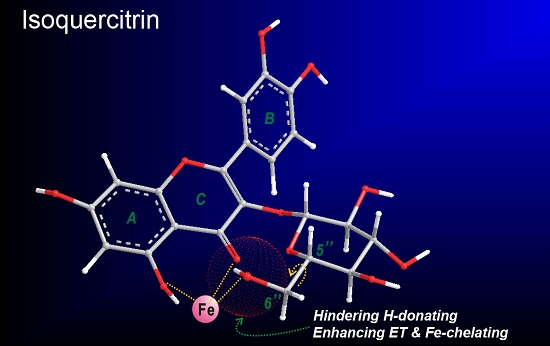
Comparison of the Antioxidant Effects of Quercitrin and Isoquercitrin: Understanding the Role of the 6″-OH Group
Abstract
:1. Introduction
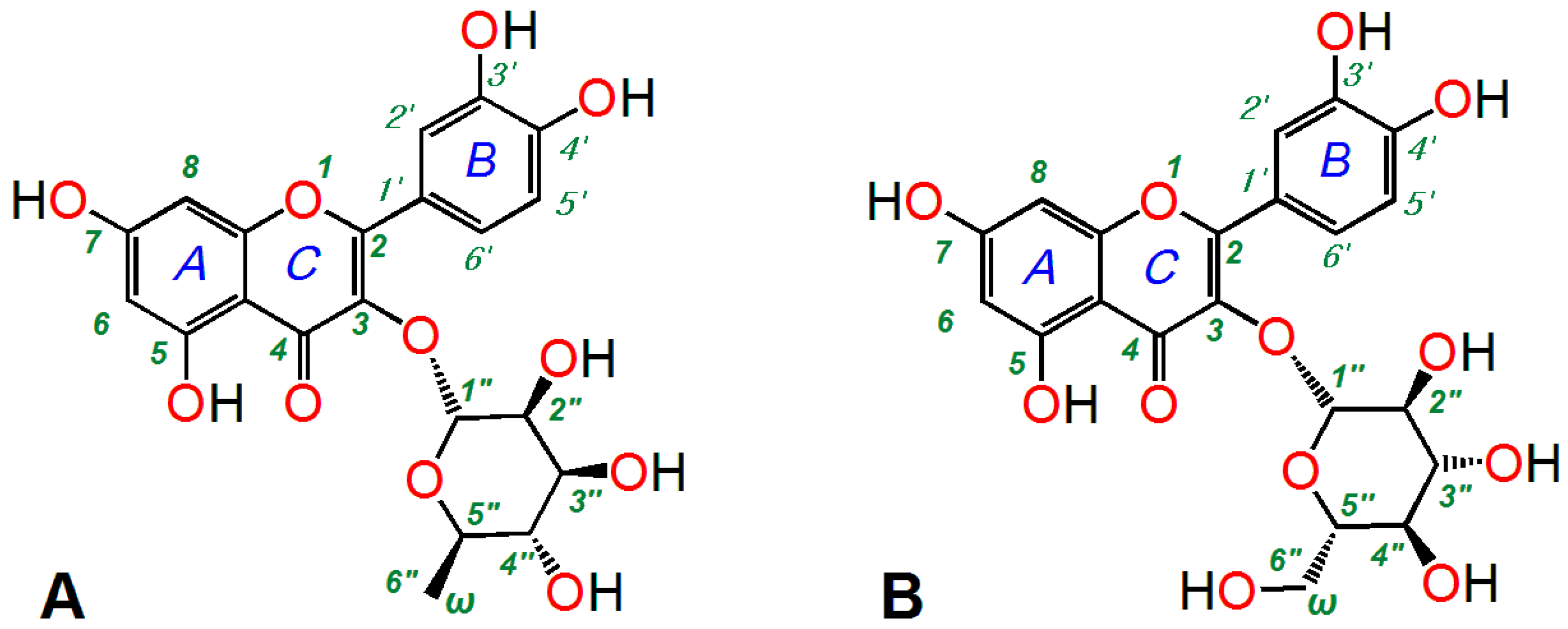
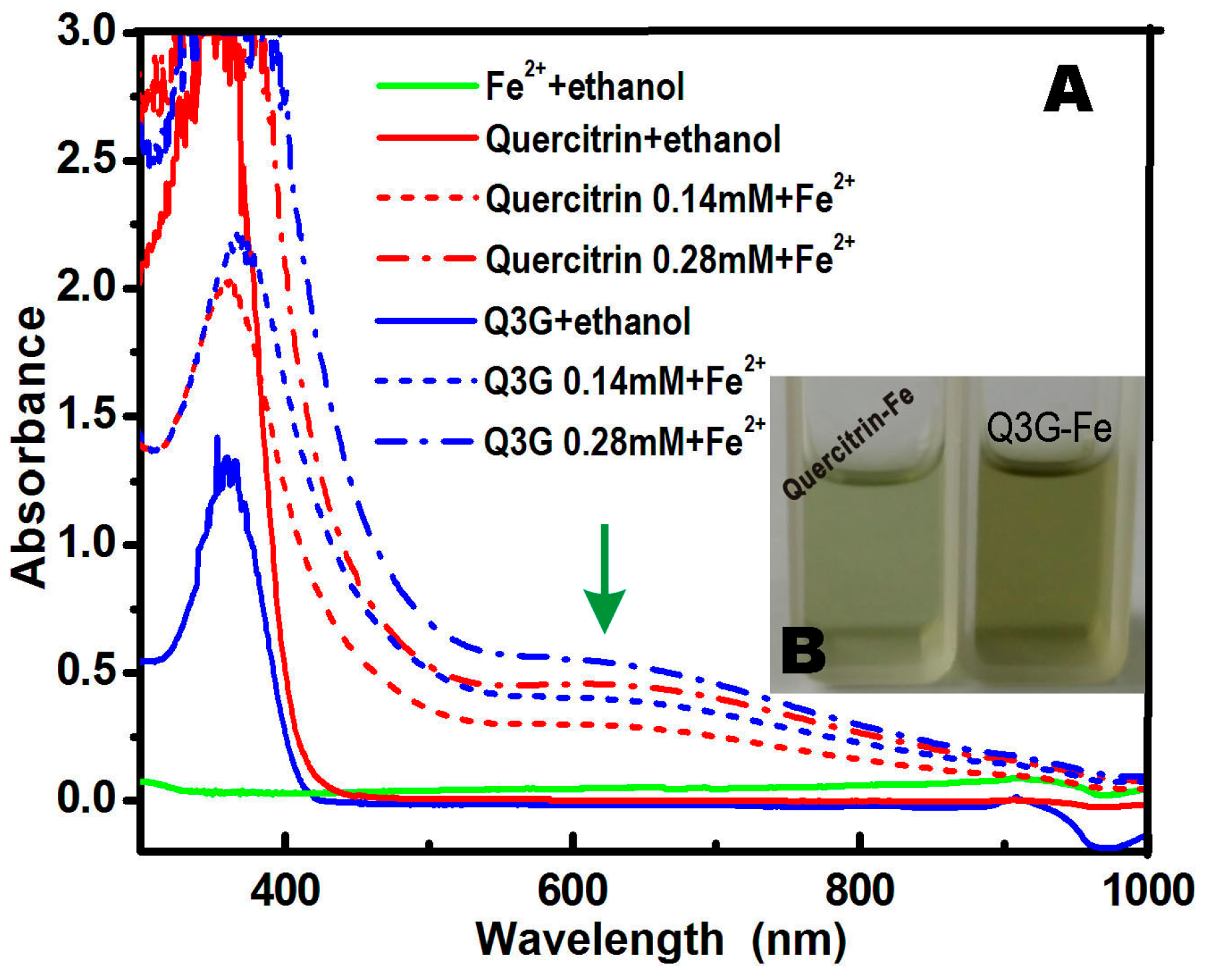
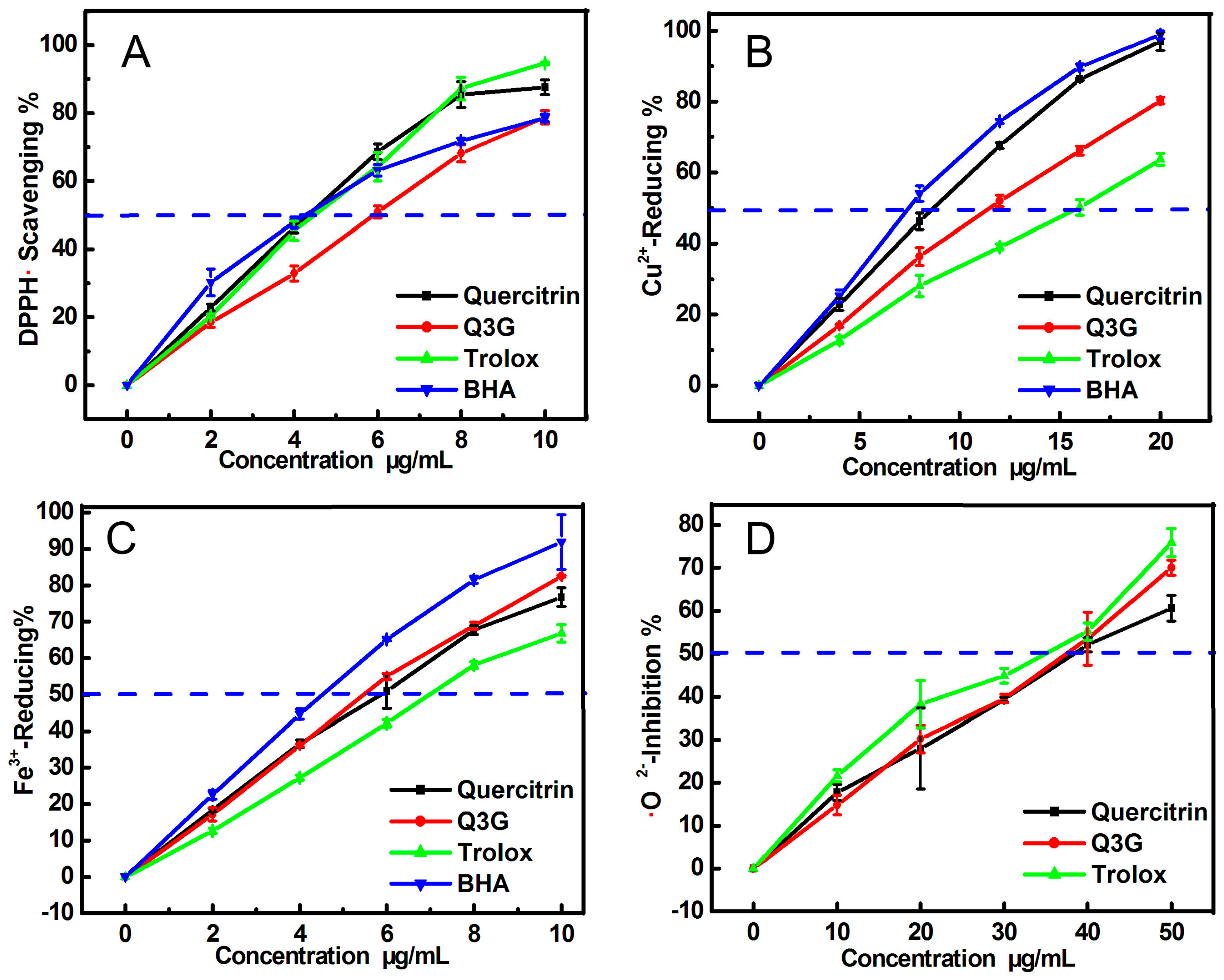
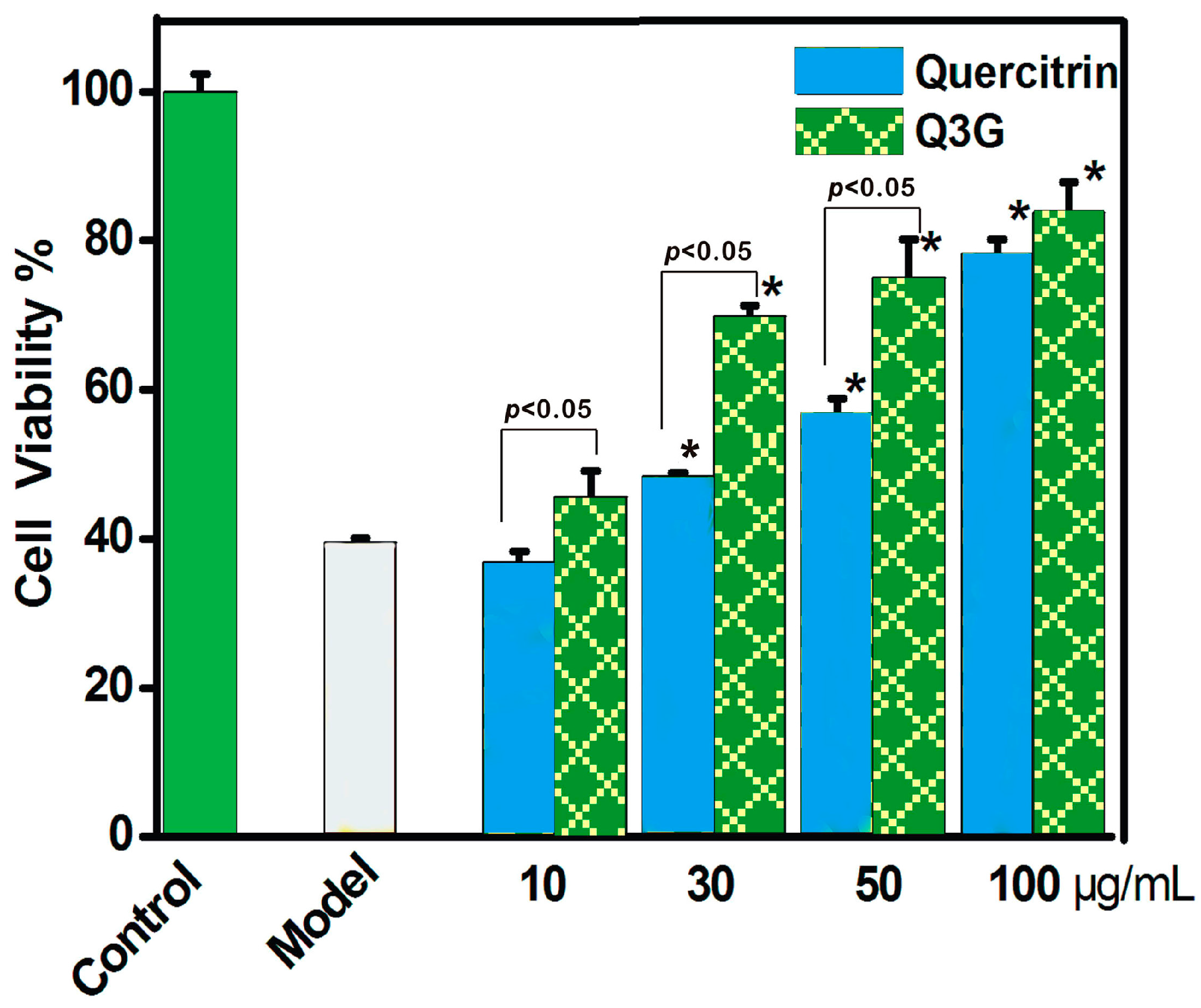
2. Results and Discussion
3. Materials and Methods
3.1. Animals and Chemicals
3.2. Ultraviolet (UV) Spectra Determination of Fe2+-Binding
3.3. DPPH• Radical-Scavenging Assay and Cu2+-Reducing Power Assay
3.4. Ferric Ion Reducing Antioxidant Power (FRAP) Assay
3.5. Scavenging Ability towards •O2− Radicals (Pyrogallol Autoxidation Assay)
3.6. Protective Effect towards the ROS-Induced Damage of MSCs (MTT Assay)
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BHA | butylated hydroxyanisole |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMSO | dimethyl sulfoxide |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl radical |
| EDTA | ethylene diamine tetraacetic acid |
| ET | electron transfer |
| FBS | fetal bovine serum |
| FRAP | Ferric ion reducing power assay |
| MSCs | mesenchymal stem cells |
| MTT | [3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl] |
| Q3G | quercetin-3-O-glucoside |
| RAF | radical adduct formation |
| ROS | reactive oxygen species |
| SD | standard deviation |
| SPSS | statistical product and service solutions |
