109.1 | 112.2 | 112.0 | 108.6 | 109.6 |
| CH | ||||||||||
| 3 | 52.4 | 49.6 | 56.1 | 53.2 | 55.1 | 54.5 | 57.8 | 58.2 | 56.4 | 56.7 |
| 5 | - | - | - | 60.1 | - | - | - | - | - | - |
| 9 | 118.8 | 117.8 | 117.4 | 118.8 | 118.7 | 119.3 | 119.2 | 104.4 | 118.1 | 118.0 |
| 10 | 120.1 | 118.7 | 118.1 | 120.1 | 120.2 | 121.3 | 124.2 | 155.1 | 123.2 | 123.2 |
| 11 | 122.7 | 121.2 | 120.3 | 122.6 | 122.6 | 122.9 | 125.5 | 114.2 | 124.6 | 124.6 |
| 12 | 112.0 | 111.5 | 110.8 | 112.1 | 112.3 | 114.8 | 116.0 | 116.8 | 115.4 | 115.2 |
| 15 | 32.5 | 31.8 | 30.6 | 32.4 | 24.9 | 27.9 | 35.7 | 35.6 | 29.7 | 29.2 |
| 17 | 156.1 | 150.0 | 151.8 | 156.1 | 149.2 | 149.2 | 155.7 | 155.5 | 158.0 | 156.4 |
| 19 | 135.7 | 135.6 | 135.6 | 135.2 | 134.4 | 133.4 | 135.1 | 135.0 | 70.2 | 69.4 |
| 20 | 45.6 | 44.3 | 44.0 | 45.7 | 44.7 | 44.1 | 45.4 | 45.4 | 51.8 | 53.9 |
| 21 | 97.5 | 95.1 | 95.9 | 97.6 | 98.1 | 97.5 | 97.4 | 97.3 | - | - |
| CH2 | ||||||||||
| 5 | 42.9 | 40.0 | 45.2 | 60.1 | 44.8 | 41.6 | 46.4 | 46.7 | 45.5 | 45.5 |
| 6 | 21.0 | 19.2 | 15.9 | 25.2 | 22.1 | 22.3 | 18.8 | 18.8 | 17.6 | 17.7 |
| 14 | 35.9 | 33.7 | 35.3 | 35.6 | 27.3 | 35.6 | 34.4 | 34.1 | 39.1 | 35.3 |
| 18 | 119.5 | 117.8 | 117.8 | 119.6 | 120.6 | 120.7 | 119.2 | 119.3 | - | - |
| CH3 | ||||||||||
| MeN- | - | - | 39.8 | - | - | - | 41.4 | 41.2 | - | 41.5 |
| Me | - | - | - | - | - | - | - | - | 18.3 | 19.3 |
| Glucose | ||||||||||
| 1′ | 100.3 | 98.9 | 98.7 | 100.5 | 100.5 | 99.6 | 100.5 | 100.5 | - | - |
| 2′ | 78.6 | 69.8 | 73.0 | 74.7 | 74.3 | 74.9 | 74.7 | 74.7 | - | - |
| 3′ | 78.0 | 73.1 | 77.2 | 78.0 | 77.9 | 77.9 | 78.6 | 78.6 | - | - |
| 4′ | 74.6 | 77.2 | 70.0 | 71.9 | 71.3 | 71.6 a | 71.6 | 71.7 | - | - |
| 5′ | 71.7 | 76.5 | 76.6 | 78.6 | 78.2 | 78.3 | 78.0 | 78.0 | - | - |
| 6′ | 62.9 | 61.0 | 61.0 | 63.1 | 62.6 | 62.7 | 62.9 | 62.9 | - | - |
| 1″ | - | - | - | - | - | 87.6 | - | - | - | - |
| 2″ | - | - | - | - | - | 71.9 | - | - | - | - |
| 3″ | - | - | - | - | - | 75.1 | - | - | - | - |
| 4″ | - | - | - | - | - | 71.6 a | - | - | - | - |
| 5″ | - | - | - | - | - | 81.2 | - | - | - | - |
| 6″ | - | - | - | - | - | 62.9 | - | - | - | - |
| CHO | - | - | - | - | - | - | - | - | 199.5 | 199.2 |
| CO2Me | 52.4 | - | - | 52.6 | - | - | - | - | - | - |
| CO2H | - | - | - | 176.5 | - | - | - | - | - | - |
| Carbons | Compounds/δC (ppm) | |||||||||
| 11 II | 12 I | 13 I | 14 I | 15 I | 16 I | 17 II | 18 I | 19 I | 20 I | |
| C | ||||||||||
| 2 | 136.2 | 134.6 | 136.0 | 132.4 | 134.4 | 132.2 | 127.4 | 145.7 | 134.4 | 136.0 |
| 3 | - | - | - | - | 139.7 | 139.5 | 140.8 | - | 148.1 | - |
| 7 | 108.0 | 117.4 | 117.0 | 116.2 | 124.6 | 132.9 | 116.9 | 138.0 | 134.0 | 111.3 |
| 8 | 126.8 | 130.6 | 131.0 | 130.1 | 118.9 | 119.7 | 125.7 | 123.9 | 125.2 | 129.7 |
| 13 | 137.1 | 137.7 | 137.3 | 137.7 | 146.9 | 144.6 | 139.0 | 140.0 | 142.3 | 136.0 |
| 14 | - | - | - | - | 191.6 | - | - | - | - | - |
| 15 | - | - | - | - | - | - | 141.1 | - | - | - |
| 16 | 111.2 | - | 112.7 | 111.8 | - | - | 117.1 | 113.5 | 114.5 | 95.3 |
| 19 | - | - | - | - | - | - | 199.6 | - | - | - |
| 20 | - | - | - | - | - | - | 138.8 | - | - | - |
| 21 | - | 194.3 | - | - | - | - | - | - | - | - |
| 22 | 167.5 | 174.8 | 168.6 | 168.1 | - | - | 161.6 | 167.9 | 168.8 | 171.8 |
