Long-Term Sodium Ferulate Supplementation Scavenges Oxygen Radicals and Reverses Liver Damage Induced by Iron Overloading
Abstract
:1. Introduction
2. Results
2.1. SF Attenuates Mice Liver Damage Induced by Iron Overload
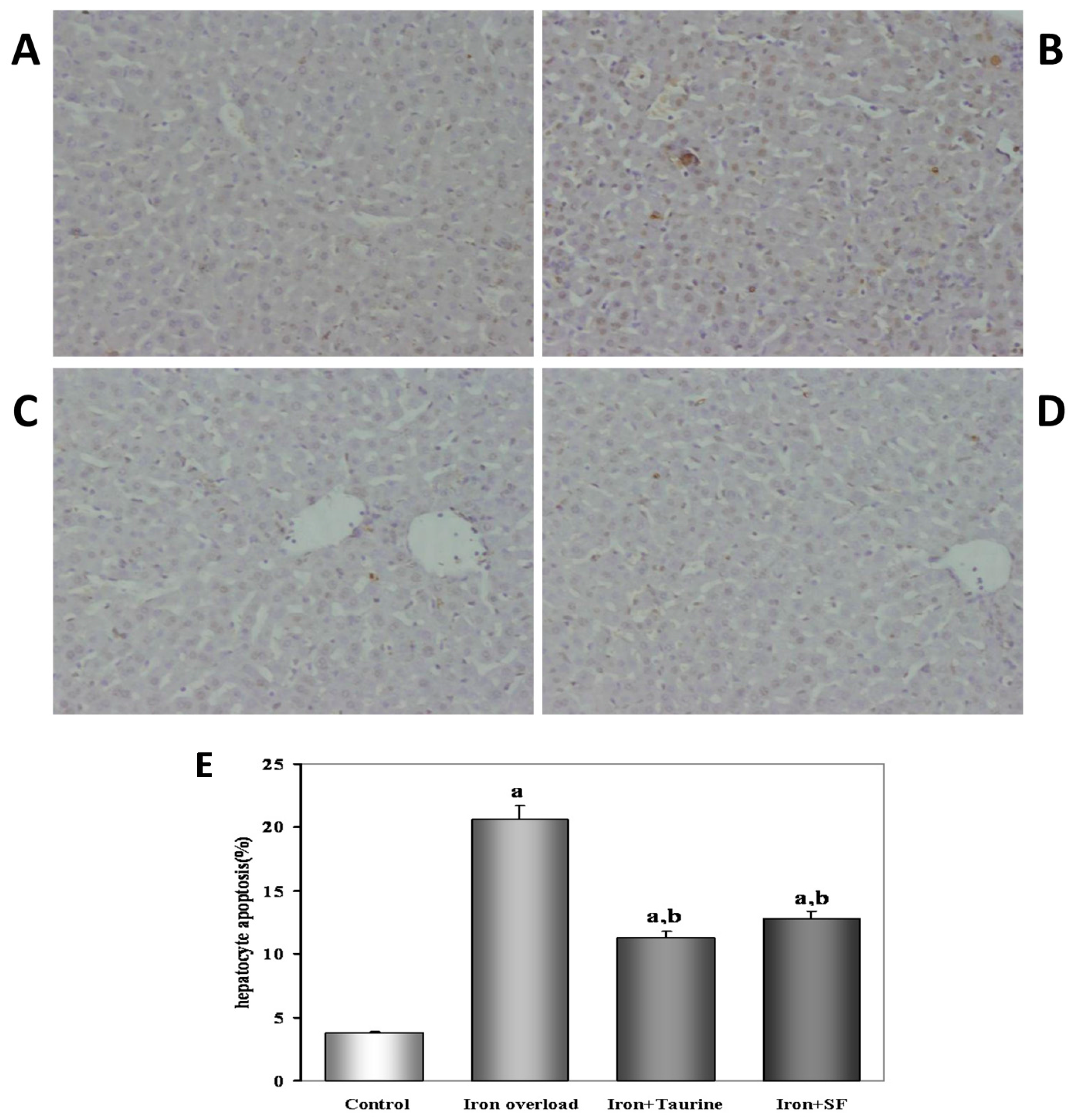
2.2. SF Reduces Hepatocyte Apoptosis
2.3. SF Elevates Enzymatic Antioxidants Activity
2.4. SF Increases Non-Enzymatic Antioxidants
2.5. SF Reduces the ROS Production
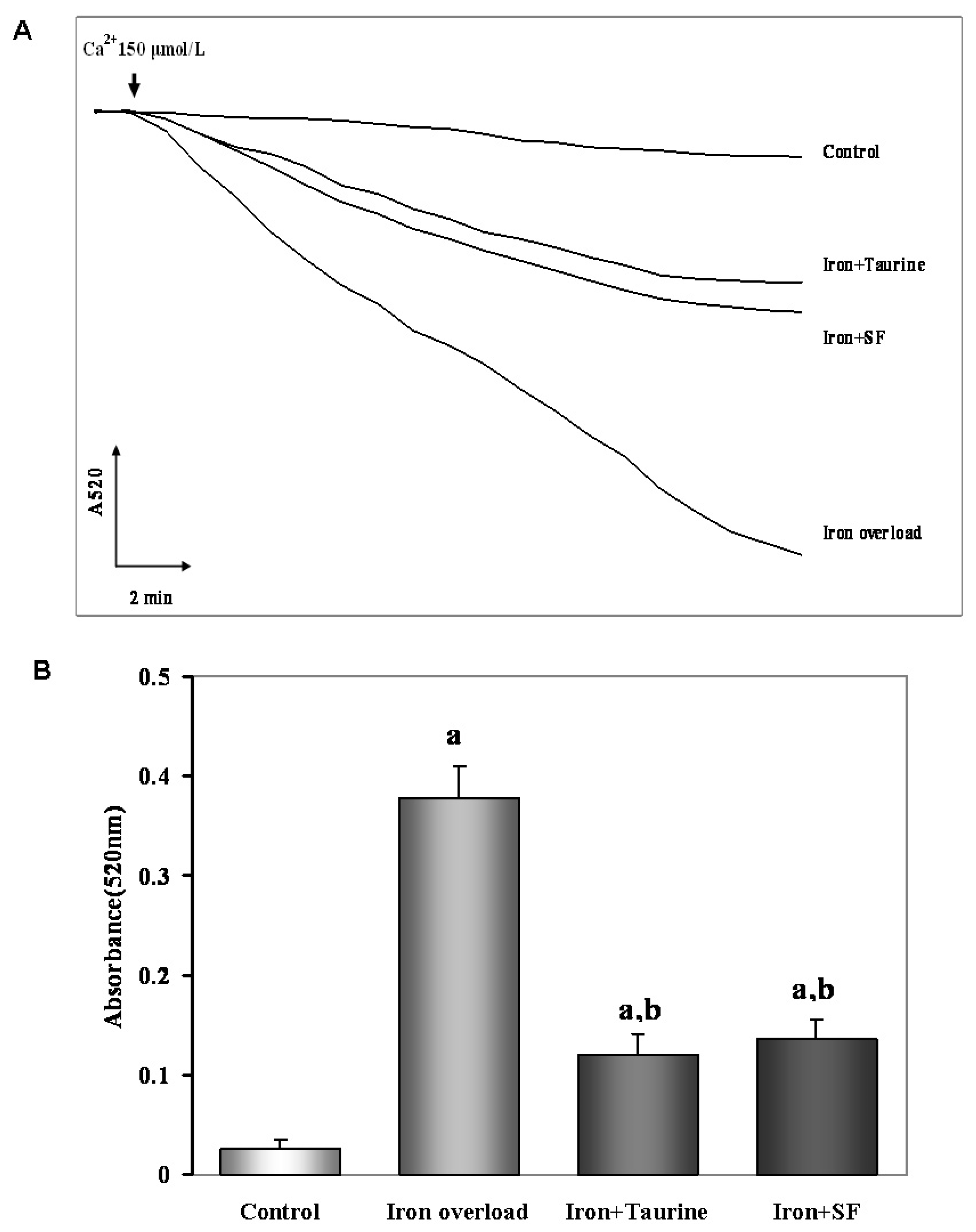
2.6. SF Inhibits the Mitochondrial Swelling
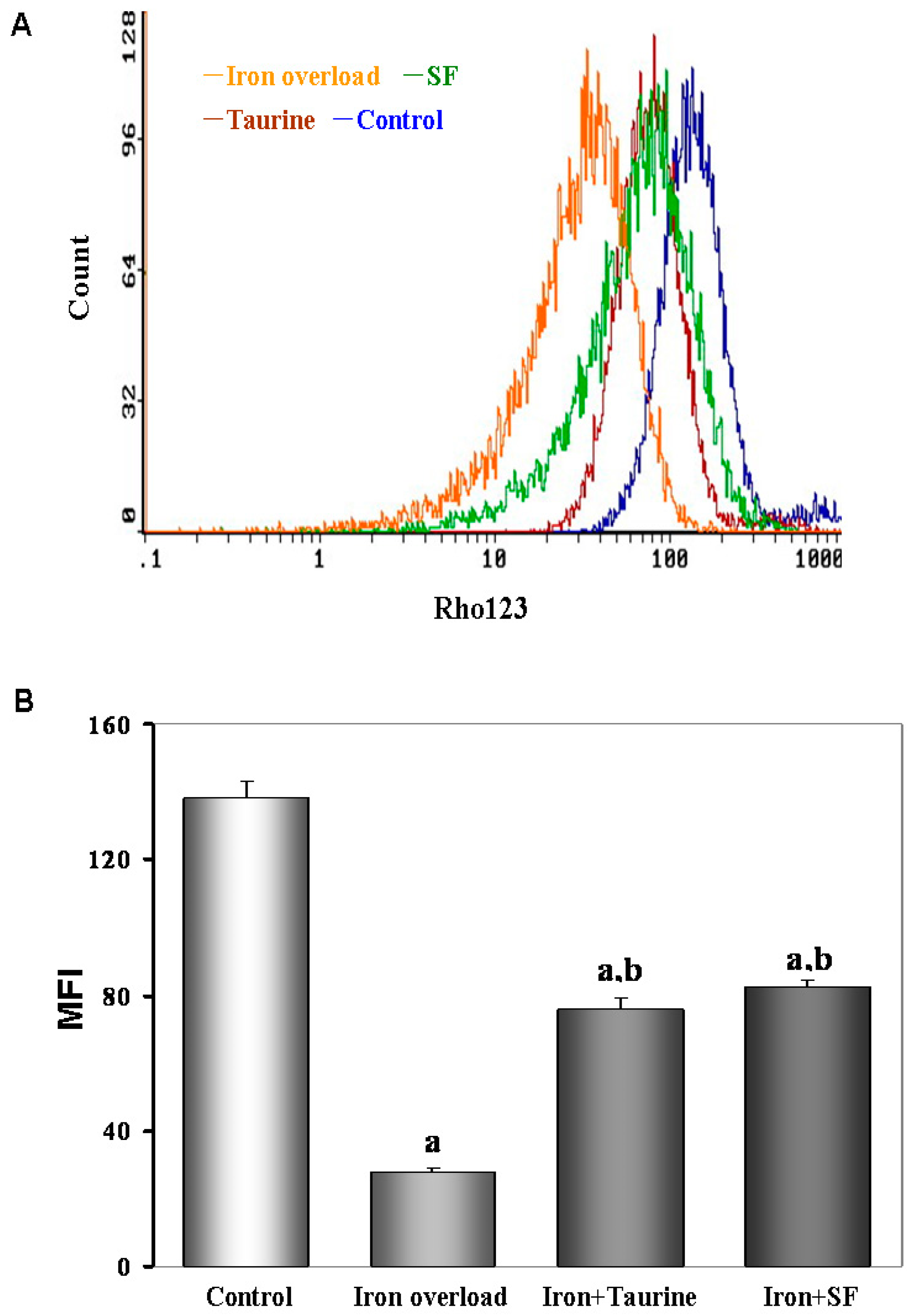
2.7. SF Inhibits the Loss of MMP (Mitochondrial Membrane Potential)
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Study Protocol
4.3. Measurement of Biochemical Parameters and Indices of Liver Injury
4.4. Determination of Antioxidant Enzymes Activities and Lipid Peroxidation
4.5. Estimation of Non-Enzymatic Antioxidants (GSH and GSSH)
4.6. Hepatocyte Preparation
4.7. Measurement of Intracellular ROS
4.8. Determination of Mitochondrial Membrane Potential (MMP)
4.9. Isolation of Mitochondria from Mouse Liver
4.10. Evaluation of Mitochondrial Swelling
4.11. TUNEL Assay
4.12. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bao, W.D.; Fan, Y.; Deng, Y.Z.; Long, L.Y.; Wang, J.J.; Guan, D.X.; Qian, Z.Y.; An, P.; Feng, Y.Y.; He, Z.Y.; et al. Iron overload in hereditary tyrosinemia type 1 induces liver injury through the Sp1/Tfr2/hepcidin axis. J. Hepatol. 2016, 65, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghe, C.N.; Kolnagou, A.; Kontoghiorghes, G.J. Phytochelators Intended for Clinical Use in Iron Overload, Other Diseases of Iron Imbalance and Free Radical Pathology. Molecules 2015, 20, 20841–20872. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.C.; Crawford, D.H.; Jaskowski, L.A.; Subramaniam, V.N.; Clouston, A.D.; Crane, D.I.; Bridle, K.R.; Anderson, G.J.; Fletcher, L.M. Excess iron modulates endoplasmic reticulum stress-associated pathways in a mouse model of alcohol and high-fat diet-induced liver injury. Lab. Investig. 2013, 93, 1295–1312. [Google Scholar] [CrossRef] [PubMed]