| TPTZ | 2,4,6-tripyridyl triazine |
| Tris | tris-hydroxymethyl amino methane |
| Trolox | (±)-6-hydroxyl-2,5,7,8-tetramethlychroman-2-carboxylic acid |
References
- Xiao, J.; Capanoglu, E.; Jassbi, A.R.; Miron, A. Advance on the flavonoid C-glycosides and health benefits. Crit. Rev. Food Sci. Nutr. 2016, 56, S29–S45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Huang, H.T.; Huang, S.Y.; Lin, Z.H.; Shen, C.C.; Tsai, W.J.; Kuo, Y.H. Antioxidant and anti-inflammatory phenolic glycosides from Clematis tashiroi. J. Nat. Prod. 2015, 78, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Halabalaki, M.; Urbain, A.; Paschali, A.; Mitakou, S.; Tillequin, F.; Skaltsounis, A.L. Quercetin and kaempferol 3-O-[α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranoside]-7-O-α-l-rhamnopyranosides from Anthyllis hermanniae: Structure determination and conformational studies. J. Nat. Prod. 2011, 74, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Shu, B.W.; Zhang, X.Q.; Li, Y.H.; Zhu, K.X.; Pei, H.H.; Tan, W.H. Quercitrin content analysis in 7 different species of mistletoe medicinal plants from persimmon host Loranthaceae. World Sci. Technol. Mod. Tradit. Chin. Med. Mater. 2014, 16, 368–373. [Google Scholar]
