3.2.1. Preparation of Isoquinolinequinone-Amino Acid Derivatives. General Procedure
Suspensions of isoquinolinequinone 1 (1 equiv.), l- or d-α-amino acid methyl esters hydrochloride (2 equiv.) and NaOAc (2 equiv.) in ethanol (15 mL) were left with stirring at room temperature (rt) after completion of the reaction as indicated by TLC. The solvent was removed under reduced pressure and the residue was purified by chromatography over silica gel (90:10 CH2Cl2/EtOAc) to yield mixtures of regioisomers, in ratios determined by 1H-NMR in CDCl3. Further column chromatography of the mixture of isomers over silica gel (CH2Cl2), provided pure samples of the regioisomers.
Methyl 6- and 7-(1-methoxy-1-oxopropan-2-ylamino)-3-methyl-5,8-dioxo-5,8-dihydroisoquinoline-4-carboxylate (2a, 2b). The mixture of regioisomers was prepared from 1 (200 mg, 0.87 mmol) and l-alanine methyl ester hydrochloride (2 h). Compound 2a (less polar, 104 mg, 0.31 mmol, 53%): orange solid, mp: 141–143 °C; IR νmax: 3370 and 3324 (N-H), 2954 and 2930 (C-H), 1745 (C=O ester); 1687 (C=O quinone); 1H-NMR (400 MHz, CDCl3): δ 1.57 (d, 3H, J = 6.8 Hz, 12-Me), 2.65 (s, 3H, 3-Me), 3.81 (s, 3H, 12-CO2Me), 4.03 (s, 3H, 4-CO2Me), 4.13 (m, 1H, 12-H), 5.70 (s, 1H, 6-H), 6.46 (d, 1H, J = 7.2 Hz, NH), 9.19 (s, 1H, 1-H); 13C-NMR (100 MHz, CDCl3): δ 17.6 (CH3, 12-CH3), 23.1 (CH3, C-9), 50.7 (CH, C-12), 53.1 (CH3, C-10), 53.2 (CH3, C-14), 102.4 (CH, C-6), 122.0 (C, C-8a), 126.2 (C, C-4), 135.8 (C, C-4a), 146.4 (C, C-7), 148.3 (CH, C-1), 163.0 (C, C-3), 168.8 (C, C-11), 171.7 (C, C-13), 180.1 (C, C-5), 180.7 (C, C-8); HRMS (M+): m/z calcd. for C16H16N2O6: 332.10084; found: 332.10707. Attempts to isolate a pure sample of 2b were unsuccessful.
Methyl 6- and 7-(1-methoxy-3-methyl-1-oxobutan-2-ylamino)-3-methyl-5,8-dioxo-5,8-dihydro-isoquinoline-4-carboxylate (3a, 3b). The mixture of regioisomers was prepared from 1 (200 mg, 0.87 mmol) and l-valine methyl ester hydrochloride (2 h). Compound 3a (less polar, 128 mg, 0.36 mmol, 58%): orange solid, mp: 91.5–93 °C; IR νmax: 3368 (N-H), 2970 and 2930 (C-H), 1734 (C=O ester); 1686 (C=O quinone); 1H-NMR (400 MHz, CDCl3): δ 1.02 (d, 3H, J = 6.8 Hz, 12-C-Me), 1.08 (d, 3H, J = 6.8 Hz, 12-C-Me), 2.31 (m, 1H, 12-C-CH), 2.65 (s, 3H, 3-Me), 3.80 (s, 3H, 12-CO2Me), 3.92 (m, 1H, 12-H), 4.03 (s, 3H, 4-CO2Me), 5.73 (s, 1H, 6-H), 6.48 (d, 1H, J = 8.0 Hz, NH), 9.19 (s, 1H, 1-H); 13C-NMR (100 MHz, CDCl3): δ 18.4 (CH3, 12-CHCH3), 18.8 (CH3, 12-CHCH3), 22.9 (CH3, C-9), 31.2 (CH, 12-CH), 52.6 (CH3, C-14), 53.1 (CH3, C-10), 60.5 (CH, C-12), 102.2 (CH, C-6), 121.8 (C, C-8a), 126.0 (C, C-4), 135.6 (C, C-4a), 147.0 (C, C-7), 148.1 (CH, C-1), 162.8 (C, C-3), 168.6 (C, C-11), 170.5 (C, C-13), 180.0 (C, C-5), 180.5 (C, C-8); HRMS (M+): m/z calcd. for C18H20N2O6: 360.13214; found: 360.13813.
Compound 3b (more polar, 15 mg, 0.04 mmol, 7%): orange solid, mp: 81.5–83 °C; IR νmax: 3375 (N-H), 2959 and 2931 (C-H), 1739 (C=O ester); 1686 (C=O quinone); 1H-NMR (400 MHz, CDCl3): δ 1.01 (d, 3H, J = 6.8 Hz, 12-C-Me), 1.06 (d, 3H, J = 6.8 Hz, 12-C-Me), 2.29 (m, 1H, 12-C-CH), 2.66 (s, 3H, 3-Me), 3.80 (s, 3H, 12-CO2Me), 3.89 (m, 1H, 12-H), 4.06 (s, 3H, 4-CO2Me), 5.72 (s, 1H, 7-H), 6.22 (d, 1H, J = 8.4 Hz, NH), 9.28 (s, 1H, 1-H); 13C-NMR (100 MHz, CDCl3): δ 18.5 (CH3, 12-CHCH3), 18.9 (CH3, 12-CHCH3), 22.7 (CH3, C-9), 31.3 (CH, 12-CH), 52.7 (CH3, C-14), 53.4 (CH3, C-10), 60.6 (CH, C-12), 102.3 (CH, C-7), 122.7 (C, C-8a), 125.1 (C, C-4), 132.3 (C, C-4a), 146.9 (C, C-6), 148.8 (CH, C-1), 160.3 (C, C-3), 168.4 (C, C-11), 170.7 (C, C-13), 181.0 (C, C-5), 181.8 (C, C-8); HRMS (M+): m/z calcd. for C18H20N2O6: 360.13214; found: 360.13788.