- Bonkovsky, H.L.; Lambrecht, R.W. Iron-induced liver injury. Clin. Liver Dis. 2000, 4, 409–429. [Google Scholar] [CrossRef]
- Kowdley, K.V. Iron, hemochromatosis, and hepatocellular carcinoma. Gastroenterology 2004, 127, S79–S86. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Guo, J.; Zhang, Y.; Zhang, J. The beneficial effects of taurine in preventing metabolic syndrome. Food Funct. 2016, 7, 1849–1863. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.W.; Ramila, K.C.; Jong, C.J.; Shetewy, A.; Shimada, K.; Ito, T.; Azuma, J.; Cioffi, E. Does taurine prolong lifespan by improving heart function? Adv. Exp. Med. Biol. 2015, 803, 555–570. [Google Scholar] [PubMed]
- Pasantes-Morales, H.; Ramos-Mandujano, G.; Hernández-Benítez, R. Taurine enhances proliferation and promotes neuronal specification of murine and human neural stem/progenitor cells. Adv. Exp. Med. Biol. 2015, 803, 457–472. [Google Scholar] [PubMed]
- Heidari, R.; Rasti, M.; Shirazi Yeganeh, B.; Niknahad, H.; Saeedi, A.; Najibi, A. Sulfasalazine-induced renal and hepatic injury in rats and the protective role of taurine. Bioimpacts 2016, 6, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Eghbal, M.A.; Taziki, S.; Sattari, M.R. Mechanisms of phenytoin-induced toxicity in freshly isolated rat hepatocytes and the protective effects of taurine and/or melatonin. J. Biochem. Mol. Toxicol. 