| CH | ||||||||||
| 3 | 61.4 | 58.5 | 50.6 | 47.9 | - | - | - | 50.0 | - | 50.2 |
| 5 | - | - | - | - | 134.1 | 132.5 | - | 137.0 | 142.6 | - |
| 6 | - | - | - | - | 120.6 | 116.0 | - | 116.2 | 114.5 | - |
| 9 | 118.5 | 117.8 | 119.2 | 119.5 | 123.6 | 122.8 | 119.3 | 123.9 | 122.3 | 119.0 |
| 10 | 119.8 | 119.0 | 124.4 | 124.6 | 123.4 | 122.4 | 119.9 | 126.3 | 125.5 | 121.0 |
| 11 | 121.5 | 125.0 | 125.5 | 126.0 | 137.2 | 132.3 | 120.9 | 133.5 | 131.3 | 122.7 |
| 12 | 109.2 | 126.0 | 116.4 | 116.4 | 113.7 | 113.2 | 112.0 | 119.8 | 119.3 | 114.6 |
| 14 | - | - | - | - | - | - | 95.6 | - | - | - |
| 15 | 30.8 | 34.5 | 35.7 | 35.6 | 42.8 | 25.6 | - | 30.5 | 21.2 | 33.7 |
| 16 | - | 52.0 | - | - | - | - | - | - | - | - |
| 17 | 155.2 | 67.5 | 155.6 | 156.5 | 87.9 | 86.7 | 154.0 | 157.2 | 155.9 | 149.3 |
| 19 | 74.8 | 149.7 | 135.2 | 134.9 | 132.8 | 134.9 | - | 133.6 | 134.1 | 140.8 |
| 20 | 52.0 | - | 45.6 | 45.3 | 42.0 | 41.2 | - | 46.4 | 46.7 | 55.2 |
| 21 | 75.5 | - | 97.5 | 97.6 | - | - | 155.4 | 97.9 | 97.9 | - |
| CH2 | ||||||||||
| 5 | 52.0 | 48.0 | 40.0 | 41.6 | - | - | 40.7 | - | - | 53.0 |
| 6 | 20.9 | 19.6 | 23.2 | 23.2 | - | - | 19.8 | - | - | 23.4 |
| 14 | 36.7 | 35.9 | 36.7 | 34.8 | - | 24.8 | - | 36.7 | 39.8 | 35.1 |
| 16 | - | - | - | - | 42.8 | 25.6 | - | - | - | - |
| 18 | - | 33.8 | 119.3 | 119.5 | 118.9 | 117.9 | - | 121.8 | 121.3 | 116.9 |
| 21 | - | - | - | - | 63.4 | 61.9 | - | - | - | 65.4 |
| CH3 | ||||||||||
| 18 | 18.6 | - | - | - | - | - | 29.3 | - | - | - |
| MeN- | 43.0 | 41.9 | - | - | - | - | - | - | - | - |
| Glucose | ||||||||||
| 1′ | - | - | 100.7 | 100.8 | - | - | - | 100.1 | 100.1 | 87.6 |
| 2′ | - | - | 74.9 | 74.9 | - | - | - | 74.8 | 74.7 | 72.4 |
| 3′ | - | - | 78.7 | 78.2 | - | - | - | 78.0 | 77.9 | 79.4 |
| 4′ | - | - | 71.8 | 71.7 | - | - | - | 71.7 | 71.7 | 71.8 |
| 5′ | - | - | 78.2 | 78.7 | - | - | - | 78.0 | 78.6 | 81.2 |
| 6′ | - | - | 63.1 | 63.0 | - | - | - | 62.9 | 62.9 | 63.0 |
| CHO | - | - | - | 163.9 | - | - | - | - | - | - |
| CO2Me | 51.1 | - | - | - | - | - | - | - | - | 51.2 |
| Carbons | Compounds/δC (ppm) | |||||||||
| 21 I | 22 I | 23 III | 24 III | 25 I | 26 III | 27 I | 28 I | 29 I | ||
| C | ||||||||||
| 2 | 134.0 | 133.4 | 133.1 | 133.6 | 134.0 | 126.8 | 136.9 | 130.5 | 131.0 | |
| 3 | - | - | - | - | - | 136.9 | 145.5 | - | - | |
| 7 | 108.4 | 108.4 | 106.6 | 107.4 | 106.5 | 114.8 | 130.6 | 106.0 | 105.7 | |
| 8 | 138.6 | 138.1 | 126.2 | 127.0 | 128.1 | 125.5 | 123.1 | 128.6 | 128.3 | |
| 10 | - | - | - | - | - | - | 152.6 | 151.8 | 152.0 | |
| 13 | 128.4 | 128.0 | 136.1 | 136.8 | 137.8 | 138.5 | 137.5 | 133.1 | 133.6 | |
| 15 | - | - | - | - | - | 139.0 | - | - | - | |
| 16 | 112.2 | 112.0 | 93.2 | 93.4 | 112.0 | 119.8 | - | - | - | |
| 19 | 141.0 | 142.1 | - | - | - | - | - | - | - | |
| 20 | - | - | 146.1 | 143.9 | - | 127.8 | - | - | - | |
| 22 | 169.7 | 169.0 | 166.9 | 167.6 | 169.8 | 161.1 | - | - | - | |
| CH | ||||||||||
| 3 | 61.7 | 59.3 | 48.6 | 47.9 | 58.8 | - | - | 57.0 | 71.6 | |
| 5 | - | - | - | - | - | - | 135.7 | - | - | |
| 6 | - | - | - | - | - | - | 114.6 | - | - | |
| 9 | 118.6 | 118.0 | 117.4 | 118.4 | 118.7 | 119.9 | 106.6 | 103.2 | 103.3 | |