- Taiwo, B.J.; Igbeneghu, O.A. Antioxidant and antibacterial activities of flavonoid glycosides from Ficus exasperata Vahl-Holl (Moraceae) leaves. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Z.W.; Zhang, L.; Liu, X.; Chen, X.H.; Bi, K.S. HPLC analysis and pharmacokinetic study of quercitrin and isoquercitrin in rat plasma after administration of Hypericum japonicum thunb. extract. Biomed. Chromatogr. 2008, 22, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Z.; Pan, R.L.; Li, Z.; Qi, J.Y. Determination of the contents of total flavonoids, isoquercitroside and quercitrosidein Amomum Villosum. Sci. Technol. Rev. 2009, 27, 30–33. [Google Scholar]
- Wang, K.J.; Zhang, Y.J.; Yang, G.R. Recent advance on the chemistry and bioactivity of Genus Polygonum. Nat. Prod. Res. Dev. 2006, 18, 151–164. [Google Scholar]
- Hu, S.; Yin, J.; Nie, S.; Wang, J.; Phillips, G.; Xie, M.; Cui, S.W. In vitro evaluation of the antioxidant activities of carbohydrates. Bioact. Carbohydr. Diet. Fibre 2016, 7, 19–27. [Google Scholar] [CrossRef]
- Bian, Y.Y.; Li, P. Study on scavenging activities for superoxide anion radicals and structure-activity relationship of flavonoids from Astragalus membranaceus (Fish.) Bge. var. mongholicus (Bge.) Hsia. Chin. Pharm. J. 2008, 43, 256–259. [Google Scholar]
- Saeed, H.; Ahsan, M.; Saleem, Z.; Iqtedar, M.; Islam, M.; Danish, Z.; Khan, A.M. Mesenchymal stem cells (MSCs) as skeletal therapeutics—An update. J. Biomed. Sci. 2016, 23, 41. [Google Scholar] [CrossRef] [PubMed]