2014, 28, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Fukuno, S.; Oda, A.; Konishi, H. Protective effects of taurine on doxorubicin-induced acute hepatotoxicity through suppression of oxidative stress and apoptotic responses. Anticancer Drugs 2016, 27, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, J.; Rodrigues, A.F.; Rós Ade, S.; de Castro, A.B.; de Lima, D.D.; Magro, D.D.; Zeni, A.L. Ferulic acid chronic treatment exerts antidepressant-like effect: Role of antioxidant defense system. Metab. Brain Dis. 2015, 30, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.Y.; Guo, J.; Majeed, H.; Zhu, K.X.; Guo, X.N.; Peng, W.; Zhou, H.M. Ferulic acid renders protection to HEK293 cells against oxidative damage and apoptosis induced by hydrogen peroxide. In Vitro Cell Dev. Biol. Anim. 2015, 51, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Bourne, L.C.; Rice-Evans, C. Bioavailability of ferulic acid. Biochem. Biophys. Res. Commun. 1998, 253, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Khallouki, F.; Haubner, R.; Ricarte, I.; Erben, G.; Klika, K.; Ulrich, C.M.; Owen, R.W. Identification of polyphenolic compounds in the flesh of Argan (Morocco) fruits. Food Chem. 2015, 179, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Devi, K.P.; Malar, D.S.; Sureda, A.; Daglia, M.; Nabavi, S.M. Ferulic acid and Alzheimer's disease: Promises and pitfalls. Mini Rev. Med. Chem. 2015, 15, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.W.; Jiang, J.S.; Lu, W.Q. Ferulic Acid Exerts Anti-Angiogenic and Anti-Tumor Activity by Targeting Fibroblast Growth Factor Receptor 1-Mediated Angiogenesis. Int. J. Mol. Sci. 2015, 16, 24011–24031. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Morales, M.; Espinosa-Alonso, L.G.; Espinoza-Torres, L.C.; Delgado-Vargas, F.; Medina-Godoy, S. Phenolic content and antioxidant and antimutagenic activities in tomato peel, seeds, and byproducts. J. Agric. Food Chem. 2014, 62, 5281–5289. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R.; Zhu, S.T.; Harris, P.J. Antioxidant and antigenotoxic effects of plant cell wall hydroxycinnamic acids in cultured HT-29 cells. Mol. Nutr. Food Res. 2005, 49, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Panja, S.; Chaudhuri, D.; Ghate, N.B.; Mandal, N. Phytochemical profile of a microalgae Euglena tuba and its hepatoprotective effect against iron-induced liver damage in Swiss albino mice. J. Appl. Microbiol. 2014, 117, 1773–1786. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, Y.; Yu, H.; Gao, C.; Liu, L.; Chen, S.; Xing, M.; Liu, L.; Yao, P. Quercetin prevents ethanol-induced iron overload by regulating hepcidin through the BMP6/SMAD4 signaling pathway. J. Nutr. Biochem. 2014, 25, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Ghate, N.B.; Chaudhuri, D.; Panja, S.; Mandal, N. Nerium indicum leaf alleviates iron-induced oxidative stress and hepatic injury in mice. Pharm. Biol. 2015, 53, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Hazra, B.; Mandal, N. Amelioration of iron overload-induced liver toxicity by a potent antioxidant and iron chelator, Emblica officinalis Gaertn. Toxicol. Ind. Health 2015, 31, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Britton, R.S.; Leicester, K.L.; Bacon, B.R. Iron toxicity and chelation therapy. Int. J. Hematol. 2002, 76, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Lubrano, V.; Balzan, S. Enzymatic antioxidant system in vascular inflammation and coronary artery disease. World J. Exp. Med. 2015, 5, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Kandola, K.; Bowman, A.; Birch-Machin, M.A. Oxidative stress—A key emerging impact factor in health, ageing, lifestyle and aesthetics. Int. J. Cosmet. Sci. 2015, 37, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Oyewole, A.O.; Birch-Machin, M.A. Mitochondria-targeted antioxidants. FASEB J. 2015, 29, 4766–4771. [Google Scholar] [CrossRef] [PubMed]
