Passerini Reactions on Biocatalytically Derived Chiral Azetidines
Abstract
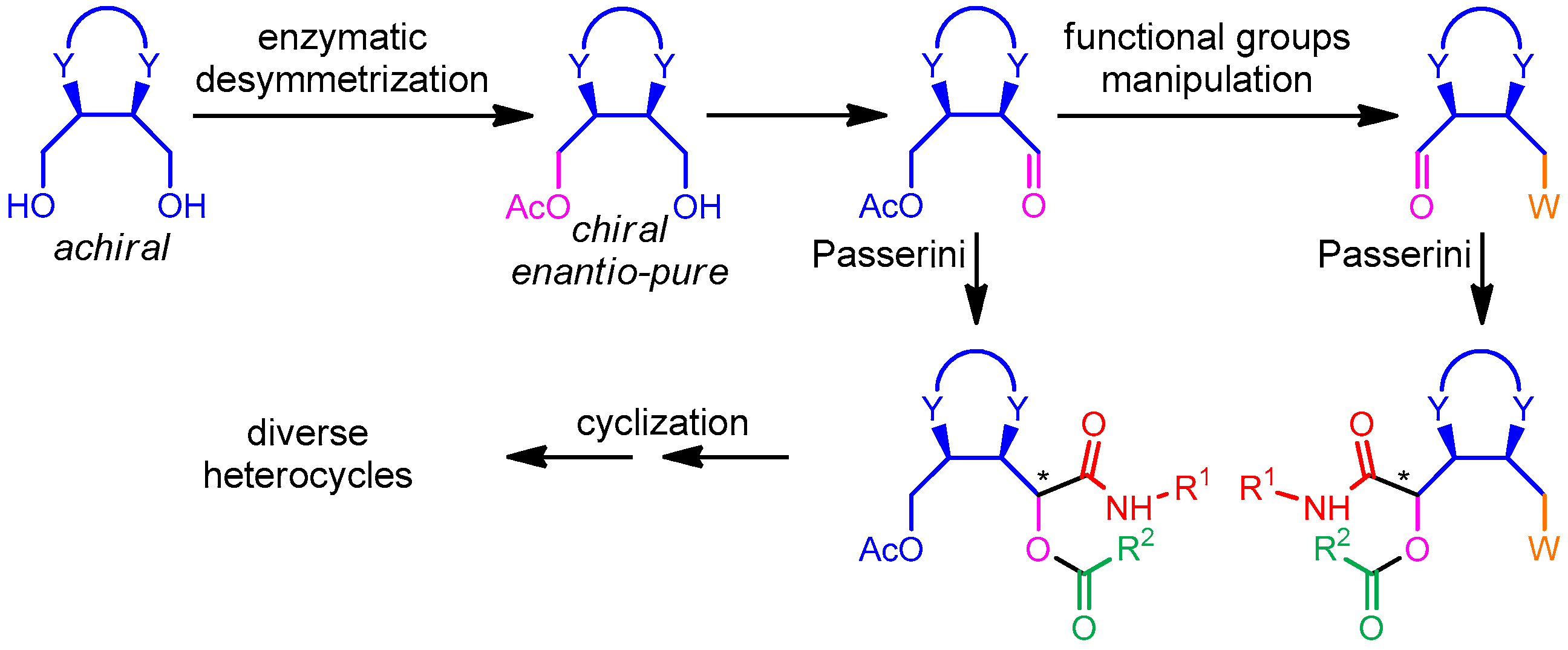
:1. Introduction
2. Results
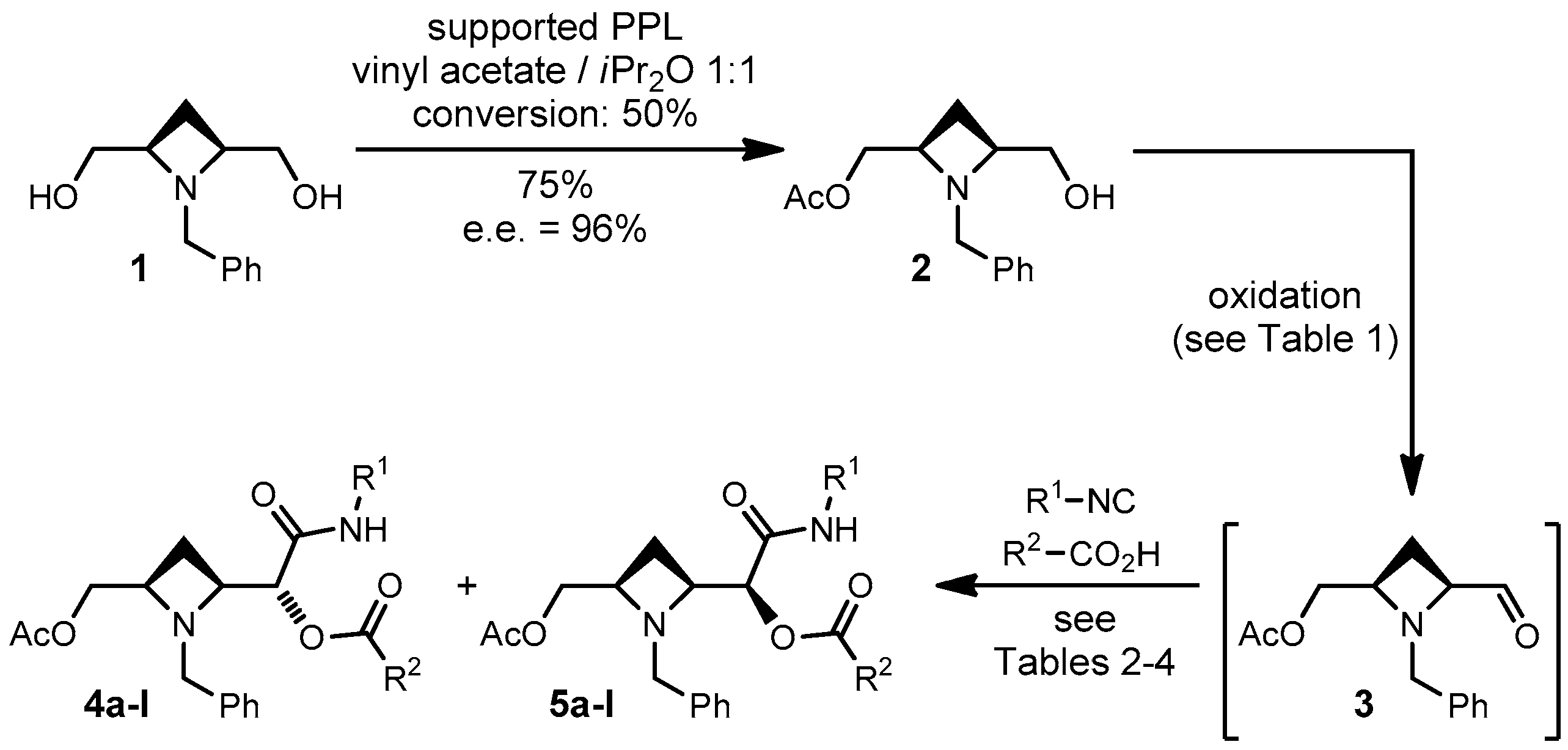
2.1. Optimization of Oxidation-Passerini on a Model Compound
2.2. Scope of the Reaction
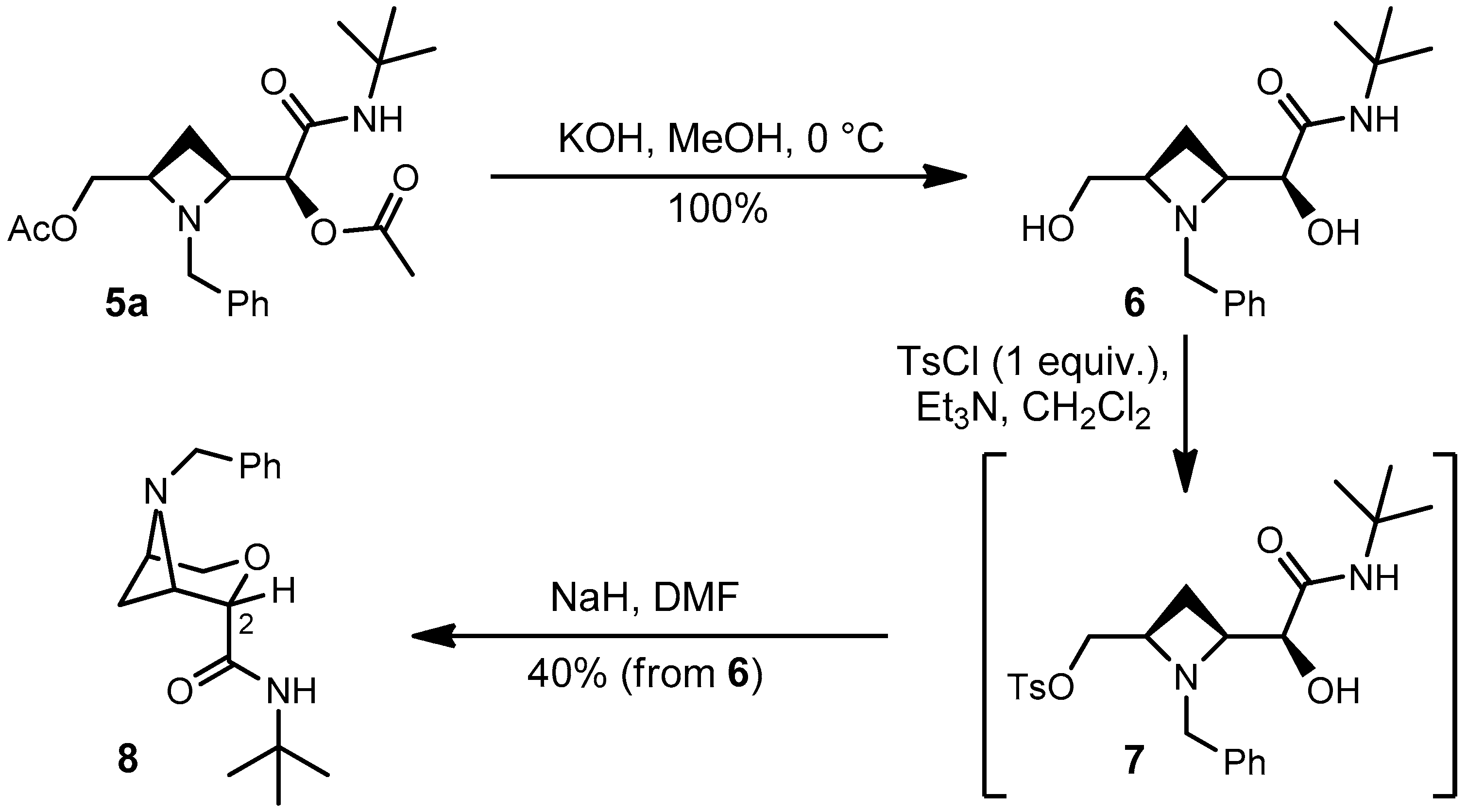
2.3. Establishment of Relative Configuration
3. Discussion
4. Materials and Methods
4.1. General Remarks
4.2. Synthesis of ((2R,4S)-1-Benzyl-4-formylazetidin-2-yl)methyl Acetate (3)
4.3. General Procedure for Passerini Reaction under Classical Conditions
4.4. General Procedure for Passerini Reaction with ZnBr2
4.5 Synthesis of (2S)-2-((2S,4R)-1-Benzyl-4-(hydroxymethyl)azetidin-2-yl)-N-(tert-butyl)-2-hydroxyacetamide (6)
4.6 Synthesis of (1S,2S,5R)-6-Benzyl-N-(tert-butyl)-3-oxa-6-azabicyclo[3.1.1]heptane-2-carboxamide (8)