Methyl 6- and 7-(1-methoxy-4-methyl-1-oxopentan-2-ylamino)-3-methyl-5,8-dioxo-5,8-dihydroisoquinoline-4-carboxylate (4a, 4b). The mixture of regioisomers was prepared from 1 (200 mg, 0.87 mmol) and l-leucine methyl ester hydrochloride (2 h). Compound 4a (less polar, 132 mg, 0.35 mmol, 56%): orange solid, mp: 90.5–92.5 °C; IR νmax: 3371 and 3326 (N-H), 2956 and 2929 (C-H), 1749 (C=O ester); 1693 (C=O quinone); 1H-NMR (400 MHz, CDCl3): δ 0.85 (d, 3H, J = 6.4 Hz, 12-CH2-C-Me), 0.91 (d, 3H, J = 6.4 Hz, 12-CH2-C-Me), 1.68 (m, 1H, 12-CH2-CH), 1.72 (m, 2H, 12-CH2), 2.57 (s, 3H, 3-Me), 3.70 (s, 3H, 12-CO2Me), 3.95 (s, 4H, 4-CO2Me and 12-H), 5.64 (s, 1H, 6-H), 6.26 (d, 1H, J = 8.0 Hz, NH), 9.10 (s, 1H, 1-H); 13C-NMR (100 MHz, CDCl3): δ 22.0 (CH3, 2-CHCH3), 22.5 (CH3, 12-CHCH3), 23.0 (CH3, C-9), 25.0 (CH, 12-CH2CH), 40.7 (CH, 12-CH), 52.8 (CH3, C-14), 53.1 (CH3, C-10), 53.6 (CH, C-12), 102.2 (CH, C-6), 121.8 (C, C-8a), 126.0 (C, C-4), 135.6 (C, C-4a), 146.8 (C, C-7), 148.1 (CH, C-1), 162.9 (C, C-3), 168.6 (C, C-11), 171.5 (C, C-13), 180.0 (C, C-5), 180.6 (C, C-8); HRMS (M+): m/z calcd. for C19H22N2O6: 374.14779; found: 374.15351.
Compound 4b (more polar, 17 mg, 0.04 mmol, 7%): orange solid, mp: 58.5–60.5 °C; IR νmax: 3367 (N-H), 2956 and 2928 (C-H), 1739 (C=O ester); 1687 (C=O quinone); 1H-NMR (400 MHz, CDCl3): δ 0.93 (d, 3H, J = 6.0 Hz, 12-CH2-C-Me), 0.99 (d, 3H, J = 6.0 Hz, 12-CH2-C-Me), 1.76 (m, 3H, 12-CH2 and 12-CH2-CH), 2.66 (s, 3H, 3-Me), 3.78 (s, 3H, 12-CO2Me), 4.03 (s, 4H, 4-CO2Me and 12-H), 5.72 (s, 1H, 7-H), 6.01 (d, 1H, J = 8.0 Hz, NH), 9.28 (s, 1H, 1-H); 13C-NMR (100 MHz, CDCl3): δ 22.1 (CH3, C-9), 22.7 (2CH3, 12-CHCH3), 25.0 (CH, 12-CH2CH), 40.9 (CH, 12-CH), 53.0 (CH3, C-14), 53.4 (CH3, C-10), 53.7 (CH, C-12), 102.4 (CH, C-7), 122.7 (C, C-8a), 125.0 (C, C-4), 132.3 (C, C-4a), 146.6 (C, C-6), 148.8 (CH, C-1), 160.4 (C, C-3), 168.4 (C, C-11), 171.7 (C, C-13), 181.0 (C, C-5), 181.9 (C, C-8); HRMS (M+): m/z calcd. for C19H22N2O6: 374.14779; found: 374.15335.
Methyl 6- and 7-(1-methoxy-4-(methylthio)-1-oxobutan-2-ylamino)-3-methyl-5,8-dioxo-5,8-dihydroisoquinoline-4-carboxylate (5a, 5b). The mixture of regioisomers was prepared from 1 (200 mg, 0.87 mmol) and l-methionine methyl ester hydrochloride (1 h 30 min). Compound 5a (less polar, 125 mg, 0.32 mmol, 52%): orange solid, mp: 66.5–68 °C; IR νmax: 3353 (N-H), 2951 and 2918 (C-H), 1738 (C=O ester); 1681 (C=O quinone); 1H-NMR (400 MHz, CDCl3): δ 2.11 (s, 3H, Me-S), 2.23 (m, 2H, S-CH2), 2.60 (m, 2H, 12-CH2), 2.66 (s, 3H, 3-Me), 3.82 (s, 3H, 12-CO2Me), 4.03 (s, 3H, 4-CO2Me), 4.33 (m, 1H, 12-H), 5.830 (s, 1H, 6-H), 6.59 (d, 1H, J = 8.4 Hz, NH), 9.19 (s, 1H, 1-H); 13C-NMR (100 MHz, CDCl3): δ 15.5 (CH3, S-CH3), 22.9 (CH3, C-9), 29.8 (CH2, 12-CH2), 30.6 (CH2, S-CH2), 53.0 (CH3, C-14), 53.1 (CH3, C-10), 53.6 (CH, C-12), 102.4 (CH, C-6), 121.8 (C, C-8a), 126.0 (C, C-4), 135.5 (C, C-4a), 146.7 (C, C-7), 148.0 (CH, C-1), 162.8 (C, C-3), 168.5 (C, C-11), 170.8 (C, C-13), 180.0 (C, C-5), 180.5 (C, C-8); HRMS (M+): m/z calcd. for C18H20N2O6S: 392.10421; found: 392.10987. Attempts to isolate a pure sample of 5b were unsuccessful.
