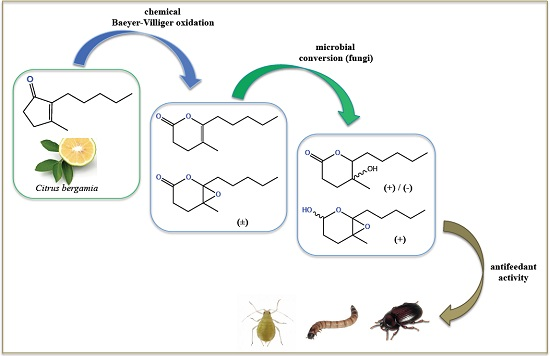
Alkyl-Substituted δ-Lactones Derived from Dihydrojasmone and Their Stereoselective Fungi-Mediated Conversion: Production of New Antifeedant Agents
Abstract
:1. Introduction
2. Results and Discussion
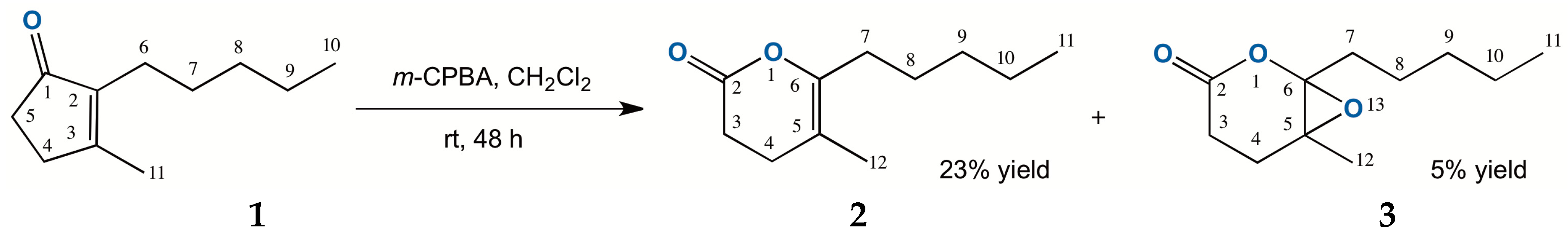
2.1. Chemical Oxidation of Dihydrojasmone (1)
2.2. Biotransformation of 3,4-Dihydro-5-methyl-6-pentyl-2H-pyran-2-one (2)
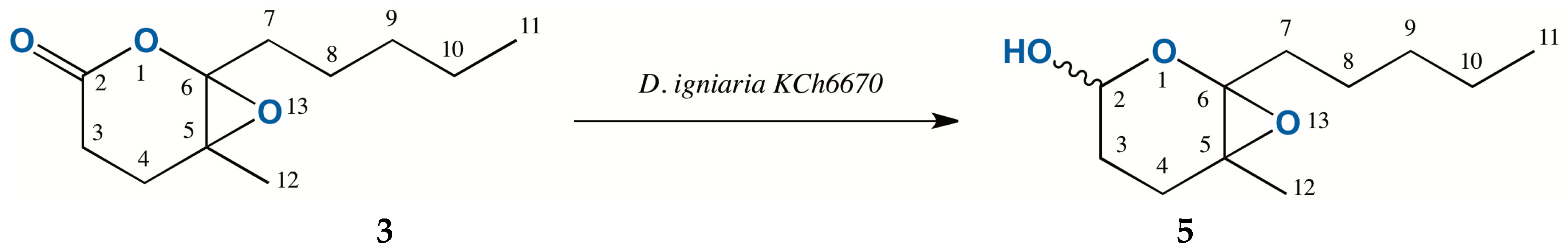
2.3. Biotransformation of 5-Methyl-6-pentyl-1,13-dioxabicyclo[4.1.0]heptan-2-one (3)
2.4. Feeding Deterrent Activity Against the Lesser Mealworm
2.5. Deterrent Activity against the Peach Potato Aphid
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Chemical Synthesis
3.3. Microorganisms
3.4. Screening Procedure
3.5. Preparative Biotransformation
3.5.1. Identification of Product
3.5.2. Feeding Deterrent Activity—Bioassays
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Marco, J.A.; Carda, M.; Murga, J.; Falomir, E. Stereoselective synthesis of naturally occurring 5,6-dihydropyran-2-ones. Tetrahedron 2007, 63, 2929–2958. [Google Scholar] [CrossRef]
- Fink, M.J.; Rudroff, F.; Mihovilovic, M.D. Baeyer-Villiger monooxygenases in aroma compound synthesis. Bioorg. Med. Chem. Lett. 2011, 21, 6135–6138. [Google Scholar] [CrossRef] [PubMed]
- Fink, M.J.; Schön, M.; Rudroff, F.; Schnürch, M.; Mihovilovic, M.D. Single operation stereoselective synthesis of Aerangis lactones: Combining continuous flow hydrogenation and biocatalysts in a chemoenzymatic sequence. ChemCatChem 2013, 5, 724–727. [Google Scholar] [CrossRef]
- Rolli, E.; Marieschi, M.; Maietti, S.; Guerrini, A.; Grandini, A.; Sacchetti, G.; Bruni, R. Phytotoxic effects and phytochemical fingerprinting of hydrodistilled oil, enriched fractions, and isolated compounds obtained from Cryptocarya massoy (OKEN) KOSTERM. Bark. Chem. Biodivers. 2016, 13, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.P.; Milligan, K.E.; Gerwick, W.H. Tanikolide, a toxic and antifungal lactone from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 1999, 62, 1333–1335. [Google Scholar] [CrossRef] [PubMed]
- Cavill, G.W.K.; Clark, D.V.; Whitefield, F.B. Insect venoms, attractants, and repellents. XI. Massoilactone from two species of formicine ants, and some observations on constituents of the bark oil of Cryptocarya massoia. Aust. J. Chem. 1968, 21, 2819–2823. [Google Scholar] [CrossRef]
- Lopez, L.; Morgan, E.D. Explanation of bitter taste of venom of ponerine ant, Pachycondyla apicalis. J. Chem. Ecol. 1997, 23, 705–712. [Google Scholar] [CrossRef]
- Li, G.; Roze, U.; Locke, D. Warning odor of the North American Porcupine (Erethizon dorsatum). J. Chem. Ecol. 1997, 23, 2737–2754. [Google Scholar] [CrossRef]
- Bernardi, R.; Ghiringhelli, D. Synthesis of enantiomerically pure (S)-5,6-dihydro- and (S)-tetrahydro-6-methyl-2H-pyran-2-one. Gazz. Chim. Tal. 1992, 122, 395–396. [Google Scholar] [CrossRef]
