Synthesis and Anti-HIV-1 Activity Evaluation for Novel 3a,6a-Dihydro-1H-pyrrolo[3,4-c]pyrazole-4,6-dione Derivatives
Abstract
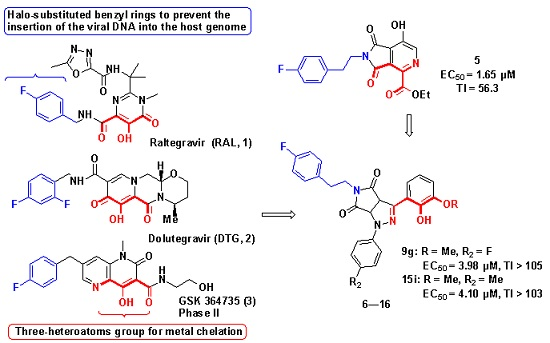
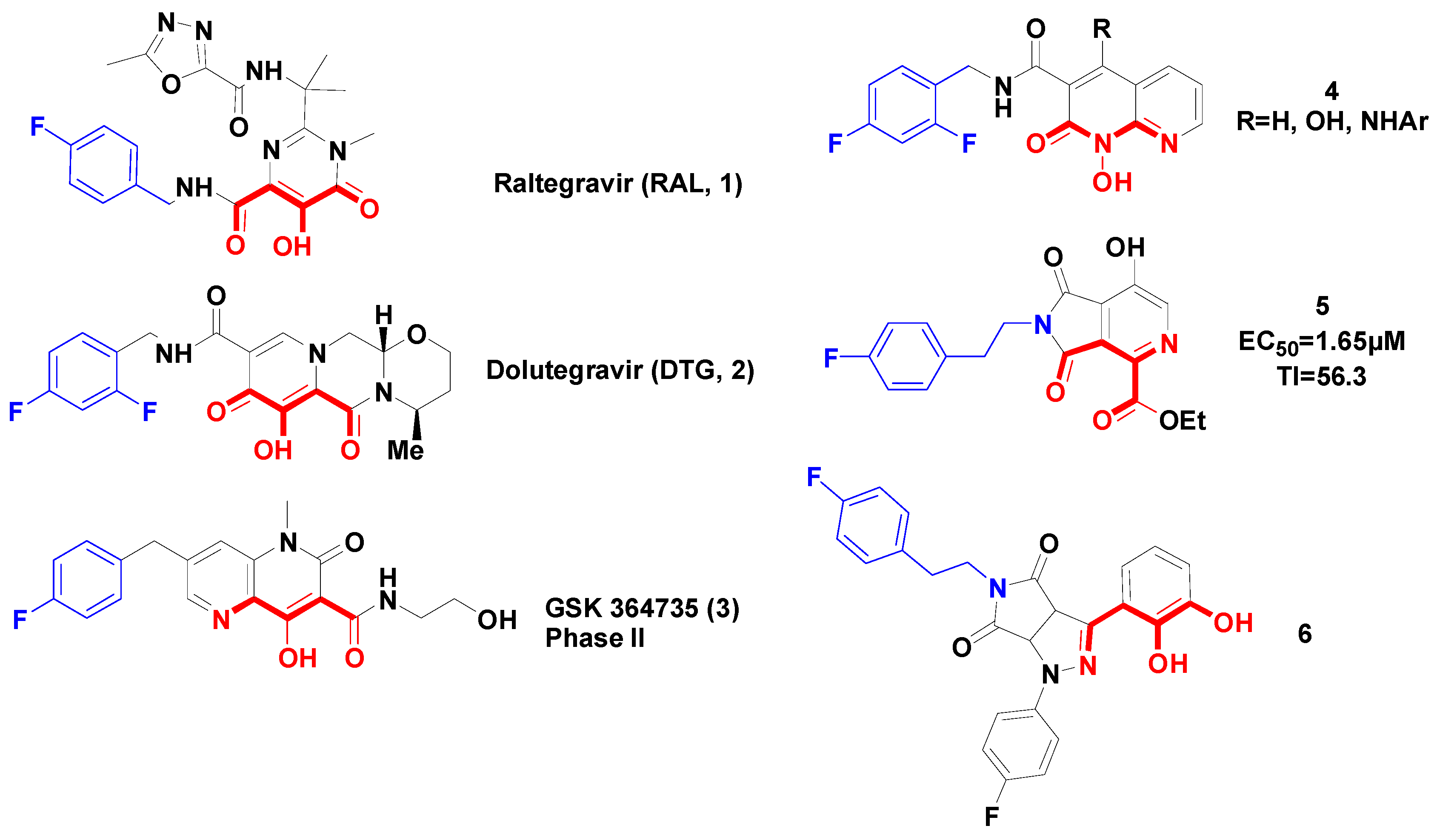
:1. Introduction
2. Results and Discussion
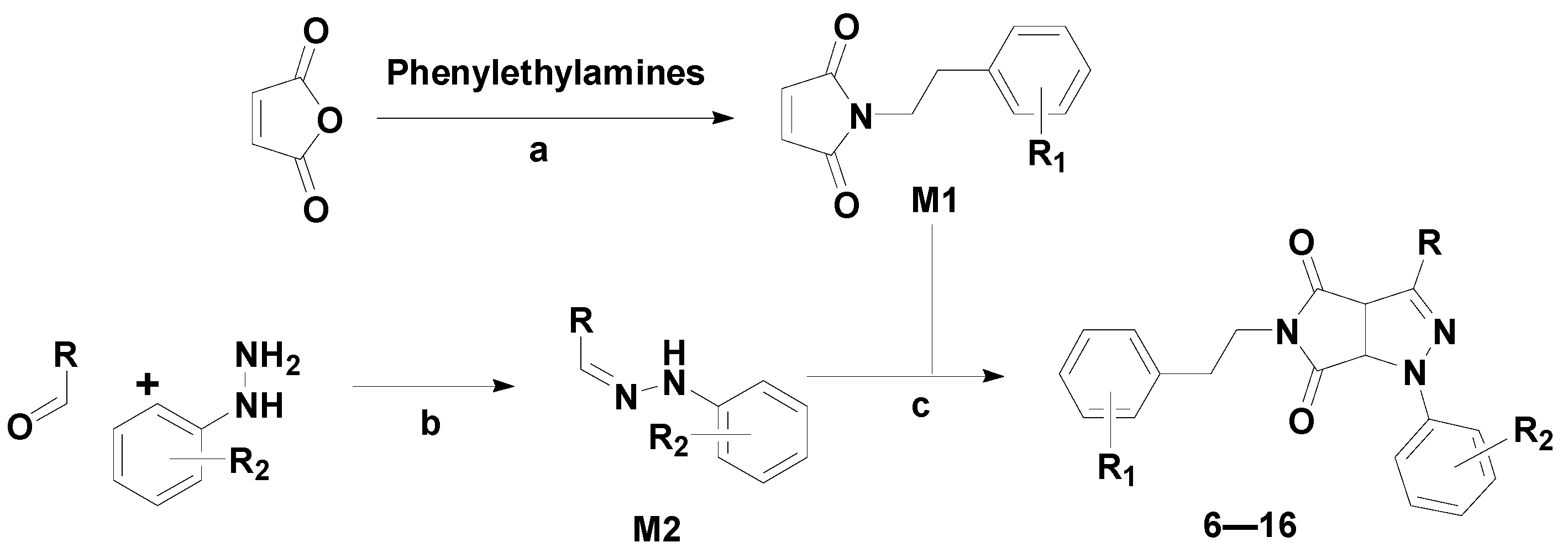
2.1. Chemical Synthesis
2.2. Anti-HIV-1 Evaluation
3. Experimental Section
3.1. Chemistry
3.1.1. General Information
3.1.2. General Procedure for the Preparation of M1
3.1.3. General Procedure for the Preparation of M2
3.1.4. General Procedure for the Preparation of Target Compounds 6–16
3.2. Biological Evaluation
3.2.1. Cytotoxicty Assay
3.2.2. Syncytia Inhibition Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhan, P.; Pannecouque, C.; de Clercq, E.; Liu, X. Anti-HIV drug discovery and development: Current innovations and future trends. J. Med. Chem. 2016, 59, 2849–2878. [Google Scholar] [CrossRef] [PubMed]
- Di Santo, R. Inhibiting the HIV integration process: Past, present, and the future. J. Med. Chem. 2014, 57, 539–566. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y.; Johnson, A.A.; Marchand, C. Integrase inhibitors to treat HIV/AIDS. Nat. Rev. Drug Discov. 2005, 4, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Farnet, C.M.; Wang, B.; Russell Lipford, J.; Bushman, F.D. Differential inhibition of HIV-1 preintegration complexs and purified integrase protein by small molecules. Proc. Natl. Acad. Sci. USA 1996, 93, 9742–9747. [Google Scholar] [CrossRef] [PubMed]
- Summa, V.; Petrocchi, A.; Bonelli, F.; Crescenzi, B.; Donghi, M.; Ferrara, M.; Fiore, F.; Gardelli, C.; Gonzalez Paz, O.; Hazuda, D.J.; et al. Discovery of raltegravir, a potent, selective orally bioavailable HIV-integrase inhititor for the treatment of HIV-AIDS infection. J. Med. Chem. 2008, 51, 5843–5855. [Google Scholar] [CrossRef] [PubMed]
