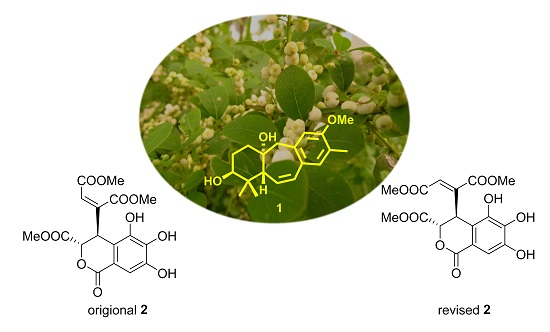
Chemical Constituents from Flueggea virosa and the Structural Revision of Dehydrochebulic Acid Trimethyl Ester
Abstract
:1. Introduction
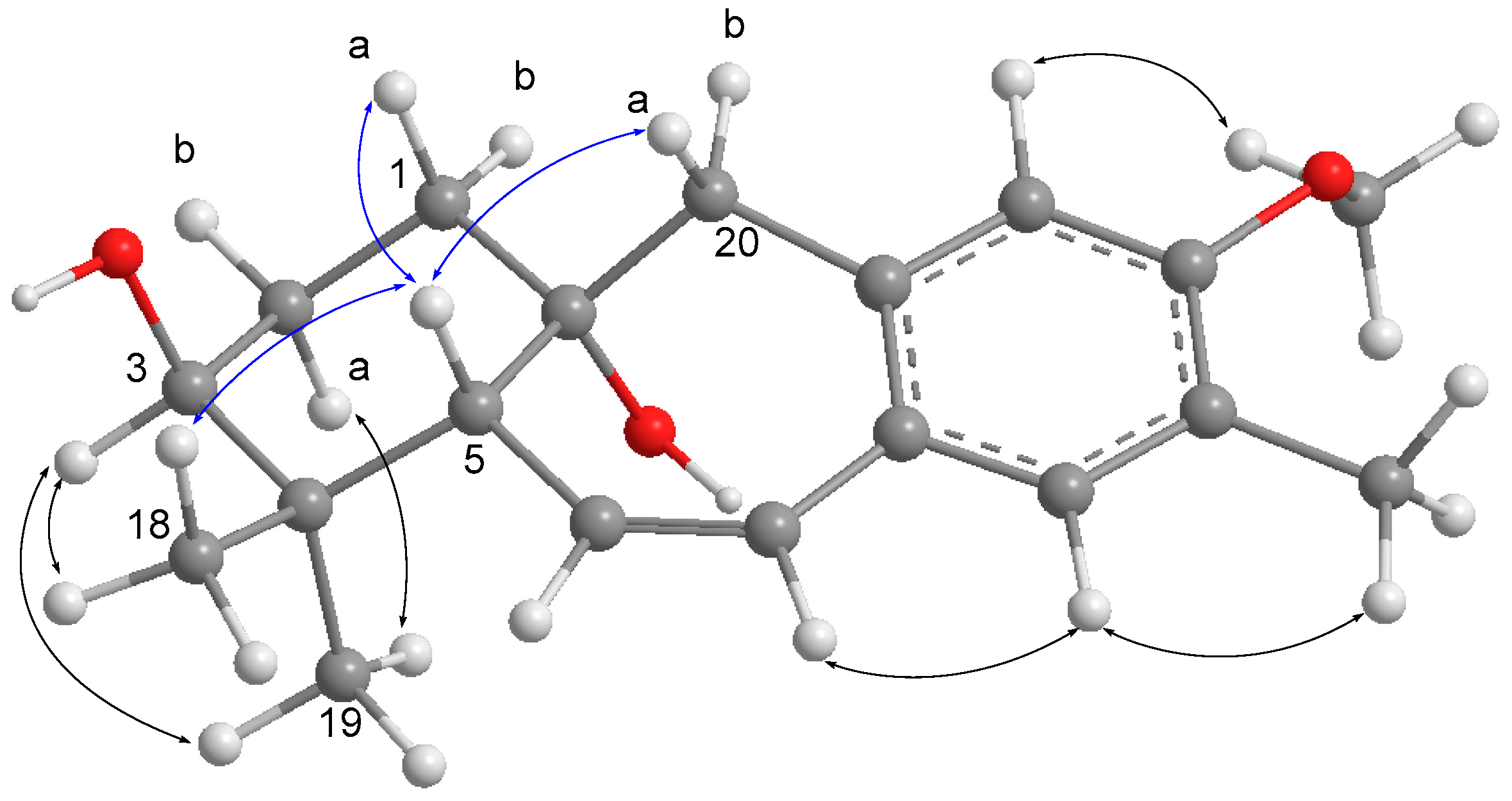
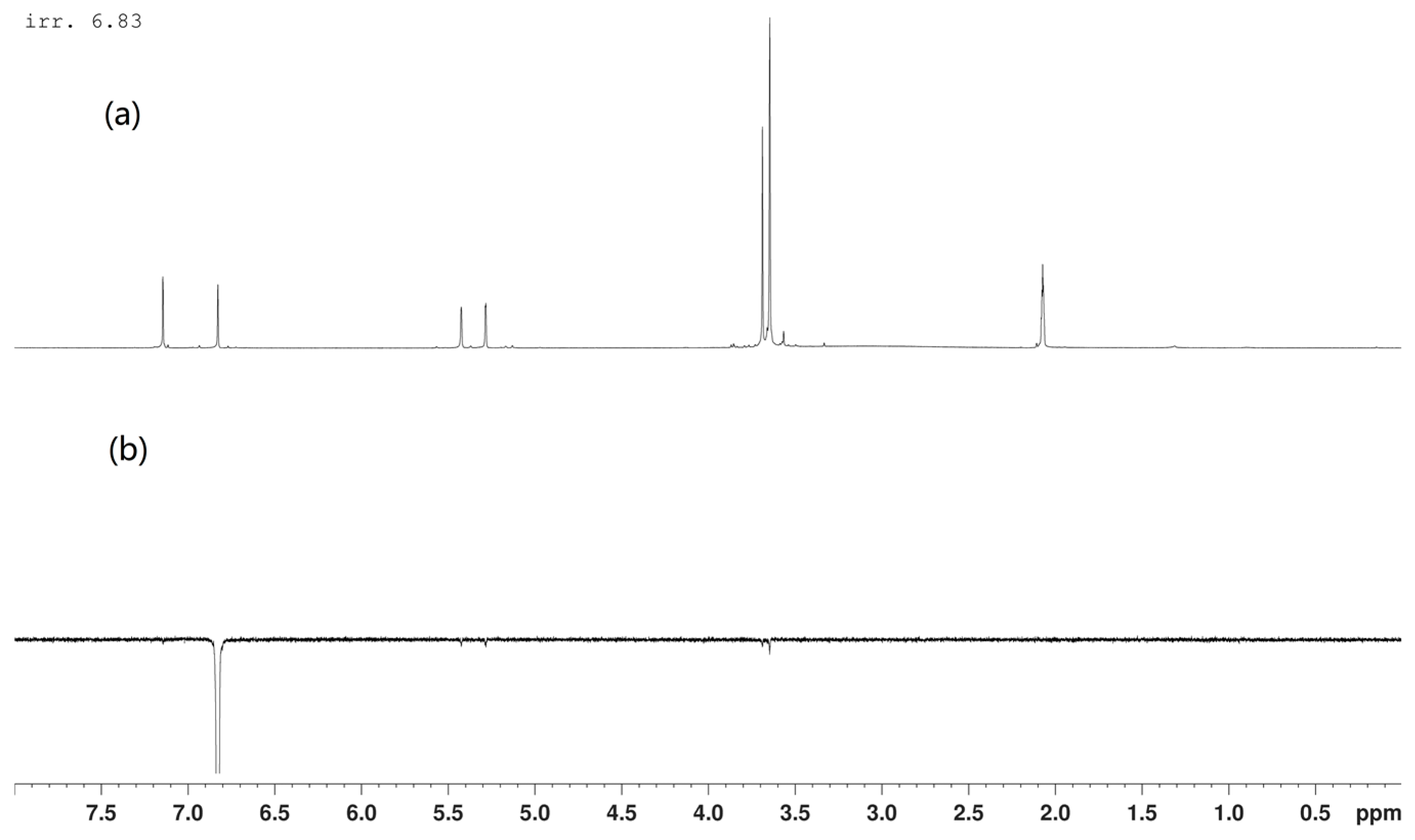
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
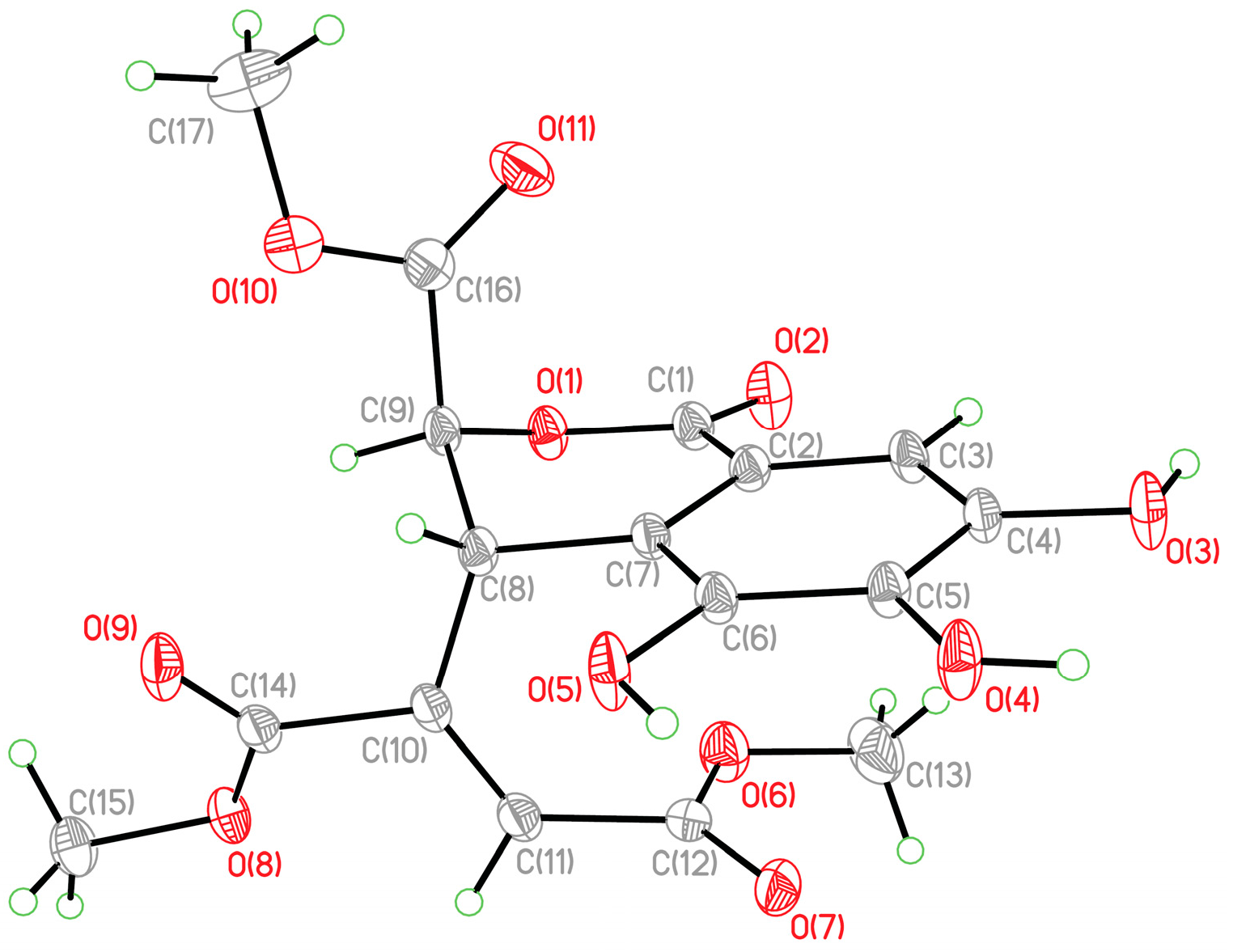
3.4. Crystallographic Data of 2
3.5. Infection Inhibition Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kamatenesi-Mugisha, M.; Oryem-Origa, H. Traditional herbal remedies used in the management of sexual impotence and erectile dysfunction in western Uganda. Afr. Health Sci. 2005, 5, 40–49. [Google Scholar] [PubMed]
- Chen, S.-K.; Chen, B.-Y.; Li, H. Flora of China; Science Press: Beijing, China, 1997; Volume 44, pp. 68–71. [Google Scholar]
- Wu, Z.-L.; Zhao, B.-X.; Huang, X.-J.; Tang, G.-Y.; Shi, L.; Jiang, R.-W.; Liu, X.; Wang, Y.; Ye, W.-C. Suffrutines A and B: a pair of Z/E isomeric indolizidine alkaloids from the roots of Flueggea suffruticosa. Angew. Chem. Int. Ed. 2014, 53, 5796–5799. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-H.; Cheng, J.-C.; Shen, D.-Y.; Wu, T.-S. Anti-hepatitis C virus dinorditerpenes from the roots of Flueggea virosa. J. Nat. Prod. 2014, 77, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-H.; Cheng, J.-C.; Hwang, T.-L.; Shen, D.-Y.; Wu, T.-S. Trinorditerpenes from the roots of Flueggea virosa. Bioorg. Med. Chem. Lett. 2014, 24, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-H.; Cheng, J.-C.; Shen, D.-Y.; Huang, H.-C.; Wu, Y.-C.; Wu, T.-S. Terpenoids from Flueggea virosa and their anti-hepatitis C virus activity. Phytochemistry 2016, 128, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-H.; Cheng, J.-C.; Shen, D.-Y.; Huang, H.-C.; Wu, Y.-C.; Wu, T.-S. 13-Methyl-3,4-seco-ent- podocarpanes, rare C18-diterpenoids from the roots of Flueggea virosa. RSC Adv. 2016, 6, 34708–34714. [Google Scholar] [CrossRef]
- Camacho-Luis, A.; Gayosso-De-Lucio, J.A.; Torres-Valencia, J.M.; Munoz-Sanchez, J.L.; Alarcon-Hernandez, E.; Lopez, R.; Barron, B.L. Antioxidant Constituents of Geranium bellum Rose. J. Mex. Chem. Soc. 2008, 52, 103–107. [Google Scholar]
- Yao, Q.-Q.; Zuo, C.-X. Chemical studied on the constituents of Phyllanthus urinaria L. Acta Pharm. Sin. 1993, 28, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Lu, Z.; Liu, Y.; Meng, C.; Cheng, K.-D.; Zhu, P. Three new podocarpane-type diterpenoids from callus of Securinega suffruticosa. Chem. Pharm. Bull. 2005, 53, 1610–1612. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Lundgren, L.N.; Andersson, R. Triterpene caffeates from bark of Betula pubescens. Phytochemsitry 1994, 37, 795–799. [Google Scholar] [CrossRef]
- Yang, J.-H.; Lu, H.; Huang, R.; Wang, Y.S. Flavonoids from leaves and twigs of Stachyurus himalaicus var. himalaicus. Chem. Nat. Compd. 2011, 47, 112–113. [Google Scholar] [CrossRef]
- Lan, K.-H.; Wang, Y.-W.; Lee, W.-P.; Lan, K.-L.; Tseng, S.-H.; Hung, L.-R.; Yen, S.-H.; Lin, H.-C.; Lee, S.-D. Multiple effects of Honokiol on the life cycle of hepatitis C virus. Liver Int. 2012, 32, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.-C.; Hwang, T.-L.; Chen, H.-C.; Kuo, P.-C.; Lee, E.-J.; Lee, K.-H.; Wu, T.-S. Honokiol dimers and magnolol derivatives with new carbon skeletons from the roots of Magnolia officinalis and their inhibitory effects on superoxide anion generation and elastase release. PLoS ONE 2013, 8, e59502. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-T.; Tseng, C.-P.; Liao, M.-H.; Lu, S.-C.; Yeh, W.-Z.; Sakamoto, N.; Chen, C.-M.; Cheng, J.-C. Hepatitis C virus replication is modulated by the interaction of nonstructural protein NS5B and fatty acid synthase. J. Virol. 2013, 87, 4994–5004. [Google Scholar] [CrossRef] [PubMed]
- Blight, K.J.; McKeating, J.A.; Rice, C.M. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J. Virol. 2002, 76, 13001–13014. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1 and 2 are available from the authors.