- Meijer, T.M. The essential oil of Massoi bark. Recl. Trav. Chim. Pays-Bas 1940, 59, 191–201. [Google Scholar] [CrossRef]
- Kaiser, R.; Lamparsky, D. Lactone of 5-hydroxy-cis-2-cis-7-decadienic acid and other lactones from essential oils of Polianthes-tuberosa L. flowers. Tetrahedron Lett. 1976, 17, 1659–1660. [Google Scholar] [CrossRef]
- Goh, S.H.; Ec, G.C.L.; Chuah, C.H.; Chen, W. Styrylpyrone derivatives from Goniothalamus dolichocarpus. Aust. J. Chem. 1995, 48, 199–205. [Google Scholar]
- Senthil-Nathan, S.; Choi, M.-Y.; Paik, C.-H.; Kalaivani, K. The toxicity and physiological effect of goniothalamin, a styryl-pyrone, on the generalist herbivore, Spodoptera exigua Hübner. Chemosphere 2008, 72, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Kabir, K.E.; Khan, A.R.; Mosaddik, M.A. Goniothalamin—A potent mosquito larvicide from Bryonopsis laciniosa L. J. Appl. Entomol. 2003, 127, 112–115. [Google Scholar] [CrossRef]
- Fatima, A.; Kohn, L.K.; de Carvalho, J.E.; Pilli, R.A. Cytotoxic activity of (S)-goniothalamin and analogues against human cancer cells. Bioorg. Med. Chem. 2006, 14, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Al-Qubaisi, M.; Rozita, R.; Yeap, S.-K.; Omar, A.-R.; Ali, A.-M.; Alitheen, N.B. Selective cytotoxicity of goniothalamin against hepatoblastoma HepG2 cells. Molecules 2011, 16, 2944–2959. [Google Scholar] [CrossRef] [PubMed]
- Birkett, M.A.; Campbell, C.A.M.; Chamberlain, K.; Guerrieri, E.; Hick, A.J.; Martin, J.L.; Matthes, M.; Napier, J.A.; Pettersson, J.; Pickett, J.A.; et al. New roles for cis-jasmone as an insect semiochemical and in plant defence. Proc. Natl. Acad. Sci. USA 2000, 97, 9329–9334. [Google Scholar] [CrossRef] [PubMed]
- Gliszczyńska, A.; Gładkowski, W.; Dancewicz, K.; Gabryś, B. Enantioselective microbialhydroxylation as a useful tool in the production of jasmonate derivatives with aphid deterrent activity. Curr. Microbiol. 2015, 71, 83–94. [Google Scholar]
- Wasternack, C.; Strnad, M. Jasmonate signaling in plant stress responses and development—Active and inactive compounds. New Biotechnol. 2016, 33, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.K.; Jawalkar, D.G. Studies towards the syntheses of functionally substituted γ-butyrolactones and spiro-γ-butyrolactones and their reaction with strong acids: A novel route to α-pyrones. J. Org. Chem. 1989, 54, 2364–2369. [Google Scholar] [CrossRef]
- Gliszczyńska, A.; Gładkowski, W.; Dancewicz, K.; Gabryś, B.; Szczepanik, M. Transformation of β-damascone to (+)-(S)-4-hydroxy-β-damascone by fungal strains and its evaluation as a potential insecticide against aphids Myzus persicae and lesser mealworm Alphitobius diaperinus Panzer. Catal. Commun. 2016, 80, 39–43. [Google Scholar] [CrossRef]