- Aikemu, A.; Yusup, A.; Umar, A.; Berké, B.; Moore, N.; Upur, H. The impact of the Uighur medicine abnormal savda munziq on antitumor and antioxidant activity in a S180 and Ehrlich ascites carcinoma mouse tumor model. Pharmacogn. Mag. 2012, 8, 141–148. [Google Scholar] [PubMed]
- Jia, H.; Liu, J.W.; Ufur, H.; He, G.S.; Liqian, H.; Chen, P. The antihypertensive effect of ethyl acetate extract from red raspberry fruit in hypertensive rats. Pharmacogn Mag. 2011, 7, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.W.; Green, D.R. Mitochondrial regulation of cell death. Cold Spring Harb. Perspect. Biol. 2013, 5, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.F.; Neuzil, J. Mitochondria in cancer: Why mitochondria are a good target for cancer therapy. Prog. Mol. Biol. Transl. Sci. 2014, 127, 211–227. [Google Scholar] [PubMed]
- Liu, D.; He, H.; Yin, D.; Que, A.; Tang, L.; Liao, Z.; Huang, Q.; He, M. Mechanism of chronic dietary iron overload-induced liver damage in mice. Mol. Med. Rep. 2013, 7, 1173–1179. [Google Scholar] [PubMed]
- Galleano, M.; Puntarulo, S. Hepatic chemiluminescence and lipid peroxidation in mild iron overload. Toxicology 1992, 76, 27–38. [Google Scholar] [CrossRef]
- Brumby, P.E.; Massey, V. Determination of nonheme iron, total iron, and copper. Methods Enzymol. 1967, 10, 463–474. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Sun, Y.; Oberley, L.W.; Ying, L. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [PubMed]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1974; pp. 673–684. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Okhawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar]