- Mou, F.H.; Liu, Y.Y.; Xing, Y.; Li, W.L.; Yang, J.Z. Effects of quercitrin and its aglycone on radical scavenging. Spec. Wild Econ. Anim. Plant Res. 2015, 1, 9–13. [Google Scholar]
- Li, X.C. Improved pyrogallol autoxidation method: A reliable and cheap superoxide-scavenging assay suitable for all antioxidants. J. Agric. Food Chem. 2012, 60, 6418–6424. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.Z.; Zheng, R.L. Reactive oxygen species in theory and application of free radical biology. In Theory and Application of Free Radical Biology, 1st ed.; Science Press: Beijing, China, 2002; p. 124. [Google Scholar]
- Fang, Y.Z.; Zheng, R.L. Reactive oxygen species in theory and application of free radical biology. In Theory and Application of Free Radical Biology, 1st ed.; Science Press: Beijing, China, 2002; p. 98. [Google Scholar]
- Devos, D.; Moreau, C.; Devedjian, J.C.; Kluza, J.; Petrault, M.; Laloux, C.; Jonneaux, A.; Ryckewaert, G.; Garçon, G.; Rouaix, N.; et al. Targeting chelatable Iron as a therapeutic modality in Parkinson’s disease. Antioxid. Redox Signal. 2014, 21, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Bondet, V.; Williams, W.; Berset, C. Kinetics and mechanisms of antioxidant activity using the DPPH• free radical method. LWT-Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Foti, M.C.; Daquino, C.; Geraci, C. Electron-transfer reaction of cinnamic acids and their methyl esters with the DPPH center dot radical in alcoholic solutions. J. Org. Chem. 2004, 69, 2309–2314. [Google Scholar] [CrossRef] [PubMed]