- Gliszczyńska, A.; Dancewicz, K.; Hnatejko, M.; Szczepanik, M.; Gabryś, B. Synthesis of β-damascone derivatives with a lactone ring and their feeding deterrent activity against aphids and lesser mealworms. RSC Adv. 2014, 4, 39248–39256. [Google Scholar] [CrossRef]
- Pavela, R. Natural products as allelochemicals in pest management. In Natural Products in Plant Pest Management; Nawal, K.D., Ed.; CABI Head Office: Oxfordshire, UK, 2011; pp. 134–148. [Google Scholar]
- Tjallingii, W.F. Regulation of phloem sap feeding by aphids. In Regulatory Mechanisms in Insect Feeding; Chapman, R.F., de Gerrit, B., Eds.; Chapman & Hall: New York, NY, USA, 1995; pp. 190–209. [Google Scholar]
- Pettersson, J.; Tjallingii, W.F.; Hardie, J. Host-plant selection and feeding. In Aphids as Crop Pests; van Emden, H.F., Harrington, R., Eds.; CABI: Wallingford, UK, 2007; pp. 87–113. [Google Scholar]
- Gabryś, B.; Dancewicz, K.; Gliszczyńska, A.; Kordan, B.; Wawrzeńczyk, C. Systemic deterrence of aphid probing and feeding by novel β-damascone analogues. J. Pest Sci. 2015, 88, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Halbert, S.E.; Corsini, D.; Wiebe, M.; Vaughn, S.F. Plant-derived compounds and extracts with potential as aphid repellents. Ann. Appl. Biol. 2009, 154, 303–307. [Google Scholar] [CrossRef]
- Dancewicz, K.; Kordan, B.; Szumny, A.; Gabryś, B. Aphid behaviour-modifying activity of essential oils from Lamiaceae and Apiaceae. Aphids Other Homopterous Insects 2012, 18, 93–100. [Google Scholar]
- Wróblewska-Kurdyk, A.; Nowak, L.; Dancewicz, K.; Szumny, A.; Gabryś, B. In search of biopesticides: The effect of caraway Carum carvi essential oil and its major constituents on peach potato aphid Myzus persicae probing behavior. Acta Biol. Zesz. Naukowe Uniw. Szczec. 2015, 846, 51–62. [Google Scholar]
- Dancewicz, K.; Gabryś, B.; Dams, I.; Wawrzeńczyk, C. Enantiospecific effect of pulegone and pulegone−derived lactones on settling and feeding of Myzus persicae (Sulz.). J. Chem. Ecol. 2008, 34, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Grudniewska, A.; Dancewicz, K.; Białońska, A.; Wawrzeńczyk, C.; Gabrys, B. Piperitone-derived saturated lactones: Synthesis and aphid behavior-modifying activity. J. Agric. Food Chem. 2013, 61, 3364–3372. [Google Scholar] [CrossRef] [PubMed]
- Pickett, J.A.; Woodcock, C.M.; Midega, C.A.O.; Khan, Z.R. Push–pull farming systems. Curr. Opin. Biotechnol. 2014, 26, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.J.; Lambkin, T.A. A new culture method for lesser mealworm, Alphitobius diaperinus. J. Appl. Entomol. 2009, 133, 67–72. [Google Scholar] [CrossRef]
- Hardie, J.; Holyoak, M.; Taylor, N.J.; Griffits, D.C. The combination of electronic monitoring and video-assisted observations of plant penetration by aphids and behavioural effects of polygodial. Entomol. Exp. Appl. 1992, 62, 233–239. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–3 are available from the authors.