- Lu, Y.; Liu, S.; Wang, Y.; Wang, D.; Gao, J.; Zhu, L. Asiatic acid uncouples respiration in isolated mouse liver mitochondria and induces HepG2 cells death. Eur. J. Pharmacol. 2016, 786, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, D.; Yi, B.; Liao, Z.; Tang, L.; Yin, D.; He, M. Taurine supplementation reduces oxidative stress and protects the liver in an iron-overload murine model. Mol. Med. Rep. 2014, 10, 2255–2262. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.



| Group | Control | Iron | Iron + TAU | Iron + SF |
|---|---|---|---|---|
| Serum iron concentration (μmol/L) | 35.03 ± 1.26 | 415.26 ± 14.21 a | 395.18 ± 15.18 a | 398.64 ± 16.21 a |
| Hepatic iron concentration (mg/g dry weight) | 0.058 ± 0.002 | 1.102 ± 0.032 a | 1.081 ± 0.029 a | 1.076 ± 0.031 a |
| LW/BW (mg/g) | 45.6 ± 2.2 | 102.6 ± 4.3 a | 62.3 ± 2.2 a,b | 65.1 ± 2.6 a,b |
| ALT (U/L) | 46.82 ± 1.35 | 235.21 ± 8.62 a | 120.32 ± 4.61 a,b | 135.06 ± 5.25 a,b |
| AST (U/L) | 100.65 ± 3.25 | 405.28 ± 13.06 a | 224.52 ± 9.31 a,b | 240.61 ± 9.53 a,b |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, Y.; He, H.; Zhang, Z.; Liao, Z.; Yin, D.; Liu, D.; Yi, B.; He, M. Long-Term Sodium Ferulate Supplementation Scavenges Oxygen Radicals and Reverses Liver Damage Induced by Iron Overloading. Molecules 2016, 21, 1219. https://doi.org/10.3390/molecules21091219
Qiao Y, He H, Zhang Z, Liao Z, Yin D, Liu D, Yi B, He M. Long-Term Sodium Ferulate Supplementation Scavenges Oxygen Radicals and Reverses Liver Damage Induced by Iron Overloading. Molecules. 2016; 21(9):1219. https://doi.org/10.3390/molecules21091219
Chicago/Turabian StyleQiao, Yang, Huan He, Zeyu Zhang, Zhangping Liao, Dong Yin, Dan Liu, Bo Yi, and Ming He. 2016. "Long-Term Sodium Ferulate Supplementation Scavenges Oxygen Radicals and Reverses Liver Damage Induced by Iron Overloading" Molecules 21, no. 9: 1219. https://doi.org/10.3390/molecules21091219