Methyl 6- and 7-(1-methoxy-1-oxo-3-phenylpropan-2-ylamino)-3-methyl-5,8-dioxo-5,8-dihydroisoquinoline-4-carboxylate (6a, 6b). The mixture of regioisomers was prepared from 1 (200 mg, 0.87 mmol) and l-phenylalanine methyl ester hydrochloride (2 h 30 min).Compound 6a (less polar, 149 mg, 0.37 mmol, 60%): orange solid, mp: 118–119.5 °C; IR νmax: 3353 and 3287 (N-H), 2947 and 2927 (C-H), 1735 (C=O ester); 1685 (C=O quinone); 1H-NMR (400 MHz, CDCl3): δ 2.64 (s, 3H, 3-Me), 3.17 (dd, 1H, J = 6.4; 14 Hz, 12-CH2), 3.26 (dd, 1H, J = 5.6; 14 Hz, 12-CH2), 3.76 (s, 3H, 12-CO2Me), 4.02 (s, 3H, 4-CO2Me), 4.31 (m, 1H, 12-H), 5.68 (s, 1H, 6-H), 6.42 (d, 1H, J = 7.6 Hz, NH), 7.11 (m, 2H, arom), 7.28 (m, 3H, arom), 9.16 (s, 1H, 1-H); 13C-NMR (100 MHz, CDCl3): δ 23.0 (CH3, C-9), 37.5 (CH2, 12-CH2), 52.9 (CH3, C-14), 53.2 (CH3, C-10), 56.1 (CH, C-12), 102.5 (CH, C-6), 121.8 (C, C-8a), 126.1 (C, C-4), 127.8 (C, C-4’), 129.0 (2CH, C-2’ and C-6’), 129.1 (2CH, C-3’ and C-5’), 134.8 (C, 12-CH2, C-1’), 135.6 (C, C-4a), 146.4 (C, C-7), 148.2 (CH, C-1), 163.0 (C, C-3), 168.7 (C, C-11), 170.3 (C, C-13), 179.9 (C, C-8), 180.6 (C, C-5); HRMS (M+): m/z calcd. for C22H20N2O6: 408.13214; found: 408.13756.
Compound 6b (more polar, 17 mg, 0.04 mmol, 7%): orange solid, mp: 72–73.5 °C; IR νmax: 3350 and 3284 (N-H), 2946 and 2929 (C-H), 1735 (C=O ester); 1684 (C=O quinone); 1H-NMR (400 MHz, CDCl3): δ 2.65 (s, 3H, 3-Me), 3.14 (dd, 1H, J = 6.8; 14 Hz, 12-CH2), 3.25 (dd, 1H, J = 5.6; 14 Hz, 12-CH2), 3.76 (s, 3H, 12-CO2Me), 4.04 (s, 3H, 4-CO2Me), 4.31 (m, 1H, 12-H), 5.67 (s, 1H, 7-H), 6.18 (d, 1H, J = 7.6 Hz, NH), 7.13 (m, 2H, arom), 7.29 (m, 3H, arom), 9.26 (s, 1H, 1-H); 13C-NMR (100 MHz, CDCl3): δ 22.7 (CH3, C-9), 37.7 (CH2, 12-CH2), 53.0 (CH3, C-14), 53.4 (CH3, C-10), 56.3 (CH, C-12), 102.6 (CH, C-7), 122.7 (C, C-8a), 125.1 (C, C-4), 127.8 (C, C-4’), 129.1 (2CH, C-2’ and C-6’), 129.2 (2CH, C-3’ and C-5’), 132.3 (C, C-4a), 134.8 (C, 12-CH2, C-1’), 146.3 (C, C-6), 148.8 (CH, C-1), 160.4 (C, C-3), 168.4 (C, C-11), 170.5 (C, C-13), 180.8 (C, C-5), 181.8 (C, C-8); HRMS (M+): m/z calcd. for C22H20N2O6: 408.13214; found: 408.13762.