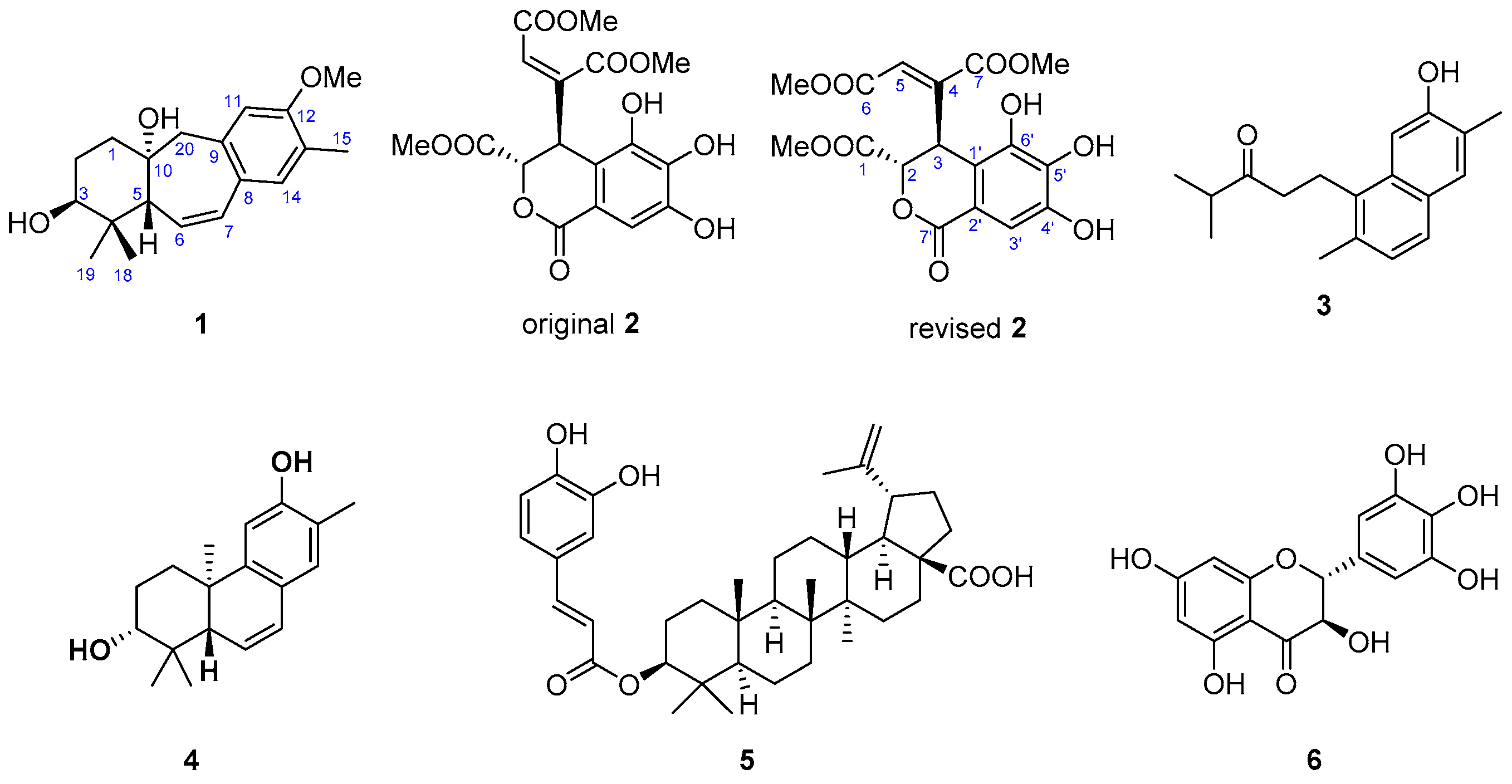

| 1 | |||
|---|---|---|---|
| δH (J in Hz) | δC (Mult.) | HMBC | |
| 1 | 2.05 td (14.1, 4.4) | 33.0 (CH2) | 2, 3, 20 |
| 1.61 m | |||
| 2 | 2.27 m | 25.5 (CH2) | 1 |
| 1.62 m | |||
| 3 | 3.51 t (2.8) | 75.6 (CH) | 1, 5 |
| 4 | 38.2 (C) | ||
| 5 | 2.45 dd (4.4, 2.6) | 49.6 (CH) | 4, 6, 7, 10, 18, 19 |
| 6 | 5.78 dd (12.1, 4.4) | 128.0 (CH) | 4, 5, 8, 10 |
| 7 | 6.58 dd (12.1, 2.6) | 131.3 (CH) | 5, 9, 14 |
| 8 | 128.4 (C) | ||
| 9 | 135.2 (C) | ||
| 10 | 76.4 (C) | ||
| 11 | 6.56 s | 112.2 (CH) | 8, 12, 13, 20 |
| 12 | 156.9 (C) | ||
| 13 | 124.4 (C) | ||
| 14 | 6.95 s | 132.4 (CH) | 7, 9, 12 |
| 15 | 2.17 s | 15.6 (CH3) | 12, 13, 14 |
| 18 | 1.00 s | 27.2 (CH3) | 3, 4, 5, 19 |
| 19 | 1.09 s | 22.4 (CH3) | 3, 4, 5, 18 |
| 20 | 2.96 d (14.2) | 51.6 (CH2) | 1, 5, 8, 9, 10, 11 |
| 2.68 d (14.2) | |||
| 12-OMe | 3.82 s | 55.3 (CH3) | 12 |
| Compound | EC50 (μM) a | IC50 (μM) b | (TI) c |
|---|---|---|---|
| 1 | 27.4 ± 1.4 | >100 | - d |
| 2 | 98.4 ± 2.1 | >100 | - d |
| 3 | 12.8 ± 3.1 | 87.1 ± 5.1 | 6.8 |
| 4 | 7.7 ± 2.7 | 70.5 ± 4.2 | 9.2 |
| 5 | 20.8 ± 2.0 | 60.7 ± 2.0 | 2.9 |
| 6 | 66.7 ± 1.8 | >100 | - d |