- Shimura, K.; Kodama, E.; Sakagami, Y.; Matsuzaki, Y.; Watanabe, W.; Yamataka, K.; Watanabe, Y.; Ohata, Y.; Doi, S.; Sato, M.; et al. Broad antiretroviral activity and resistance profile of the novel human immunodeficiency virus integrase inhibitor elvitegravir (JTK-303/GS-9137). J. Virol. 2008, 82, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Delelis, O.; Thierry, S.; Subra, F.; Simon, F.; Malet, I.; Alloui, C.; Sayon, S.; Calvez, V.; Deprez, E.; Marcelin, A.-G.; et al. Impact of Y143 HIV-1 integrase mutations on resistance to raltegravir in vitro and in vivo. Antimicrob. Agents Chemother. 2009, 54, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Geretti, A.M.; Armenia, D.; Ceccherini-Silberstein, F. Emerging patterns and implications of HIV-1 integrase inhibitor resistance. Curr. Opin. Infect. Dis. 2012, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Karmon, S.L.; Markowitz, M. Next-generation integrase inhibitors: Where to After Raltegravir? Drugs 2013, 73, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Metifiot, M.; Marchand, C.; Pommier, Y. HIV integrase inhibitors: 20-year landmark and challenges. Adv. Pharmacol. 2013, 67, 75–105. [Google Scholar] [PubMed]
- Metifiot, M.; Marchand, C.; Maddali, K.; Pommier, Y. Resistance to integrase inhibitors. Viruses 2010, 2, 1347–1366. [Google Scholar] [CrossRef] [PubMed]
- Cherepanov, P.; Maertens, G.N.; Hare, S. Structural insights into the retroviral DNA integration apparatus. Curr. Opin. Struct. Biol. 2011, 21, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Reviriego, C. Elvitegravir for the treatment of HIV infection. Drugs Today 2014, 50, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Johns, B.A.; Kawasuji, T.; Weatherhead, J.G.; Boros, E.E.; Thompson, J.B.; Koble, C.S.; Garvey, E.P.; Foster, S.A.; Jeffrey, J.L.; Fujiwara, T. Naphthyridinone (NTD) integrase inhibitors: N1 protio and methyl combination substituent effects with C3 amide groups. Bioorg. Med. Chem. Lett. 2013, 23, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Fardis, M.; Jin, H.; Chen, X.; Tsiang, M.; Chen, J.; Kim, C.; Wright, M. Conformationally constrained tricyclic HIV integrase inhibitors. In HIV-1 Integrase: Mechanism and Inhibitor Design, 1st ed.; Neamati, N., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 239–254. [Google Scholar]