Methyl 6- and 7-(3-(4-hydroxyphenyl)-1-methoxy-1-oxopropan-2-ylamino)-3-methyl-5,8-dioxo-5,8-dihydroisoquinoline-4-carboxylate (7a, 7b). The mixture of regioisomers was prepared from 1 (200 mg, 0.87 mmol) and l-tyrosine methyl ester hydrochloride (1 h 40 min). Compound 7a (less polar, 141 mg, 0.33 mmol, 56%): orange solid, mp: 93–94.5 °C; IR νmax: 3358 (N-H), 2953 and 2851 (C-H), 1738 (C=O ester); 1683 (C=O quinone); 1H-NMR (400 MHz, CDCl3): δ 2.63 (s, 3H, 3-Me), 3.06 (dd, 1H, J = 6.4; 14 Hz, 12-CH2), 3.14 (dd, 1H, J = 5.6; 14 Hz, 12-CH2), 3.75 (s, 3H, 12-CO2Me), 4.01 (s, 3H, 4-CO2Me), 4.26 (m, 1H, 12-H), 5.68 (s, 1H, 6-H), 6.49 (d, 1H, J = 8.0 Hz, NH), 6.71 (d, 2H, J = 8.4 Hz, arom), 6.91 (d, 2H, J = 8.4 Hz, arom), 7.75 (br s, 1H, OH), 9.12 (s, 1H, 1-H); 13C-NMR (100 MHz, CDCl3): δ 22.7 (CH3, C-9), 36.4 (CH2, 12-CH2), 52.9 (CH3, C-14), 53.3 (CH3, C-10), 56.2 (CH, C-12), 102.1 (CH, C-6), 116.0 (2CH, C-3’ and C-5’), 122.0 (C, C-8a), 125.9 (C, C-4), 126.2 (C, 12-CH2, C-1’), 130.2 (2CH, C-2’ and C-6’), 135.8 (C, C-4a), 146.6 (C, C-7), 147.9 (CH, C-1), 155.9 (CH, C4’-OH), 162.7 (C, C-3), 168.7 (C, C-11), 170.5 (C, C-13), 179.5 (C, C-8), 180.6 (C, C-5); HRMS (M+): m/z calcd. for C22H20N2O7: 424.12705; found: 424.13256.
Compound 7b (more polar, 13 mg, 0.03 mmol, 5%): orange solid, mp: 76.5–78.5 °C; IR νmax: 3360 (N-H), 2953 and 2848 (C-H), 1739 (C=O ester); 1685 (C=O quinone); 1H-NMR (400 MHz, CDCl3): δ 2.65 (s, 3H, 3-Me), 3.09 (dd, 1H, J = 6.4; 14 Hz, 12-CH2), 3.16 (dd, 1H, J = 5.6; 14 Hz, 12-CH2), 3.76 (s, 3H, 12-CO2Me), 4.04 (s, 3H, 4-CO2Me), 4.25 (m, 1H, 12-H), 5.23 (br s, 1H, OH), 5.67 (s, 1H, 7-H), 6.19 (d, 1H, J = 7.6 Hz, NH), 6.76 (d, 2H, J = 8.4 Hz, arom), 6.97 (d, 2H, J = 8.4 Hz, arom), 9.27 (s, 1H, 1-H); 13C-NMR (100 MHz, CDCl3): δ 22.6 (CH3, C-9), 36.7 (CH2, 12-CH2), 52.9 (CH3, C-14), 53.35 (CH3, C-10), 56.2 (CH, C-12), 102.3 (CH, C-7), 115.9 (2CH, C-3’ and C-5’), 122.6 (C, C-8a), 125.3 (C, C-4), 126.3 (C, 12-CH2, C-1’), 130.3 (2CH, C-2’ and C-6’), 134.9 (C, C-4a), 146.6 (C, C-6), 147.4 (CH, C-1), 155.9 (CH, C4’-OH), 162.8 (C, C-3), 168.6 (C, C-11), 170.5 (C, C-13), 179.5 (C, C-8), 179.6 (C, C-5); HRMS (M+): m/z calcd. for C22H20N2O7: 424.12705; found: 424.13239.
Methyl 6- and 7-(3-(1H-indol-3-yl)-1-methoxy-1-oxopropan-2-ylamino)-3-methyl-5,8-dioxo-5,8-dihydroisoquinoline-4-carboxylate (8a, 8b). The mixture of regioisomers was prepared from 1 (200 mg, 0.87 mmol) and l-tryptophan methyl ester hydrochloride (2 h). Compound 8a (less polar, 214 mg, 0.48 mmol, 67%): orange solid, mp: 98.5–100 °C; IR νmax: 3360 (N-H), 2952 and 2927 (C-H), 1737 (C=O ester); 1681 (C=O quinone); 1H-NMR (400 MHz, CDCl3): δ 2.63 (s, 3H, 3-Me), 3.35 (dd, 1H, J = 6.4; 15 Hz, 12-CH2), 3.40 (dd, 1H, J = 5.2; 15 Hz, 12-CH2), 3.70 (s, 3H, 12-CO2Me), 4.01 (s, 3H, 4-CO2Me), 4.33 (m, 1H, 12-CH), 5.64 (s, 1H, 6-H), 6.47 (d, 1H, J = 7.6 Hz, NH), 6.98 (m, 1H, CH), 7.07 (m, 1H, arom), 7.13 (m, 1H, arom), 7.28 (d, 1H, J = 8.0 Hz, arom), 7.45 (d, 1H, J = 8.0 Hz, arom), 8.45 (br s, 1H, NH), 9.10 (s, 1H, 1-H); 13C-NMR (100 MHz, CDCl3): δ 23.0 (CH3, C-9), 27.5 (CH2, 12-CH2), 53.0 (CH3, C-14), 53.2 (CH3, C-10), 55.5 (CH, C-12), 102.2 (CH, C-6), 108.8 (C, 12-CH2C), 111.6 (CH, HN-CCH), 118.2 (CH, 12-CH2CCCH), 120.0 (CH, HN-CCHCH), 121.8 (C, C-8a), 122.6 (CH, CH2CCCHCH), 123.2 (CH, HNCH), 126.0 (C, C-4), 127.0 (C, 12-CH2CC), 135.7 (C, C-4a), 136.2 (C, HNC), 146.7 (C, C-7), 148.1 (CH, C-1), 162.8 (C, C-3), 168.8 (C, C-11), 170.8 (C, C-13), 179.8 (C, C-8), 180.6 (C, C-5); HRMS (M+): m/z calcd. for C24H21N3O6: 447.14304; found: 447.14845.