- İlhami, G. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar]
- Fisher, A.E.; Hague, T.A.; Clarke, C.L.; Naughton, D.P. Catalytic superoxide scavenging by metal complexes of the calcium chelator EGTA and contrast agent EHPG. Biochem. Biophys. Res. Commun. 2004, 323, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Holtomo, O.; Nsangou, M.; Fifen, J.J.; Motapon, O. DFT study of the effect of solvent on the H- atom transfer involved in the scavenging of the free radicals •HO2 and •O2− by caffeic acid phenethyl ester and some of its derivatives. J. Mol. Model. 2014, 20, 2509. [Google Scholar] [CrossRef] [PubMed]
- Bielski, B.H.J.; Cabelli, D.E.; Arudi, R.L.; Ross, A.B. Reactivity of HO2/O2− radicals in aqueous solution. J. Phys. Chem. Ref. Data 1985, 14, 1041–1100. [Google Scholar] [CrossRef]
- Andrew, B.D.; Thomas, N. Rapid reaction of superoxide within sulin-tyrosyl radicals to generate a hydroperoxide with subsequent glutathione addition. Free Radic. Biol. Med. 2014, 70, 86–95. [Google Scholar]
- Eun, M.C. Protective effect of quercitrin against hydrogen peroxide-induced dysfunction in osteoblastic MC3T3-E1 cells. Exp. Toxicol. Pathol. 2012, 64, 211–216. [Google Scholar]