| 10 | 119.5 | 120.0 | 118.3 | 119.2 | 119.9 | 119.9 | - | - | - | |
| 11 | 121.9 | 122.0 | 120.7 | 121.6 | 122.3 | 124.6 | 120.4 | 112.9 | 113.2 | |
| 12 | 112.0 | 112.0 | 110.8 | 111.8 | 111.8 | 112.0 | 113.7 | 112.8 | 113.0 | |
| 14 | - | - | - | - | - | 93.8 | - | - | - | |
| 15 | 33.0 | 35.3 | 27.4 | 30.5 | 30.5 | - | 36.4 | 31.1 | 30.6 | |
| 17 | 153.3 | 153.0 | 147.2 | 148.5 | 154.0 | 149.7 | - | - | - | |
| 18 | 132.6 | 131.0 | - | - | - | - | - | - | - | |
| 19 | - | - | 152.0 | 146.3 | 135.8 | 130.2 | 138.1 | 138.7 | 138.2 | |
| 20 | 49.0 | 48.4 | - | - | 45.5 | - | 51.0 | 50.8 | 52.3 | |
| 21 | 95.6 | 97.0 | - | - | 97.8 | 147.7 | - | - | - | |
| 25 | 65.1 | 66.3 | - | - | - | - | - | - | - | |
| CH2 | ||||||||||
| 5 | 49.0 | 49.6 | 49.8 | 50.7 | 47.9 | 40.4 | - | 52.4 | 69.0 | |
| 6 | 21.4 | 19.7 | 21.3 | 22.2 | 17.9 | 19.2 | - | 18.1 | 20.6 | |
| 14 | 39.4 | 39.5 | 32.9 | 32.9 | 34.5 | - | 37.0 | 31.6 | 28.5 | |
| 17 | - | - | - | - | - | - | 61.4 | 48.0 | 59.1 | |
| 18 | - | - | - | - | 119.8 | 119.8 | 118.7 | 118.5 | 118.5 | |
| 21 | - | - | - | - | - | - | 64.4 | 64.0 | 63.8 | |
| 24 | 62.4 | 61.6 | - | - | - | - | - | - | - | |
| CH3 | ||||||||||
| 18 | - | - | 14.3 | 13.8 | - | - | - | - | - | |
| 26 | 20.7 | 21.2 | - | - | - | - | - | - | - | |
| MeN- | - | - | - | - | 40.6 | - | - | - | - | |
| Glucose | ||||||||||
| 1′ | 100.1 | 100.1 | - | - | 100.5 | - | - | - | - | |
| 2′ | 74.6 | 74.8 | - | - | 74.7 | - | - | - | - | |
| 3′ | 78.0 | 78.0 | - | - | 78.6 | - | - | - | - | |
| 4′ | 78.2 | 71.6 | - | - | 71.6 | - | - | - | - | |
| 5′ | 76.2 | 78.5 | - | - | 78.0 | - | - | - | - | |
| 6′ | 62.7 | 62.5 | - | - | 62.9 | - | - | - | - | |
| CHO | - | - | 195.5 | 191.5 | - | - | - | - | ||
| CO2Me | 51.6 | 51.7 | 49.7 | 50.8 | 51.9 | - | - | - | - | |
| Carbons | Compounds/δC (ppm) | |||||||||
| 30 I | 31 | 32 III | 33 I | 34 I | 35 I | 36 I | 37 I | 38 I | ||
| C | ||||||||||
| 2 | 137.1 | 140.3 | 134.8 | 130.7 | 131.1 | 128.8 | 135.6 | 135.0 | 138.0 | |
| 3 | - | 143.8 | - | - | - | 158.9 | 142.9 | - | - | |
| 5 | - | - | - | - | - | - | 135.6 | - | - | |
| 7 | 118.4 | 121.0 | 121.0 | 108.3 | 107.9 | 118.8 | 128.4 | 107.3 | 106.6 | |
| 8 | 129.2 | 126.9 | 121.5 | 127.7 | 127.6 | 139.9 | 121.7 | 128.4 | 128.0 | |
| 13 | 139.1 | 134.6 | 140.2 | 112.3 | 138.1 | 126.1 | 141.6 | 137.8 | 138.0 | |
| 15 | ||||||||||
| 19 | - | - | - | - | 140.0 | 67.3 | - | - | - | |
| 16 | 114.8 | 109.9 | 112.0 | 111.8 | 112.2 | 110.2 | 108.7 | 112.1 | 111.5 | |
| 21 | 169.0 | - | - | - | - | - | - | - | - | |
| 22 | - | 166.6 | 170.0 | 169.1 | 169.1 | 168.9 | 171.3 | 169.7 | 170.0 | |
| 22b | - | - | - | - | - | - | 169.5 | 169.4 | ||
| CH | ||||||||||
| 3 | 51.7 | - | 48.5 | 54.7 | 53.7 | - | - | 58.8 | 59.6 | |
| 5 | - | 137.3 | 137.0 | - | - | - | - | - | - | |
| 6 | - | 112.6 | 118.0 | - | - | - | 114.2 | - | - | |
| 9 | 119.7 | 121.4 | 126.6 | 118.9 | 119.0 | 121.2 | 121.4 | 120.6 | 118.7 | |
| 10 | 125.2 | 119.0 | 118.9 | 120.2 | 120.3 | 121.1 | 119.9 | 119.7 | 120.0 | |
| 11 | 126.8 | 127.6 | 127.5 | 123.2 | 123.6 | 126.2 | 128.4 | 122.0 | 122.5 | |
| 12 | 118.3 | 111.8 | 112.5 | 112.3 | 112.3 | 113.6 | 111.6 | 112.0 | 112.0 | |
| 15 | 32.9 | 30.1 | - | 35.5 | 37.5 | 31.7 | 34.5 | 31.5 | 31.6 | |