Compound 8b (more polar, 29 mg, 0.06 mmol, 9%): orange solid, mp: 89–91 °C; IR νmax: 3370 (N-H), 2952 and 2925 (C-H), 1737 (C=O ester); 1686 (C=O quinone); 1H-NMR (400 MHz, CDCl3): δ 2.64 (s, 3H, 3-Me), 3.36 (dd, 1H, J = 6.4; 15 Hz, 12-CH2), 3.43 (dd, 1H, J = 5.2; 15 Hz, 12-CH2), 3.71 (s, 3H, 12-CO2Me), 4.01 (s, 3H, 4-CO2Me), 4.36 (m, 1H, 12-H), 5.66 (s, 1H, 7-H), 6.26 (d, 1H, J = 8.0 Hz, NH), 7.02 (m, 1H, CH), 7.12 (m, 1H, arom), 7.19 (m, 1H, arom), 7.35 (d, 1H, J = 8.0 Hz, arom), 7.50 (d, 1H, J = 8.0 Hz, arom), 8.23 (br s, 1H, NH), 9.24 (s, 1H, 1-H); 13C-NMR (100 MHz, CDCl3): δ 22.7 (CH3, C-9), 27.7 (CH2, 12-CH2), 53.0 (CH3, C-14), 53.4 (CH3, C-10), 55.5 (CH, C-12), 102.4 (CH, C-7), 109.1 (C, 12-CH2C), 111.6 (CH, HN-CCH), 118.4 (CH, 12-CH2CCCH), 120.1 (CH, HN-CCHCH), 122.7 (C and CH, C-8a y CH2CCCHCH), 123.1 (CH, HNCH), 125.0 (C, C-4), 127.1 (C, 12-CH2CC), 132.3 (C, C-4a), 136.3 (C, HNC), 146.4 (C, C-6), 148.7 (CH, C-1), 160.3 (C, C-3), 168.4 (C, C-11), 170.9 (C, C-13), 180.8 (C, C-5), 181.8 (C, C-8); HRMS (M+): m/z calcd. for C24H21N3O6: 447.14304; found: 447.14818.
Methyl 6- and 7-(1-methoxy-1-oxopropan-2-ylamino)-3-methyl-5,8-dioxo-5,8-dihydroisoquinoline-4-carboxylate (9a, 9b). The mixture of regioisomers was prepared from 1 (200 mg, 0.87 mmol) and d-alanine methyl ester hydrochloride (2 h). Compound 9a (less polar, 104 mg, 0.31 mmol, 50%): orange solid, mp: 140.5–142 °C; IR νmax 3370 and 3324 (N-H), 2954 and 2930 (C-H), 1745 (C=O ester); 1687 (C=O quinone); 1H-NMR (400 MHz, CDCl3): δ 1.57 (d, 3H, J = 6.8 Hz, Me), 2.65 (s, 3H, 3-Me), 3.81 (s, 3H, 12-CO2Me), 4.03 (s, 3H, 4-CO2Me), 4.13 (m, 1H, 12-H), 5.70 (s, 1H, 6-H), 6.46 (d, 1H, J = 7.2 Hz, NH), 9.19 (s, 1H, 1-H); 13C-NMR (100 MHz, CDCl3): δ 17.6 (CH3, 12-CH3), 23.1 (CH3, C-9), 50.7 (CH, C-12), 53.1 (CH3, C-10), 53.2 (CH3, C-14), 102.4 (CH, C-6), 122.0 (C, C-8a), 126.2 (C, C-4), 135.8 (C, C-4a), 146.4 (C, C-7), 148.3 (CH, C-1), 163.0 (C, C-3), 168.8 (C, C-11), 171.7 (C, C-13), 180.1 (C, C-5), 180.7 (C, C-8); HRMS (M+): m/z calcd. for C16H16N2O6: 332.10084; found: 332.10193. Attempts to isolate a pure sample of 9b were unsuccessful.
Methyl 6- and 7-(1-methoxy-1-oxo-3-phenylpropan-2-ylamino)-3-methyl-5,8-dioxo-5,8-dihydroisoquinoline-4-carboxylate (10a, 10b). The mixture of regioisomers was prepared from 1 (200 mg, 0.87 mmol) and d-phenylalanine methyl ester hydrochloride (2 h 30 min).Compound 10a (less polar, 139 mg, 0.34 mmol, 58%): orange solid, mp: 118.5–120 °C; IR νmax: 3353 and 3287 (N-H), 2947 and 2927 (C-H), 1735 (C=O ester); 1685 (C=O quinone); 1H-NMR (400 MHz, CDCl3): δ 2.64 (s, 3H, 3-Me), 3.17 (dd, 1H, J = 6.4; 14 Hz, 12-CH2), 3.26 (dd, 1H, J = 5.6; 14 Hz, 12-CH2), 3.76 (s, 3H, 12-CO2Me), 4.02 (s, 3H, 4-CO2Me), 4.31 (m, 1H, 12-H), 5.68 (s, 1H, 6-H), 6.42 (d, 1H, J = 7.6 Hz, NH), 7.11 (m, 2H, arom), 7.28 (m, 3H, arom), 9.16 (s, 1H, 1-H); 13C-NMR (100 MHz, CDCl3): δ 23.0 (CH3, C-9), 37.5 (CH2, 12-CH2), 52.9 (CH3, C-14), 53.2 (CH3, C-10), 56.1 (CH, C-12), 102.5 (CH, C-6), 121.8 (C, C-8a), 126.1 (C, C-4), 127.8 (C, C-4’), 129.0 (2CH, C2’ and C6’), 129.1 (2CH, C-3’ and C-5’), 134.8 (C, 12-CH2C1’), 135.6 (C, C-4a), 146.4 (C, C-7), 148.2 (CH, C-1), 163.0 (C, C-3), 168.7 (C, C-11), 170.3 (C, C-13), 179.9 (C, C-8), 180.6 (C, C-5); HRMS (M+): m/z calcd. for C22H20N2O6: 408.13214; found: 408.13759.