- Hightower, K.E.; Wang, R.; DeAnda, F.; Johns, B.A.; Weaver, K.; Shen, Y.; Tomberlin, G.H.; Carter, H.L., III; Broderick, T.; Sigethy, S.; et al. Dolutegravir (S/GSK1349572) exhibits significantly slower dissociation than raltegravir and elvitegravir from wild-type and integrase inhibitor-resistant HIV-1 integrase-DNA complexes. Antimicrob. Agents Chemother. 2011, 55, 4552–4559. [Google Scholar] [CrossRef] [PubMed]
- Katlama, C.; Murphy, R. Dolutegravir for the treatment of HIV. Expert Opin. Investig. Drugs 2012, 21, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, A.D.; Perry, C.M. Dolutegravir: First global approval. Drugs 2013, 73, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Z.; Smith, S.J.; Metifiot, M.; Johnson, B.C.; Marchand, C.; Pommier, Y.; Hughes, S.H.; Burke, T.R., Jr. Bicyclic 1-hydroxy-2-oxo-1,2-dihydropyridine-3-carboxamide-containing HIV-1 integrase inhibitors having high antiviral potency against cells harboring raltegravir-resistant integrase mutants. J. Med. Chem. 2014, 57, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Z.; Smith, S.J.; Metifiot, M.; Marchand, C.; Boyer, P.L.; Pommier, Y.; Hughes, S.H.; Burke, T.R., Jr. 4‑Amino-1-hydroxy-2-oxo-1,8-naphthyridine-containing compounds having high potency against raltegravir-resistant integrase mutants of HIV‑1. J. Med. Chem. 2014, 57, 5190–5202. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.N.; Luo, R.H.; Zhang, X.J.; Zhou, Y.; Li, J.; Zheng, Y.T.; Liu, H. Synthesis and evaluation of anti-HIV-1 activities of novel 7-hydroxy-1,3-dioxo-2,3-dihydro-1H-pyrrolo[3,4-c]pyridine-4-carboxylate derivatives. Med. Chem. 2014, 4, 573–580. [Google Scholar]
- Zhao, X.Z.; Maddali, K.; Vu, B.C.; Marchand, C.; Hughes, S.H.; Pommier, Y.; Burke, T.R., Jr. Examination of halogen substituent effects on HIV-1 integrase inhibitors derived from 2,3-dihydro-6,7-dihydroxy-1H-isoindol-1-ones and 4,5-dihydroxy-1H-isoindole-1,3(2H)-diones. Bioorg. Med. Chem. Lett. 2009, 19, 2714–2717. [Google Scholar] [CrossRef] [PubMed]