| 17 | 148.7 | 151.6 | 151.0 | 153.5 | 153.4 | 153.1 | 153.2 | 154.0 | 154.7 | |
| 18 | - | - | - | 74.3 | 138.5 | 62.5 | - | - | - | |
| 19 | 53.3 | 134.0 | 134.5 | 49.0 | - | - | 133.8 | 136.2 | 136.1 | |
| 20 | 95.5 | 42.9 | 45.5 | 41.9 | 49.0 | 43.8 | 44.4 | 45.5 | 45.4 | |
| 21 | - | 95.9 | 95.4 | 99.0 | 99.4 | 95.2 | 96.1 | 98.2 | 97.9 | |
| 15b | - | - | - | - | - | - | 30.3 | 30.5 | ||
| 17b | - | - | - | - | - | - | 153.2 | 153.5 | ||
| 19b | - | - | - | - | - | - | 135.7 | 135.5 | ||
| 20b | - | - | - | - | - | - | 44.8 | 44.8 | ||
| 21b | - | - | - | - | - | - | 98.5 | 98.3 | ||
| CH2 | ||||||||||
| 5 | 48.0 | - | - | 41.8 | 42.1 | 48.2 | - | 44.8 | 44.8 | |
| 6 | 21.2 | - | - | 24.4 | 20.5 | 20.1 | - | 17.6 | 17.4 | |
| 14 | 43.7 | 32.1 | 45.6 | 43.5 | 40.5 | 34.7 | 34.0 | 36.9 | 36.7 | |
| 15 | - | - | 30.0 | - | - | - | - | - | - | |
| 17 | - | - | - | - | - | - | - | - | - | |
| 18 | 72.4 | 118.6 | 118.9 | - | - | - | 117.6 | 119.8 | 119.8 | |
| 3b | - | - | - | - | - | - | 52.0 | 51.9 | ||
| 14b | - | - | - | - | - | - | - | 28.0 | 27.4 | |
| 18b | - | - | - | - | - | - | - | 120.1 | 120.1 | |
| CH3 | ||||||||||
| Me | - | - | 10.4 | - | - | - | - | - | - | |
| Glucose | ||||||||||
| 1′ | 100.3 | 98.79 | 98.6 | 100.6 | 101.5 | 99.4 | 99.0 | 100.4 | 100.4 | |
| 2′ | 74.9 | 73.1 | 73.0 | 74.0 | 71.0 | 74.7 | 73.2 | 74.6 | 74.8 c | |
| 3′ | 78.5 | 77.3 | 69.9 | 71.1 | 78.6 | 78.1 | 76.6 | 78.0 | 78.6 a | |
| 4′ | 71.9 | 71.10 | 77.3 | 78.3 | 74.6 | 72.1 | 70.4 | 71.6 | 71.7 d | |
| 5′ | 78.5 | 77.8 | 76.8 | 77.7 | 77.6 | 78.8 | 76.6 | 78.4 | 78.2 b | |
| 6′ | 63.0 | 61.2 | 61.0 | 62.1 | 61.8 | 63.3 | 61.8 | 62.9 | 62.9 e | |
| 1″ | - | - | - | - | - | - | - | 100.3 | 100.4 | |
| 2″ | - | - | - | - | - | - | - | 74.8 | 74.6 c | |
| 3″ | - | - | - | - | - | - | - | 78.1 | 78.4 a | |
| 4″ | - | - | - | - | - | - | - | 71.6 | 71.6 d | |
| 5″ | - | - | - | - | - | - | - | 78.3 | 78.0 b | |
| 6″ | - | - | - | - | - | - | - | 62.8 | 62.8 e | |
| CO2Me | - | 50.7 | - | 51.8 | 51.9 | 51.9 | 50.6 | 52.1 | 52.1 | |
| Compounds | Species | References | 13C-NMR Data |
|---|---|---|---|
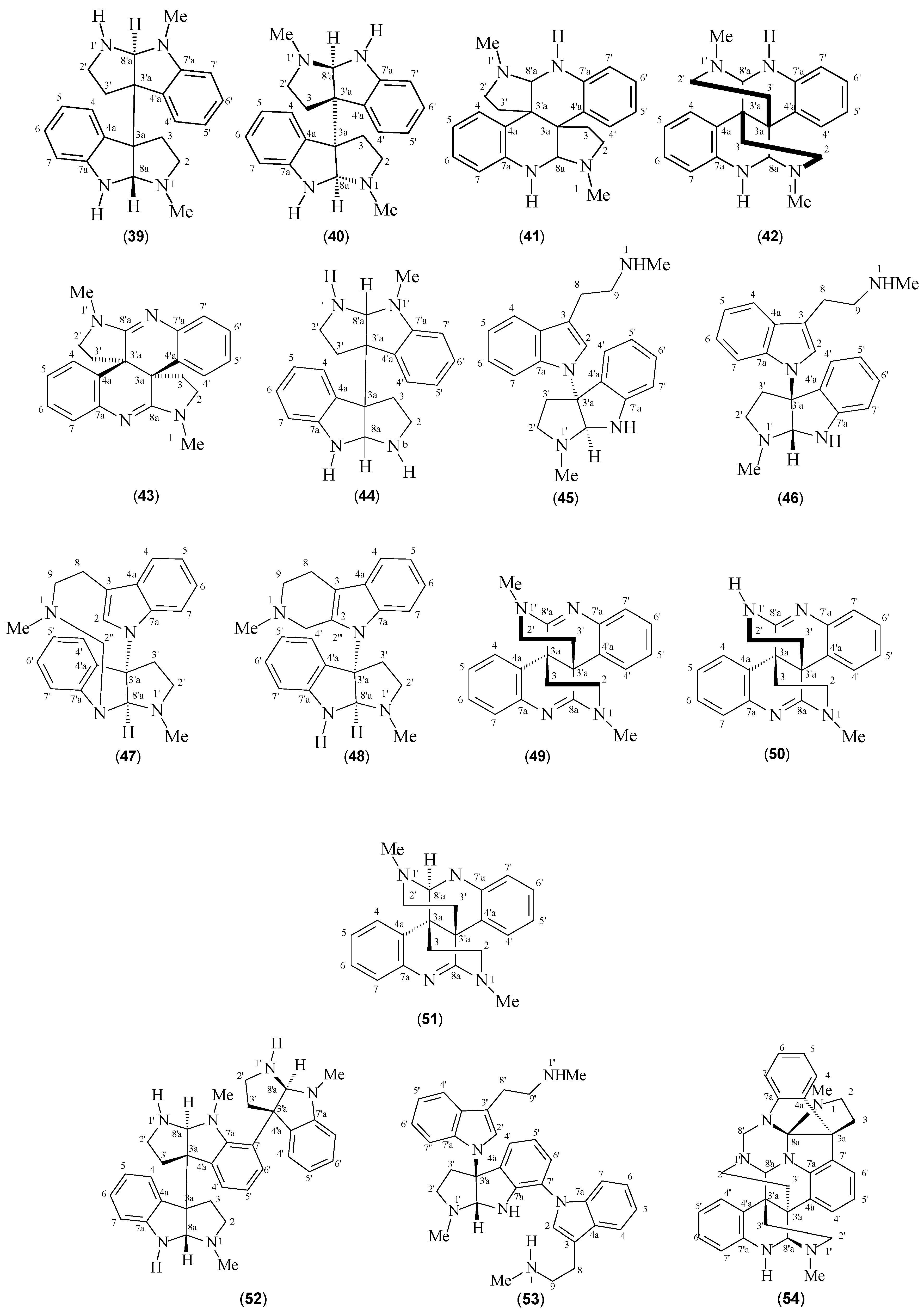
| Meso-chimonanthine (39) | P. forsteriana P. muscosa | [41,49,50] | [50] |
| (+)-Chimonanthine (40) | P. colorata P. muscosa P. rostrata P. hoffmannseggiana | [40,41,42] | [40] |
| Iso-calycanthine (41) | P. forsteriana | [50] | [50] |
| Calycanthine (42) | P. forsteriana | [50] | [50] |
| (8-8a),(8’-8’a)-tetradehydroisocalycanthine 3a(R), 3’a(R) (43) | P. colorata | [42] | [42] |
| Nb-desmethyl-meso-chimonanthine (44) | P. lyciiflora | [49] | [49] |
| Psychotriasine (45) | P. calocarpa | [43] | [43] |
| Psychohenin (46) | P. henryi | [47] | [47] |
| Compound (47) | P. henryi | [48] | [48] |
| Compound (48) | P. henryi | [48] | [48] |
| Glomerulatine A (49) | P. glumerulata | [51] | [51] |
| Glomerulatine B (50) | P. glumerulata | [51] | [51] |
| Glomerulatine C (51) | P. glumerulata | [51] | [51] |
| Hodgkinsine (52) | P. colorata P. oleoides P. lyciiflora P. muscosa P. beccarioides P. rostrata | [41,42,43,44,45,46] | [42] |
| Psychotrimine (53) | P. rostrata | [2] | [2] |
| Psychotripine (54) | P. pilifera | [52] | [52] |