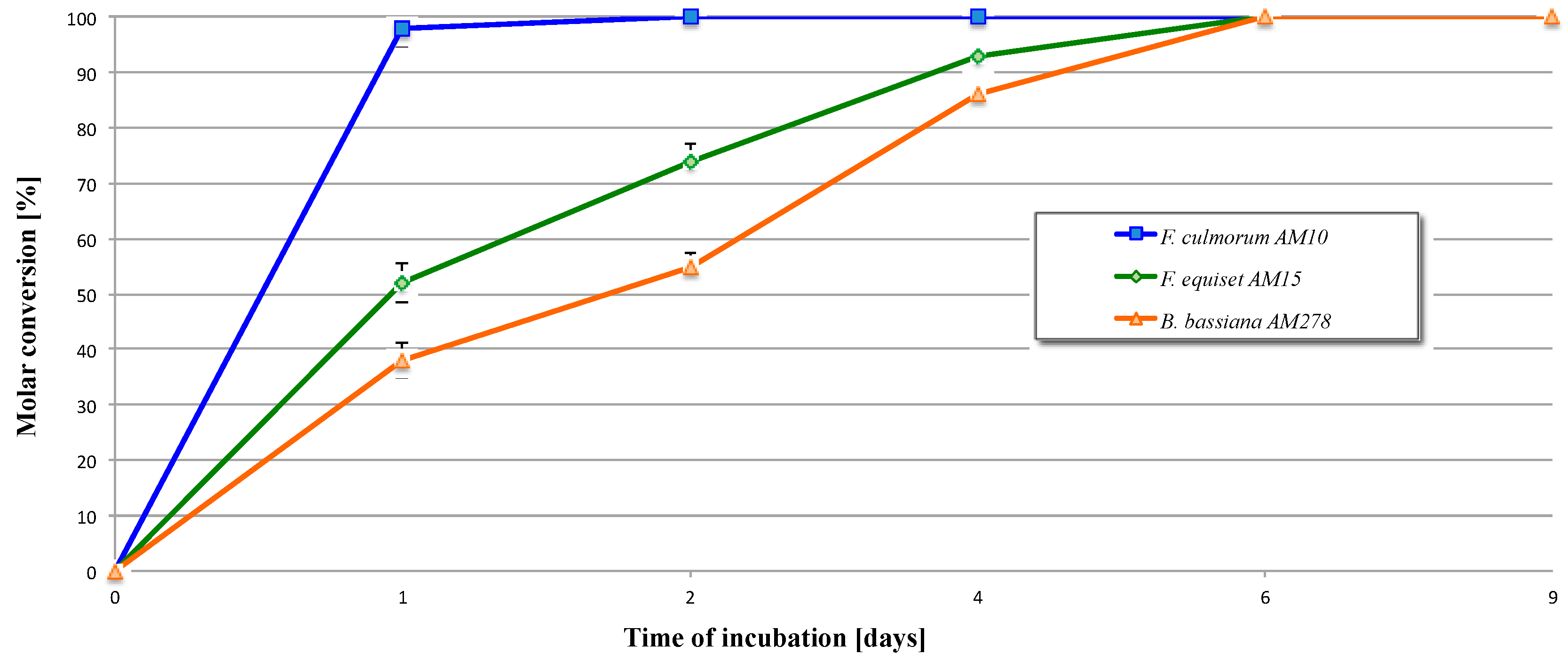
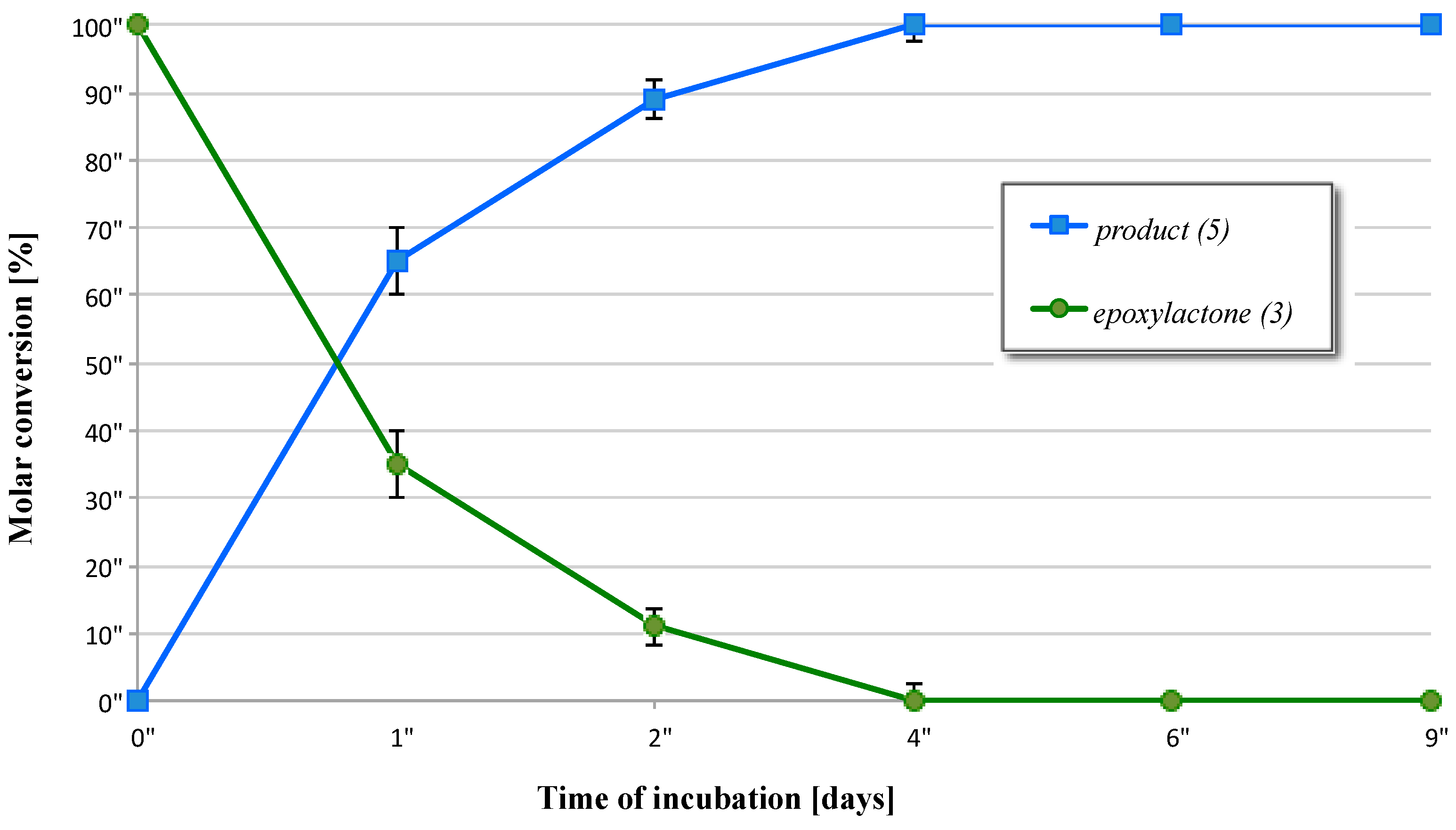
| Entry | Microorganism | Transformation Period (Days) | Reaction Rate (mg/h) of Product |
|---|---|---|---|
| 1 | F. culmorum AM10 | 1 | 4.1 × 10−1 |
| 2 | 8.3 × 10−3 | ||
| 4 | Full conversion | ||
| 6 | Full conversion | ||
| 9 | Full conversion | ||
| 2 | F. equiseti AM15 | 1 | 2.2 × 10−1 |
| 2 | 9.2 × 10−2 | ||
| 4 | 3.9 × 10−2 | ||
| 6 | 1.5 × 10−2 | ||
| 9 | Full conversion | ||
| 3 | B. bassiana AM278 | 1 | 1.6 × 10−1 |
| 2 | 7.1 × 10−2 | ||
| 4 | 6.5 × 10−2 | ||
| 6 | 2.9 × 10−2 | ||
| 9 | Full conversion |
| Compound | Deterrence Coefficients ± SE a | |||||
|---|---|---|---|---|---|---|
| Larvae | Adults | |||||
| A | R | T | A | R | T | |
| (1) | 29.37 ± 0.91bc | 71.40 ± 4.17bc | 100.77 ± 3.70b | −20.69 ± 7.61a | 91.48 ± 1.02b | 70.79 ± 8.04a |
| (2) | 40.88 ± 3.77c | 88.84 ± 0.65c | 129.72 ± 3.97bc | 57.17 ± 14.42b | 93.19 ± 0.51b | 150.36 ± 11.87b |
| (−)-(4) | 74.95 ± 3.57d | 78.29 ± 3.59c | 153.24 ± 7.09c | 90.21 ± 0.3b | 72.26 ± 7.51a | 162.46 ± 7.69b |
| (+)-(4) | −8.99 ± 2.67a | 53.21 ± 9.14ab | 44.22 ± 8.36a | 82.69 ± 3.27b | 97.02 ± 0.49b | 179.71 ± 2.81b |