Compound 10b (more polar, 12 mg, 0.03 mmol, 5%): orange solid, mp: 71.5–73 °C; IR νmax: 3350 and 3284 (N-H), 2946 and 2929 (C-H), 1735 (C=O ester); 1684 (C=O quinone); 1H-NMR (400 MHz, CDCl3): δ 2.65 (s, 3H, 3-Me), 3.14 (dd, 1H, J = 6.8; 14 Hz, 12-CH2), 3.25 (dd, 1H, J = 5.6; 14 Hz, 12-CH2), 3.76 (s, 3H, 12-CO2Me), 4.04 (s, 3H, 4-CO2Me), 4.31 (m, 1H, 12-H), 5.67 (s, 1H, 7-H), 6.18 (d, 1H, J = 7.6 Hz, NH), 7.13 (m, 2H, arom), 7.29 (m, 3H, arom), 9.26 (s, 1H, 1-H); 13C-NMR (100 MHz, CDCl3): δ 22.7 (CH3, C-9), 37.7 (CH2, 12-CH2), 53.0 (CH3, C-14), 53.4 (CH3, C-10), 56.3 (CH, C-12), 102.6 (CH, C-7), 122.7 (C, C-8a), 125.1 (C, C-4), 127.8 (C, C-4’), 129.1 (2CH, C-2’ and C-6’), 129.2 (2CH, C-3’ and C-5’), 132.3 (C, C-4a), 134.8 (C, 12-CH2C1’), 146.3 (C, C-6), 148.8 (CH, C-1), 160.4 (C, C-3), 168.4 (C, C-11), 170.5 (C, C-13), 180.8 (C, C-5), 181.8 (C, C-8); HRMS (M+): m/z calcd. for C22H20N2O6: 408.13214; found: 408.13757.
Methyl 6- and 7-(3-(1H-indol-3-yl)-1-methoxy-1-oxopropan-2-ylamino)-3-methyl-5,8-dioxo-5,8-dihydroisoquinoline-4-carboxylate (11a, 11b). The mixture of regioisomers was prepared from 1 (200 mg, 0.87 mmol) and d-tryptophan methyl ester hydrochloride (2 h). Compound 11a (less polar, 205 mg, 0.46 mmol, 66%): orange solid, mp: 99–100.5 °C; IR νmax: 3360 (N-H), 2952 and 2927 (C-H), 1737 (C=O ester); 1681 (C=O quinone); 1H-NMR (400 MHz, CDCl3): δ 2.63 (s, 3H, 3-Me), 3.35 (dd, 1H, J = 6.4; 15 Hz, 12-CH2), 3.40 (dd, 1H, J = 5.2; 15 Hz, 12-CH2), 3.70 (s, 3H, 12-Me), 4.01 (s, 3H, 4-CO2Me), 4.33 (m, 1H, 12-H), 5.64 (s, 1H, 6-H), 6.47 (d, 1H, J = 7.6 Hz, NH), 6.98 (m, 1H, CH), 7.07 (m, 1H, arom), 7.13 (m, 1H, arom), 7.28 (d, 1H, J = 8.0 Hz, arom), 7.45 (d, 1H, J = 8.0 Hz, arom), 8.45 (br s, 1H, NH), 9.10 (s, 1H, 1-H); 13C-NMR (100 MHz, CDCl3): δ 23.0 (CH3, C-9), 27.5 (CH2, 12-CH2), 53.0 (CH3, C-14), 53.2 (CH3, C-10), 55.5 (CH, C-12), 102.2 (CH, C-6), 108.8 (C, 12-CH2C), 111.6 (CH, HN-CCH), 118.2 (CH, 12-CH2CCCH), 120.0 (CH, HN-CCHCH), 121.8 (C, C-8a), 122.6 (CH, CH2CCCHCH), 123.2 (CH, HNCH), 126.0 (C, C-4), 127.0 (C, 12-CH2CC), 135.7 (C, C-4a), 136.2 (C, HNC), 146.7 (C, C-7), 148.1 (CH, C-1), 162.8 (C, C-3), 168.8 (C, C-11), 170.8 (C, C-13), 179.8 (C, C-8), 180.6 (C, C-5); HRMS (M+): m/z calcd. for C24H21N3O6: 447.14304; found: 447.14867. Attempts to isolate a pure sample of 11b were unsuccessful.
3.2.2. Synthesis of Bromoisoquinolinequinones 12, 13a,b, 14, 15, 16. General Procedure
A solution of the required aminoisoquinolinequinone (1 equiv.), N-bromosuccinimide (NBS) (1.1 equiv.) and methanol (15 mL) was left with stirring at rt after completion of the reaction as indicated by TLC. The solvent was removed under reduced pressure and the residue was column chromatographed over silica gel (CH2Cl2/EtOAc 90:10) to yield the corresponding bromoisoquinolinequinones.