| Quadrigemine A (55) | P. forsteriana | [53] | [53] |
| Quadrigemine B (56) | P. forsteriana P. colorata P. rostrata | [41,53] | [53] |
| Quadrigemine C (57) | P. colorata P. oleoides | [41,42,43,45,46,50,54] | [45] |
| Quadrigemine I (58) | P. oleoides | [49] | [49] |
| Psycholeine (59) | P. oleoides | [46,54] | [46] |
| Psychopentamine (60) | P. rostrata | [2] | [2] |
| Psychotridine (61) | P. forsteriana P. oleoides P. colorata P. beccarioides | [41,44,45,53] | [45] |
| Isopsychotridine C (62) | P. forsteriana | [53,55] | [55] |
| Isopsychotridine B (63) | P. oleoides | [49,50] | [45] |
| Oleoidine (64) | P. oleoides | [49] | [49] |
| Caledonine (65) | P. oleoides | [49] | [49] |
| Carbons | Compounds/δC (ppm) | ||||||||
| 39 II | 40 II | 41 ns | 42 II | 43 II | 44 II | 45 I | 46 I | 47 II | |
| C | |||||||||
| 3 | - | - | - | - | - | - | 112.7 | 110.0 | 112.3 |
| 3a | 64.7 | 63.6 | 37.8 | 36.8 | 48.9 | 62.8 | - | - | - |
| 4a | 133.7 | 128.3 | 127.0 | 125.9 | 125.6 | 132.2 | 130.4 | 130.5 | 130.0 |
| 7a | 152.5 | 150.5 | 145.3 | 146.2 | 145.8 | 151.7 | 137.7 | 138.0 | 135.0 |
| 8a | - | - | - | - | 165.0 | - | - | - | - |
| 3′a | 64.7 | 63.6 | 37.8 | 36.8 | 48.9 | 63.9 | 79.4 | 77.8 | 75.3 |
| 4′a | 133.7 | 128.3 | 127.0 | 125.9 | 125.6 | 130.0 | 131.3 | 131.3 | 128.9 |
| 7′a | 152.5 | 150.5 | 145.3 | 146.2 | 145.8 | 150.3 | 152.5 | 152.7 | 152.4 |
| 8′a | - | - | - | - | 165.0 | - | - | - | - |
| CH | |||||||||
| 2 | - | - | - | - | - | - | 125.0 | 126.1 | 123.4 |
| 4 | 125.2 | 124.9 | 118.3 | 117.1 | 123.0 | 123.9 | 124.7 | 119.5 | 119.0 |
| 5 | 119.2 | 122.3 | 122.2 b | 122.1 | 118.5 | 119.9 | 119.3 | 118.8 e | 119.1 f |
| 6 | 128.9 | 129.9 | 127.7 | 127.3 | 128.2 | 128.2 | 130.7 | 123.0 | 121.4 |
| 7 | 109.5 | 110.5 | 112.9 | 112.8 | 123.9 | 109.1 | 120.1 | 117.4 | 112.8 |
| 8a | 83.9 | 84.6 | 71.7 | 71.82 | - | 79.3 | - | - | - |
| 4′ | 125.2 | 124.9 | 118.3 | 117.1 | 123.0 | 124.4 | 112.2 | 124.9 | 126.4 |
| 5′ | 119.2 | 119.8 | 125.2 | 125.2 | 121.9 | 117.9 | 122.4 | 119.8 | 118.7 |
| 6′ | 128.9 | 129.9 | 127.7 | 127.3 | 128.2 | 128.4 | 119.6 | 130.9 | 130.2 |
| 7′ | 109.5 | 110.5 | 112.9 | 112.8 | 123.9 | 108.2 | 110.0 | 110.4 | 108.8 |
| 8′a | 83.9 | 84.6 | 71.7 | 71.82 | - | 82.4 | 87.0 | 87.3 | 86.6 |
| CH2 | |||||||||
| 2 | 53.1 | 52.4 | 46.9 | 47.3 | 48.5 | 44.9 | - | - | - |
| 3 | 36.4 | 33.2 | 34.9 | 32.5 | 29.9 | 35.3 | - | - | - |
| 2′ | 53.1 | 52.4 | 46.9 | 47.4 | 48.5 | 51.8 | 52.0 | 52.3 | 53.6 |
| 3′ | 36.4 | 33.2 | 34.9 | 32.5 | 29.9 | 38.1 | 39.9 | 40.0 | 37.7 |
| 2″ | - | - | - | - | - | - | - | - | 69.1 |
| CH3 | |||||||||
| Me-N1- | nd | 33.8 | 46.9 | 43.4 | 31.1 | - | 36.3 | 33.9 | 40.6 |
| MeN1′- | nd | 33.8 | 46.9 | 43.4 | 31.1 | 35.12 | 35.7 | 36.4 | 37.1 |
| Carbons | Compounds/δC (ppm) | ||||||||
| 48 II | 49 III | 50 III | 51 III | 52 II | 53 II | 54 I + II | 55 II,* | 56 II,* | |
| C | |||||||||
| 2 | 129.6 | - | - | - | - | - | - | - | - |
| 3 | 109.4 | - | - | - | - | 114.9 | - | - | - |
| 3a | - | 49.1 | 48.6 | 49.2 | 62.8 | - | 69.1 | 60.9 c | 60.1 c |
| 4a | 128.0 | 126.4 | 126.1 a | 129.5 | 131.7 | 128.3 | 133.8 | 132.3 d | 133.2 e |
| 7a | 137.4 | 177.3 | 147.1 | 148.6 | 150.8 | 136.1 | 152.2 | 150.9 h | 150.6 h |
| 8a | - | 165.1 | 164.7 | 166.5 | - | - | 106.9 | - | - |