- Matuszak, N.; Muccioli, G.G.; Labar, G.; Lambert, D.M. Synthesis and in Vitro Evaluation of N-substituted maleimide derivatives as selective monoglyceride lipase inhibitors. J. Med. Chem. 2009, 52, 7410–7420. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M.; Matsumoto, S.; Shirai, M.; Yamamoto, H. Generation of NH-azomethine imine intermediates through the 1,2-hydrogen shift of hydrazones and their intermolecular cycloaddition reaction with olefinic dipolarophiles. Tetrahedron 2003, 59, 4123–4133. [Google Scholar] [CrossRef]
- Wang, R.R.; Yang, Q.H.; Luo, R.H.; Peng, Y.M.; Dai, S.X.; Zhang, X.J.; Chen, H.; Cui, X.Q.; Liu, Y.J.; Huang, J.F.; et al. Azvudine, a novel nucleoside reverse transcriptase inhibitor showed good drug combination features and better inhibition on drug-resistant strains than lamivudine in vitro. PLoS ONE 2014, 9, e105617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Lu, L.H.; Wang, R.R.; Wang, Y.P.; Luo, R.H.; Lai, C.C.; Yang, L.M.; He, Y.P.; Zheng, Y.T. DB-02, a C-6-cyclohexylmethyl substituted pyrimidinone HIV-1 reverse transcriptase inhibitor with nanomolar activity, displays an improved sensitivity against K103N or Y181C than S-DABOs. PLoS ONE 2013, 8, e81489. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
| Compd. | R | CC50 b (μM) | EC50 c (μM) | TI d |
|---|---|---|---|---|
| 6 | 2,3-OHPh | >431.92 | 26.39 | >16.37 |
| 7a | 2-OMe,3-OHPh | 39.14 | 5.37 | 7.29 |
| 7b | Ph | 147.64 | 9.21 | 16.03 |
| 8 | 2-OHPh | >447.35 | 10.11 | >44.25 |
| 9a | 2,4-OHPh | 173.48 | 35.31 | 4.91 |
| 9b | 2-OH,3-FPh | 141.01 | 5.74 | 24.58 |
| 9c | 2-OH,5-FPh | 158.78 | 4.21 | 37.70 |
| 9d | 2-OH,3-ClPh | 331.51 | 20.42 | 16.23 |
| 9e | 2-OH,3-NO2Ph | 49.53 | 7.55 | 6.56 |
| 9f | 2-OH,3-MePh | 150.52 | 4.90 | 30.73 |
| 9g | 2-OH,3-OMePh | >418.88 | 3.98 | >105.26 |
| 10a | 2-OH,4-OMePh | 21.36 | 8.52 | 2.51 |
| 10b | 2-OH,5-OMePh | 188.62 | 4.73 | 39.85 |
| 10c | 2-OH,3-OEtPh | >407.19 | 115.87 | >3.51 |