Methyl 6-bromo-7-(1-methoxy-1-oxopropan-2-ylamino)-3-methyl-5,8-dioxo-5,8-dihydroisoquinoline-4-carboxylate (12). Prepared from 2a (70 mg, 0.21 mmol) and NBS (20 min, 65 mg, 0.16 mmol, 76% yield): orange solid, mp: 120.5–122 °C; IR νmax: 3370 and 3320 (N-H), 2954 and 2924 (C-H), 1738 (C=O ester); 1685 (C=O quinone); 1H-NMR (400 MHz, CDCl3): δ 1.62 (d, 3H, J = 6.8 Hz, 12-Me), 2.65 (s, 3H, 3-Me), 3.83 (s, 3H, 12-CO2Me), 4.05 (s, 3H, 4-CO2Me), 4.13 (m, 1H, 12-H), 6.55 (bs, 1H, NH), 9.17 (s, 1H, 1-H); 13C-NMR (100 MHz, CDCl3): δ 20.2 (CH3, 12-CH3), 21.1 (CH3, C-9), 52.6 (CH, C-12), 53.1 (CH3, C-10), 53.3 (CH3, C-14), 121.3 (C, C-8a), 126.3 (C, C-4), 134.1 (C, C-4a), 148.7 (2C, C, C-7 and CH, C-1), 163.1 (C, C-3), 168.2 (C, C-11), 172.7 (C, C-13), 178.5 (2C, C-5 and C-8); HRMS (M+): m/z calcd. for C16H15BrN2O6: 410.01135; found: 410.01755.
Methyl 6-bromo-(1-methoxy-3-methyl-1-oxobutan-2-ylamino)-3-methyl-5,8-dioxo-5,8-dihydroisoquinoline-4-carboxylate (13a). Prepared from 3a (100 mg, 0.28 mmol) and NBS (20 min, 101 mg, 0.23 mmol, 82% yield): orange solid, mp: 105.2–107 °C; IR νmax: 3368 (N-H), 2965 and 2934 (C-H), 1725 (C=O ester); 1680 (C=O quinone); 1H-NMR (400 MHz, CDCl3): δ 1.02 (d, 3H, J = 6.8 Hz, 12-C-Me), 1.08 (d, 3H, J = 6.8 Hz, 12-C-Me), 2.33 (m, 1H, 12-CH), 2.65 (s, 3H, 3-Me), 4.03 (s, 3H, 12-CO2Me), 4.05 (s, 3H, 4-CO2Me), 4.13 (m, 1H, 12-H), 6.22 (bs, 1H, NH), 9.18 (s, 1H, 1-H); 13C-NMR (100 MHz, CDCl3): δ 17.9 (CH3, 12-CHCH3), 18.4 (CH3, 12-CHCH3), 23.1 (CH3, C-9), 32.5 (CH, 12-CH), 52.7 (CH3, C-14), 53.3 (CH3, C-10), 60.5 (CH, C-12), 121.4 (C, C-8a), 126.4 (C, C-4), 135.6 (C, C-4a), 148.7 (2C, C-7 and CH, C-1), 163.1 (C, C-3), 168.2 (C, C-11), 171.2 (C, C-13), 178.6 (2C, C-5 and C-8); HRMS (M+): m/z calcd. for C18H19N2O6Br: 438.04265; found: 438.04810.
Methyl 7-bromo-(1-methoxy-3-methyl-1-oxobutan-2-ylamino)-3-methyl-5,8-dioxo-5,8-dihydroisoquinoline-4-carboxylate (13b). Prepared from 3b (50 mg, 0.14 mmol) and NBS (30 min, 49 mg, 0.11 mmol, 78% yield): orange solid, mp: 105–106.6 °C; IR νmax: 3370 (N-H), 2962 and 2931 (C-H), 1743 (C=O ester); 1682 (C=O quinone); 1H-NMR (400 MHz, CDCl3): δ 1.01 (d, 3H, J = 6.8 Hz, 12-C-Me), 1.07 (d, 3H, J = 6.8 Hz, 12-C-Me), 2.29 (m, 1H, 12-C-CH), 2.65 (s, 3H, 3-Me), 3.80 (s, 3H, 12-CO2Me), 4.04 (m, 1H, 12-H), 4.05 (s, 3H, 4-CO2Me), 6.30 (bs, 1H, NH), 9.29 (s, 1H, 1-H); 13C-NMR (100 MHz, CDCl3): δ 17.8 (CH3, 12-CHCH3), 18.5 (CH3, 12-CHCH3), 22.8 (CH3, C-9), 32.5 (CH, 12-CH), 52.7 (CH3, C-14), 53.3 (CH3, C-10), 61.6 (CH, C-12), 121.8 (C, C-8a), 125.4 (2C, C-4 and C-4a), 146.9 (C, C-6), 149.0 (2C, CH, C-1 and C, C-3), 168.0 (C, C-11), 171.5 (C, C-13), 179.3 (2C, C-5 and C-8); HRMS (M+): m/z calcd. for C18H19N2O6Br: 438.04265; found: 438.04785.