| 3′a | 76.7 | 49.1 | 48.6 | 45.3 | 63.0 | 76.7 | 37.0 | 63.2 j | 63.9 i |
| 4′a | 130.5 | 126.4 | 125.4 a | 122.3 | 132.3 | 132.0 | 122.0 | 132.4 d | 132.9 e |
| 7′ | - | - | - | - | - | 121.5 | 130.9 | 108.9 g | - |
| 8′a | - | 165.1 | 164.7 | - | - | - | - | - | - |
| 3″ | - | - | - | - | - | 112.5 | - | - | - |
| 3″a | - | - | - | - | 60.0 | - | 38.4 | 62.9 j | 63.3 i |
| 8″ | - | - | - | - | - | 25.7 b | 68.0 | - | - |
| 9″ | - | - | - | - | - | 52.0 | - | - | - |
| 4″a | - | - | - | - | 131.7 | 129.8 | 122.3 | 132.6 d | - |
| 7″a | - | - | - | - | 151.1 | 136.1 | 144.4 | - | - |
| 3‴a | - | - | - | - | - | - | - | 60.8 c | 60.9 c |
| CH | |||||||||
| 2 | - | - | - | - | - | 126.0 | - | - | - |
| 4 | 117.9 | 123.7 | 120.9 | 123.0 | 126.4 | 119.4 a | 122.9 | - | 125.9 d |
| 5 | 119.2 | 122.3 | 122.2 b | 122.1 | 118.5 | 119.9 | 119.3 | 118.8 e | 119.1 f |
| 6 | 121.3 | 128.9 | 129.0 c | 128.8 | 127.9 | 122.4 | 128.2 | 127.9 f | 128.0 g |
| 7 | 112.1 | 125.0 | 125.2 d | 125.2 | 109.0 | 111.2 | 107.7 | 109.0 g | 108.9 |
| 8a | - | - | - | - | 86.4 | - | - | 86.9 i | 85.9 i |
| 4′ | 124.5 | 123.7 | 124.0 d | 117.5 | 121.9 | 123.7 | 123.7 | 122.5 | 125.1 d |
| 5′ | 119.0 | 122.3 | 122.6 b | 124.9 | 116.8 | 119.3 a | 122.0 | 116.3 k | 118.3 f |
| 6′ | 129.6 | 128.9 | 128.8 c | 127.1 | 126.0 | 127.3 | 121.1 | 125.4 | 127.8 g |
| 7′ | 108.9 | 125.0 | 124.4 d | 114.4 | - | - | - | - | - |
| 8′a | 86.5 | - | - | 76.5 | 81.7 | 86.1 | 69.7 | 86.1 i | 83.3 j |
| 2″ | - | - | - | - | - | 124.3 | - | - | - |
| 4″ | - | - | - | - | 124.2 | 119.3 a | 125.4 | - | - |
| 5″ | - | - | - | - | 117.5 | 119.3 a | 117.8 | 118.7 e | 117.2 f |
| 6″ | - | - | - | - | 127.4 | 121.7 | 127.6 | - | - |
| 7″ | - | - | - | - | 108.1 | 112.2 | 112.5 | - | - |
| 8″a | - | - | - | - | 82.3 | - | 69.4 | - | 82.3 j |
| 8‴a | - | - | - | - | - | - | - | - | 87.1 i |
| 5‴ | - | - | - | - | - | - | - | 116.2 k | 116.8 f |
| 6‴ | - | - | - | - | - | - | - | 126.4 f | - |
| CH2 | |||||||||
| 2 | - | 48.2 | 48.1 | 48.5 | 51.7 | - | 54.9 | 52.6 a | 52.3 a |
| 3 | - | 30.3 | 30.3 | 31.7 | 37.6 | - | 36.3 | 38.8 b | 38.5 b |
| 2′ | 51.2 | 48.2 | 48.1 | 50.5 | 51.9 | 51.7 | 42.3 | 52.5 a | 52.2 a |
| 3′ | 40.6 | 30.3 | 30.3 | 34.0 | 36.7 | 39.1 | 33.1 | 38.7 b | 36.6 b |
| 2″ | - | - | - | - | 51.9 | - | 45.9 | 52.2 a | - |
| 3″ | - | - | - | - | 38.0 | - | 33.7 | 38.5 b | - |
| 3‴ | - | - | - | - | - | - | - | 36.6 b | - |
| CH3 | |||||||||
| Me-N1- | 44.8 | 30.9 | 30.8 | 30.7 | 35.2 | 36.3 | 36.4 | 35.7 l | 35.8 k |
| MeN1′- | 36.1 | 30.9 | - | 36.6 | 35.0 | 36.4 | - | 35.5 l | 35.7 k |
| Me-N1″- | - | - | - | - | 35.1 | 36.4 | 41.8 | 35.0l | 35.6 k |
| Me-N1‴- | - | - | - | - | - | - | - | - | 35.2 k |
| Carbons | Compounds/δC (ppm) | ||||||||
| 57 II,* | 58 II,* | 59 II,* | 60 II | 61 II,* | 62 II,* | 63 II,* | 64 II,* | 65 II,* | |
| C | |||||||||
| 3a | 60.6 | 60.0 | 59.6 b | 61.1 | 60.1 a | 60.9 c | 63.0 a | 60.4 c | 60.0 c |
| 4a | - | 132.0 | 132.4 c | 132.9 b | - | 132.7 d | - | 132.8 | 132.1 e |
| 7a | - | - | - | 152.8 | - | 150.6 f | - | 150.7 e | 150.5 f |
| 3′a | 62.6 | 63.0 | 37.5 f | 63.1 | 62.9 | 63.7 h | 63.3 a | 63.3 c | 63.0 |
| 4′a | - | - | - | 132.8 b | - | 132.0 d | - | - | 132.4 e |