| (3) | 8.35 ± 2.85ab | 47.39 ± 4.64a | 55.74 ± 7.34a | −28.64 ± 9.50a | 64.48 ± 2.31a | 35.84 ± 8.38a |
| (+)-(5) | 4.93 ± 2.09ab | 90.05 ± 4.09c | 94.98 ± 14.68b | nt | nt | nt |
| Compound | Number of Aphids ± SE a | |||||
|---|---|---|---|---|---|---|
| 1 h | 2 h | 24 h | ||||
| C | T | C | T | C | T | |
| (1) | 7.9 ± 0.8 | 6.0 ± 0.9 | 7.8 ± 0.9 | 8.0 ± 0.8 | 7.9 ± 0.8 | 7.5 ± 0.6 |
| (2) | 8.1 ± 1.1 | 7.0 ± 1.0 | 7.6 ± 1.1 | 6.1 ± 1.3 | 8.4 ± 1.3 | 6.5 ± 1.1 |
| (−)-(4) | 5.8 ± 0.7 | 7.5 ± 0.5 | 6.4 ± 1.0 | 7.5 ± 0.7 | 7.8 ± 0.7 | 7.6 ± 0.6 |
| (3) | 5.5 ± 1.0 | 10.5 ± 0.9 * | 6.4 ± 0.9 | 10.6 ± 0.6 * | 7.0 ± 0.8 | 11.0 ± 0.7 * |
| Compounds | |||||
|---|---|---|---|---|---|
| Control | (1) | (2) | (−)-(4) | (3) | |
| Time to 1st probe (s) | 25.6 (±8.5) | 50.1 (±18.8) | 21.3 (±7.1) | 13.6 (±4.3) | 67.9 (±37.0) |
| Total time on leaf (s) | 900.0 (±0.0) | 644.1 (±118.8) * | 900.0 (±0.0) | 900.0 (±0.0) | 757.7 (±77.2) * |
| Total time out of the leaf (s) | 0.0 (±0.0) | 255.9 (±118.8) * | 0.0 (±0.0) | 0.0 (±0.0) | 142.3 (±77.2) * |
| Total probing time (s) | 735.3 (±47.4) | 443.3 (±110.0) * | 789.2 (±32.9) | 748.7 (±29.2) | 626.6 (±122.4) |