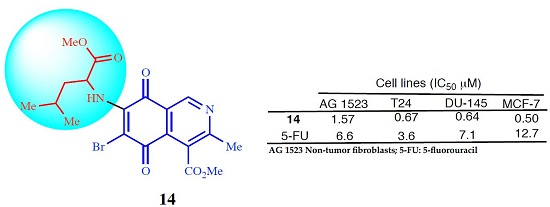
Methyl 6-bromo-(1-methoxy-4-methyl-1-oxopentan-2-ylamino)-3-methyl-5,8-dioxo-5,8-dihydroisoquinoline-4-carboxylate (14). Prepared from 4a (100 mg, 0.27 mmol) and NBS (30 min, 98 mg, 0.22 mmol, 80% yield): orange solid, mp: 104.3–106.2 °C; IR νmax: 3370 and 3326 (N-H), 2945 and 2914 (C-H), 1755 (C=O ester); 1710 (C=O quinone); 1H-NMR (400 MHz, CDCl3): δ 0.98 (d, 3H, J = 6.4 Hz, 12-CH2-C-Me), 1.01 (d, 3H, J = 6.4 Hz, 12-CH2-C-Me), 1.81 (m, 3H, 12-CH2-CH and 12-CH2), 2.65 (s, 3H, 3-Me), 3.79 (s, 3H, 12-CO2Me), 4.07 (s, 4H, 4-CO2Me and 12-H), 6.21 (bs, 1H, NH), 9.17 (s, 1H, 1-H); 13C-NMR (100 MHz, CDCl3): δ 22.5 (CH3, 2-CHCH3), 22.8 (CH3, 12-CHCH3), 23.0 (CH3, C-9), 25.1 (CH, 12-CH2CH), 42.6 (CH, 12-CH), 52.8 (CH3, C-14), 53.3 (CH3, C-10), 53.5 (CH, C-12), 121.3 (C, C-8a), 126.4 (C, C-4), 134.1 (C, C-4a), 148.7 (2C, C-7 and CH, C-1), 163.0 (C, C-3), 168.1 (C, C-11), 171.1 (C, C-13), 178.5 (2C, C-5 and C-8); HRMS (M+): m/z calcd. for C19H21N2O6Br: 452.05830; found: 452.06328.
Methyl 6-bromo-(1-methoxy-1-oxo-3-phenylpropan-2-ylamino)-3-methyl-5,8-dioxo-5,8-dihydroisoquinoline-4-carboxylate (15). Prepared from 6a (100 mg, 0.25 mmol) and NBS (40 min, 83 mg, 0.17 mmol, 68% yield): red solid, mp: 125.2–127.5 °C; IR νmax: 3350 and 3285 (N-H), 2944 and 2921 (C-H), 1746 (C=O ester); 1705 (C=O quinone); 1H-NMR (400 MHz, CDCl3): δ 2.63 (s, 3H, 3-Me), 3.25 (dd, 1H, J = 6.4; 14 Hz, 12-CH2), 3.27 (dd, 1H, J = 5.6; 14 Hz, 12-CH2), 3.79 (s, 3H, 12-CO2Me), 4.03 (s, 4H, 4-CO2Me and 12-H), 6.31 (bs, 1H, NH), 7.13 (m, 2H, arom), 7.28 (m, 3H, arom), 9.09 (s, 1H, 1-H); 13C-NMR (100 MHz, CDCl3): δ 22.9 (CH3, C-9), 39.9 (CH2, 12-CH2), 52.9 (CH3, C-14), 53.3 (CH3, C-10), 57.7 (CH, C-12), 121.5 (C, C-8a), 126.1 (C, C-4), 127.8 (C, C-4’), 129.1 (2CH, C-2’ and C-6’), 129.4 (2CH, C-3’ and C-5’), 134.6 (2C, 12-CH2C1’ and C, C-4a), 148.6 (2C, C-7 and CH, C-1), 163.0 (C, C-3), 168.2 (C, C-11), 171.3 (C, C-13), 178.5 (2C, C-5 and C-8); HRMS (M+): m/z calcd. for C22H19N2O6Br: 486.04265; found: 486.04732.
Methyl 6-bromo-(1-methoxy-1-oxo-3-phenylpropan-2-ylamino)-3-methyl-5,8-dioxo-5,8-dihydroisoquinoline-4-carboxylate (16). Prepared from 10a (80 mg, 0.19 mmol) and NBS (40 min, 63 mg, 0.13 mmol, 68% yield) red solid, mp: 126–127 °C; IR νmax: 3352 and 3285 (N-H), 2950 and 2927 (C-H), 1725 (C=O ester); 1715 (C=O quinone); 1H-NMR (400 MHz, CDCl3): δ 2.63 (s, 3H, 3-Me), 3.19 (dd, 1H, J = 6.4; 14 Hz, 12-CH2), 3.26 (dd, 1H, J = 5.6; 14 Hz, 12-CH2), 3.79 (s, 3H, 12-CO2Me), 4.05 (s, 4H, 4-CO2Me and 12-H), 6.29 (bs, 1H, NH), 7.13 (m, 2H, arom), 7.25 (m, 3H, arom), 9.08 (s, 1H, 1-H); 13C-NMR (100 MHz, CDCl3): 22.9 (CH3, C-9), 39.8 (CH2, 12-CH2), 52.9 (CH3, C-14), 53.2 (CH3, C-10), 57.7 (CH, C-12), 121.4 (C, C-8a), 126.1 (C, C-4), 127.7 (C, C-4’), 128.9 (2CH, C-2’ and C-6’), 129.4 (2CH, C-3’ and C-5’), 134.6 (2C, 12-CH2C1’ and C, C-4a), 148.5 (2C, C-7 and CH, C-1), 163.0 (C, C-3), 168.1 (C, C-11), 171.3 (C, C-13), 178.4 (2C, C-5 and C-8); HRMS (M+): m/z calcd. for C22H19N2O6Br: 486.04265; found: 486.04723.