| 7′ | - | 110.0 c | - | 123.8 | - | - | - | - | 108.8 |
| 7′a | - | - | - | 151.0 | - | 148.9 f | - | 150.3 e | 148.9 f |
| 5″ | - | - | - | 136.2 | - | 117.1 | - | - | - |
| 3″a | 62.6 | - | 38.0 f | 64.2 | 62.9 | 63.2 h | 59.8 c | 60.9 c | - |
| 4″a | - | - | - | 132.6 | - | - | - | - | - |
| 7″a | - | - | - | 149.8 | - | - | - | - | 148.6 |
| 3‴a | 60.6 | - | 60.6 b | 62.3 | 60.6 a | 60.1 c | 59.8 c | - | 60.5 c |
| 4‴a | - | - | 133.8 c | 138.6 | - | - | - | - | - |
| 7‴ | - | - | - | 120.4 | - | - | - | - | - |
| 7‴a | - | - | - | 144.7 | - | - | - | - | - |
| 3″″ | - | - | - | 114.5 | - | - | - | - | - |
| 3″″a | - | - | - | 128.3 | 60.8 a | - | 60.7 c | - | - |
| 4″″a | - | - | - | - | - | - | - | - | |
| CH | |||||||||
| 4 | - | 126.0 | - | 126.9 | - | 123.6 | - | 126.1 d | 125.3 d |
| 4′ | - | 124.0 | - | 122.1 | - | 122.2 | - | 124.1 | 125.2 d |
| 5 | - | 117.0 b | - | 118.8 | - | 119.1 e | - | 116.3 | 118.9 |
| 6 | - | 129.5 | - | 128.0 | - | 128.2 | - | 128.7 | 128.1 |
| 7 | - | 109.0 c | - | 110.5 | - | 109.0 | - | 109.3 | 107.7 |
| 8a | 85.8 a | 88.0 d | 88.5 d | 87.3 | 86.0 b | 87.2 g | 81.8 b | 86.6 | 86.9 |
| 5′ | - | 118.5 b | - | 116.2 | - | 118.4 e | - | 119.4 | 117.3 |
| 6′ | - | 127.5 | - | 126.5 | - | 126.1 | - | 126.2 | 125.3 |
| 8′a | 82.3 | 83.0 d | 74.0 g | 82.4 | 82.6 c | 85.8 g | 86.8 b | 82.8 | 86.0 |
| 4″ | - | - | - | 121.4 | - | - | - | 125.7 d | 124.1 |
| 5″ | - | 119.0 b | - | - | - | 117.1 | - | - | - |
| 6″ | - | - | - | 126.1 | - | 125.4 | - | 128.4 | - |
| 7″ | - | - | - | 108.5 | - | - | - | - | - |
| 8″a | 82.3 | - | 72.0 g | 83.4 | 82.3 c | 83.1 | 86.8 d | 83.0 | - |
| 4‴ | - | - | - | 123.3 | - | - | - | 122.7 | 123.6 |
| 5‴ | - | 119.5 b | - | 118.8 | - | - | - | - | - |
| 6‴ | - | 128.5 | - | 125.1 | - | - | - | - | - |
| 7‴ | - | - | - | 120.4 | - | - | - | - | - |
| 8‴a | 86.7 a | - | 87.5 d | 88.4 | 86.9 b | 82.1 | 85.5 d | - | - |
| 2″″ | - | - | - | 126.1 | - | - | - | - | - |
| 4″″ | - | - | - | 119.3 | - | - | - | 125.7 | 123.2 |
| 5″″ | - | - | - | 111.2 | - | - | - | - | - |
| 6″″ | - | - | - | 122.3 | - | - | - | - | - |
| 7″″ | - | - | - | 119.7 | - | - | - | - | - |
| 8″″a | - | - | - | - | 85.1 b | - | 84.8 d | - | - |
| CH2 | - | - | - | - | - | - | - | - | - |
| 2 | - | 53.0 | 47.17 a | 52.7 a | - | 52.5 a | - | 52 a | 52.1 a |
| 3 | - | 38.0 a | - | 37.8 | - | 38.8 b | - | 38.7 b | 38.3 d |
| 8 | - | - | - | - | - | - | - | - | |
| 9 | - | - | - | - | - | - | - | - | |
| 2′ | - | - | 52.7 a | - | 52.0 a | - | 52.8 a | 52.4 a | |
| 3′ | 39.0 a | 32.8 e | 35.8 | - | 38.5 b | - | 38.7 b | 38.6 b | |
| 2″ | - | - | 52.6a | - | - | - | 52.9 a | - | |
| 3″ | - | 32.4 e | 37.2 | - | - | - | - | - | |
| 2‴ | - | 48.0 a | 52.5a | - | - | - | - | - | |
| 3‴ | - | - | 39.2 | - | - | - | - | - | |
| 3″″ | - | - | 114.5 | - | - | - | - | - | |
| CH3 | - | - | - | - | - | - | - | - | |
| Me-N1- | 36.0 | 36.1 h | 34.8 | - | 35.6 i | - | 35.7 | 35.4 g | |
| Me-N1′- | - | 42.6 i | 35.3 | - | 35.1 i | - | - | 35.6 g | |
| Me-N1″- | - | 42.6 i | 35.8 | - | - | - | - | - | |
| Me-N1‴- | - | 36.1 h | 35.7 | - | - | - | - | - | |
| Me-N1″″- | - | - | 36.5 | - | - | - | - | - | |
| Compound | Reference | 13C-NMR Data |