| honokiol | 22.4 ± 0.5 | 48.8 ± 0.8 | 2.1 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, C.-H.; Lin, Y.-J.; Cheng, J.-C.; Huang, H.-C.; Yeh, Y.-J.; Wu, T.-S.; Hwang, S.-Y.; Wu, Y.-C. Chemical Constituents from Flueggea virosa and the Structural Revision of Dehydrochebulic Acid Trimethyl Ester. Molecules 2016, 21, 1239. https://doi.org/10.3390/molecules21091239
Chao C-H, Lin Y-J, Cheng J-C, Huang H-C, Yeh Y-J, Wu T-S, Hwang S-Y, Wu Y-C. Chemical Constituents from Flueggea virosa and the Structural Revision of Dehydrochebulic Acid Trimethyl Ester. Molecules. 2016; 21(9):1239. https://doi.org/10.3390/molecules21091239
Chicago/Turabian StyleChao, Chih-Hua, Ying-Ju Lin, Ju-Chien Cheng, Hui-Chi Huang, Yung-Ju Yeh, Tian-Shung Wu, Syh-Yuan Hwang, and Yang-Chang Wu. 2016. "Chemical Constituents from Flueggea virosa and the Structural Revision of Dehydrochebulic Acid Trimethyl Ester" Molecules 21, no. 9: 1239. https://doi.org/10.3390/molecules21091239
APA StyleChao, C.-H., Lin, Y.-J., Cheng, J.-C., Huang, H.-C., Yeh, Y.-J., Wu, T.-S., Hwang, S.-Y., & Wu, Y.-C. (2016). Chemical Constituents from Flueggea virosa and the Structural Revision of Dehydrochebulic Acid Trimethyl Ester. Molecules, 21(9), 1239. https://doi.org/10.3390/molecules21091239