| Number of probes | 4.3 (±0.9) | 2.8 (±0.8) | 2.6 (±0.5) | 3.7 (±0.4) | 1.6 (±0.3) * |
| Mean probing time (s) | 255.2 (±53.7) | 236.7 (±91.7) | 464.6 (±108.7) | 268.2 (±75.7) | 398.6 (±104.2) |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gliszczyńska, A.; Semba, D.; Szczepanik, M.; Dancewicz, K.; Gabryś, B. Alkyl-Substituted δ-Lactones Derived from Dihydrojasmone and Their Stereoselective Fungi-Mediated Conversion: Production of New Antifeedant Agents. Molecules 2016, 21, 1226. https://doi.org/10.3390/molecules21091226
Gliszczyńska A, Semba D, Szczepanik M, Dancewicz K, Gabryś B. Alkyl-Substituted δ-Lactones Derived from Dihydrojasmone and Their Stereoselective Fungi-Mediated Conversion: Production of New Antifeedant Agents. Molecules. 2016; 21(9):1226. https://doi.org/10.3390/molecules21091226
Chicago/Turabian StyleGliszczyńska, Anna, Damian Semba, Maryla Szczepanik, Katarzyna Dancewicz, and Beata Gabryś. 2016. "Alkyl-Substituted δ-Lactones Derived from Dihydrojasmone and Their Stereoselective Fungi-Mediated Conversion: Production of New Antifeedant Agents" Molecules 21, no. 9: 1226. https://doi.org/10.3390/molecules21091226