| 11 | 2-OH,3-OMe,5-NO2Ph | 90.29 | 41.94 | 2.15 |
| 12a | 2,3-OMePh | >406.96 | 11.13 | >36.56 |
| 12b | benzo[1,3]dioxol-4-yl | 222.45 | 7.11 | 31.29 |
| 13 | 2-OH-naphthalen-1-yl | 9.03 | 4.50 | 2.00 |
| 14a | thiazol-2-yl | 158.41 | 35.55 | 4.46 |
| 14b | pyridin-2-yl | 149.85 | 26.16 | 5.73 |
| AZT e | 3779 | 0.0085 | 444,588 |
| Compd. | R1 | R2 | CC50 b (μM) | EC50 c (μM) | TI d |
|---|---|---|---|---|---|
| 15a | 4-F | 3-F | 128.89 | 12.08 | 10.67 |
| 15b | 4-F | 2-F | >418.88 | 112.85 | >3.71 |
| 15c | 4-F | 4-Cl | 166.33 | 25.47 | 6.52 |
| 15d | 4-F | 2,4-F | >403.67 | 65.92 | >6.12 |
| 15e | 4-F | 4-CF3 | >379.17 | 32.10 | >11.81 |
| 15f | 4-F | 4-SO2Me | >372.44 | 65.34 | >5.70 |
| 15g | 4-F | 4-SO2NH2 | 46.02 | 30.43 | 1.51 |
| 15h | 4-F | H | >435.77 | 28.63 | >15.22 |
| 15i | 4-F | 4-Me | >422.83 | 4.10 | >103.09 |
| 15j | 4-F | 4-OMe | >408.61 | 21.12 | >19.34 |
| 16a | 2-F | 4-F | 19.58 | 2.52 | 7.78 |
| 16b | 3-F | 4-F | 143.35 | 13.02 | 11.01 |
| 16c | 4-Cl | 4-F | 70.13 | 10.43 | 6.73 |
| 16d | H | 4-F | >435.73 | 18.87 | >23.09 |
| 16e | 4-OMe | 4-F | 219.37 | 87.15 | 2.52 |
| AZT e | 3779 | 0.0085 | 444,588 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.-N.; Luo, R.-H.; Zhou, Y.; Zhang, X.-J.; Li, J.; Yang, L.-M.; Zheng, Y.-T.; Liu, H. Synthesis and Anti-HIV-1 Activity Evaluation for Novel 3a,6a-Dihydro-1H-pyrrolo[3,4-c]pyrazole-4,6-dione Derivatives. Molecules 2016, 21, 1198. https://doi.org/10.3390/molecules21091198
Liu G-N, Luo R-H, Zhou Y, Zhang X-J, Li J, Yang L-M, Zheng Y-T, Liu H. Synthesis and Anti-HIV-1 Activity Evaluation for Novel 3a,6a-Dihydro-1H-pyrrolo[3,4-c]pyrazole-4,6-dione Derivatives. Molecules. 2016; 21(9):1198. https://doi.org/10.3390/molecules21091198
Chicago/Turabian StyleLiu, Guan-Nan, Rong-Hua Luo, Yu Zhou, Xing-Jie Zhang, Jian Li, Liu-Meng Yang, Yong-Tang Zheng, and Hong Liu. 2016. "Synthesis and Anti-HIV-1 Activity Evaluation for Novel 3a,6a-Dihydro-1H-pyrrolo[3,4-c]pyrazole-4,6-dione Derivatives" Molecules 21, no. 9: 1198. https://doi.org/10.3390/molecules21091198