|---|---|---|
| Klugine (66) | [7] | [7] |
| 7’-O-Demethylisocephaeline (67) | [7] | [7] |
| Cephaeline (68) | [7] | [56] |
| Isocephaeline (69) | [7] | [56] |
| 7-O-Methylipecoside (70) | [7] | [57] |
| Carbons | Compound/δC (ppm) | ||||
|---|---|---|---|---|---|
| 66 ns | 67 ns | 68 II | 69 II | 70 I | |
| C | |||||
| 6 | - | - | - | - | 146.5 a |
| 7 | - | - | - | - | 147.8 a |
| 9 | 146.5 a | 146.8 | 147.2 a | 147.2 a | - |
| 10 | 147.8 b | 148.0 | 147.5 a | 147.4 a | - |
| 4a | - | - | - | - | 126.9 |
| 7a | 127.8 | 126.9 | 126.8 | 126.5 | - |
| 8a | - | - | - | - | 130.2 |
| 11a | 129.7 | 127.9 | 130.1 | 129.9 | - |
| 1′ | 79.5 | - | - | - | - |
| 4′ | - | - | - | - | 111.7 |
| 4’a | 127.7 | 123.2 | 127.6 | 127.9 | - |
| 6′ | 146.4 a | 145.6 | 143.9 b | 144.0 | - |
| 7’ | |||||
| 11’ | - | - | - | - | 169.2 |
| 8’a | 129.7 | 126.0 | 131.1 | 131.0 | - |
| CH | |||||
| 1 | - | - | - | - | 50.6 |
| 2 | |||||
| 3 | 42.5 | 41.3 | 41.7 | 61.5 | - |
| 5 | - | - | - | - | 116.2 |
| 8 | 116.2 | 112.1 | 111.5 | 111.4 | 111.1 |
| 11 | 109.7 | 109.0 | 108.6 | 108.2 | - |
| 11b | 63.8 | 62.7 | 62.4 | 62.8 | - |
| 1′ | - | 53.6 | 51.9 | 55.3 | 98.7 |
| 3′ | - | - | - | - | 153.1 |
| 4′ | 28.5 | 27.6 | 29.0 | 29.3 | - |
| 5′ | 116.4 | 115.2 | 114.7 | 114.8 | 27.5 |
| 8′ | 110.0 | 113.2 | 108.4 | 108.6 | 136.3 |
| 9′ | - | - | - | - | 45.1 |
| CH2 | |||||
| 1 | 40.6 | 36.9 | 36.9 | 39.3 | - |
| 3 | - | - | - | - | 36.1 |
| 4 | 62.2 | 61.6 | 61.3 | 52.6 | 29.1 |
| 6 | 53.3 | 51.9 | 52.3 | 52.6 | - |
| 7 | 29.3 | 25.3 | 29.2 | 29.1 | - |
| 12 | 24.4 | 23.3 | 23.6 | 24.0 | - |
| 14 | 37.0 | 38.0 | 40.9 | 40.4 | - |
| 3′ | 41.0 | 39.5 | 40.1 | 41.4 | - |
| 4′ | 28.5 | 27.6 | 29.0 | 29.3 | - |
| 6′ | - | - | - | - | 41.1 |
| 10′ | - | - | - | - | 120.1 |
| CH3 | 11.5 | 10.1 | 11.2 | 11.3 | - |
| 13 | 11.5 | 10.1 | 11.2 | 11.3 | - |
| Me7-O- | - | - | - | 56.5 | |
| Me9-O- | - | 55.4 d | 55.8 e | 55.8 f | - |
| Me10-O- | 56.8 c | 55.8 d | 56.0 e | 56.0 f | - |
| Me7′-O- | 56.6 c | - | 56.3 e | 56.0 f | - |
| Glucose | |||||
| 1″ | - | - | - | - | 100.5 |
| 2″ | - | - | - | - | 74.8 |
| 3″ | - | - | - | - | 78.2 b |
| 4″ | - | - | - | - | 71.5 |
| 5″ | - | - | - | - | 78.3 b |
| 6″ | - | - | - | - | 62.7 |
| CO2Me | 51.7 | ||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho Junior, A.R.d.; Vieira, I.J.C.; Carvalho, M.G.d.; Braz-Filho, R.; S. Lima, M.A.; Ferreira, R.O.; José Maria, E.; Oliveira, D.B.d. 13C-NMR Spectral Data of Alkaloids Isolated from Psychotria Species (Rubiaceae). Molecules 2017, 22, 103. https://doi.org/10.3390/molecules22010103
Carvalho Junior ARd, Vieira IJC, Carvalho MGd, Braz-Filho R, S. Lima MA, Ferreira RO, José Maria E, Oliveira DBd. 13C-NMR Spectral Data of Alkaloids Isolated from Psychotria Species (Rubiaceae). Molecules. 2017; 22(1):103. https://doi.org/10.3390/molecules22010103
Chicago/Turabian StyleCarvalho Junior, Almir Ribeiro de, Ivo Jose Curcino Vieira, Mario Geraldo de Carvalho, Raimundo Braz-Filho, Mary Anne S. Lima, Rafaela Oliveira Ferreira, Edmilson José Maria, and Daniela Barros de Oliveira. 2017. "13C-NMR Spectral Data of Alkaloids Isolated from Psychotria Species (Rubiaceae)" Molecules 22, no. 1: 103. https://doi.org/10.3390/molecules22010103