- Yang, H.M.; Ham, Y.M.; Yoon, W.J.; Seong, W.R.; Jeon, Y.; Tatsuya, O.; Kang, S.M.; Kang, M.C.; Kim, E.A.; Kim, D.Y.; et al. Quercitrin protects against ultraviolet B-induced cell death in vitro and in vivo zebrafish model. J. Photochem. Photobiol. B. 2012, 114, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.X.; Li, J.K.; Wang, K.; Hao, X.L.; Ge, R.; Li, Q.S. Isoquercitrin inhibits hydrogen peroxide-induced apoptosis of EA.hy926 Cells via the PI3K/Akt/GSK3β signaling pathway. Molecules 2016, 21, 356. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.X.; Sun, Z.Y. Protective effect of hypericum injection on carbon tetrachloride induced liver injury in mice. West. Pharm. J. 1992, 3, 146–149. [Google Scholar]
- Li, X.C.; Liu, J.J.; Lin, J.; Wang, T.T.; Huang, J.Y.; Lin, Y.Q.; Chen, D.F. Protective effects of dihydromyricetin against OH-induced mesenchymal stem cells damage and mechanistic chemistry. Molecules 2016, 21, 604. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.F.; Zeng, H.P.; Du, S.H.; Li, H.; Zhou, J.H.; Li, Y.W.; Wang, T.T.; Hua, Z.C. Extracts from Plastrum testudinis promote proliferation of rat bone-marrow-derived mesenchymal stem cells. Cell Prolif. 2007, 40, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Sample of the compound quercitrin and isoquercitrin are available from the authors.


| Assays | Quercitrin μg/mL (μM) | Isoquercitrin μg/mL (μM) | Positive Controls | Ratio (1) | Ratio (2) | |
|---|---|---|---|---|---|---|
| Trolox μg/mL (μM) | BHA μg/mL (μM) | |||||
| DPPH• scavenging | 4.45 ± 0.17 (9.93 ± 0.38 a) | 5.89 ± 0.25 (12.68 ± 0.54 b) | 4.53 ± 0.11 (18.10 ± 0.44 c) | 4.42 ± 0.19 (24.53 ± 1.04 d) | 1.8 | 1.4 |
| Cu2+-Reducing | 8.91 ± 0.27 (19.87 ± 0.61 a) | 11.75 ± 0.36 (25.31 ± 0.78 b) | 15.68 ± 0.63 (62.66 ± 2.51 d) | 7.96 ± 0.28 (44.19 ± 0.69 c) | 3.2 | 2.5 |
| FRAP | 6.14 ± 0.29 (13.70 ± 0.65 b) | 5.71 ± 0.16 (12.30 ± 0.34 a) | 6.98 ± 0.11 (27.88 ± 0.47 d) | 4.61 ± 0.13 (25.60 ± 0.69 c) | 2.0 | 2.3 |
| •O2− scavenging | 39.45 ± 2.43 (87.99 ± 5.43 b) | 36.30 ± 2.24 (78.16 ± 4.83 a) | 34.31 ± 0.90 (137.08 ± 3.61 c) | N.D. N.D. | 1.6 | 1.8 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Jiang, Q.; Wang, T.; Liu, J.; Chen, D. Comparison of the Antioxidant Effects of Quercitrin and Isoquercitrin: Understanding the Role of the 6″-OH Group. Molecules 2016, 21, 1246. https://doi.org/10.3390/molecules21091246
Li X, Jiang Q, Wang T, Liu J, Chen D. Comparison of the Antioxidant Effects of Quercitrin and Isoquercitrin: Understanding the Role of the 6″-OH Group. Molecules. 2016; 21(9):1246. https://doi.org/10.3390/molecules21091246
Chicago/Turabian StyleLi, Xican, Qian Jiang, Tingting Wang, Jingjing Liu, and Dongfeng Chen. 2016. "Comparison of the Antioxidant Effects of Quercitrin and Isoquercitrin: Understanding the Role of the 6″-OH Group" Molecules 21, no. 9: 1246. https://doi.org/10.3390/molecules21091246