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wessjohann, L.A.; Kaluderovic, G.; Neves Filho, R.A.W.; Morejon, M.C.; Lemanski, G.; Ziegler, T. The Ugi reaction. In Science of Synthesis: Multicomponent Reactions, Vol. 1; Müller, T.J.J., Ed.; Thieme: Stuttgart, Germany, 2013; pp. 415–497. [Google Scholar]
- Riva, R.; Banfi, L.; Basso, A. The Passerini Reaction. In Science of Synthesis: Multicomponent Reactions, Vol. 1; Müller, T.J.J., Ed.; Thieme: Stuttgart, Germany, 2013; pp. 327–414. [Google Scholar]
- Banfi, L.; Basso, A.; Chiappe, C.; De Moliner, F.; Riva, R.; Sonaglia, L. Development of a stereoselective Ugi reaction starting from an oxanorbornene beta-amino acid derivative. Org. Biomol. Chem. 2012, 10, 3819–3829. [Google Scholar] [CrossRef] [PubMed]
- Basso, A.; Banfi, L.; Riva, R.; Guanti, G. A novel highly selective chiral auxiliary for the asymmetric synthesis of l- and d-α-amino acid derivatives via a multicomponent Ugi reaction. J. Org. Chem. 2005, 70, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Hebach, C.; Kazmaier, U. Via Ugi reactions to conformationally fixed cyclic peptides. Chem. Commun. 2003. [Google Scholar] [CrossRef]
- Nenajdenko, V.G.; Gulevich, A.V.; Chernichenko, K.Y.; Sokolova, N.V.; Balenkova, E.S. R-α-Phenylglycinol and R-α-phenylglycinamide as novel chiral templates in diastereoselective Ugi reaction. Mendeleev Commun. 2011, 21, 245–246. [Google Scholar] [CrossRef]
- Ross, G.F.; Herdtweck, E.; Ugi, I. Stereoselective U-4CRs with 1-amino-5-desoxy-5-thio-2,3,4-O-isobutanoyl-β-d-xylopyranose—An effective and selectively removable chiral auxiliary. Tetrahedron 2002, 58, 6127–6133. [Google Scholar] [CrossRef]
- Caputo, S.; Basso, A.; Moni, L.; Riva, R.; Rocca, V.; Banfi, L. Diastereoselective Ugi reaction of chiral 1,3-aminoalcohols derived from an organocatalytic Mannich reaction. Beilstein J. Org. Chem. 2016, 12, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Frey, R.; Galbraith, S.G.; Guelfi, S.; Lamberth, C.; Zeller, M. First examples of a highly stereoselective Passerini reaction: A new access to enantiopure mandelamides. Synlett 2003. [Google Scholar] [CrossRef]
- Moni, L.; Banfi, L.; Basso, A.; Carcone, L.; Rasparini, M.; Riva, R. Ugi and Passerini Reactions of Biocatalytically Derived Chiral Aldehydes: Application to the Synthesis of Bicyclic Pyrrolidines and of Antiviral Agent Telaprevir. J. Org. Chem. 2015, 80, 3411–3428. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G.L.; Lawrie, K.W.M.; Morgan, P.; Willis, C.L. Ugi four component condensations using aldehydes with an asymmetric centre at C-2. Tetrahedron Lett. 2000, 41, 8001–8005. [Google Scholar] [CrossRef]
- Morana, F.; Basso, A.; Riva, R.; Rocca, V.; Banfi, L. The homo-PADAM Protocol: Stereoselective and Operationally Simple Synthesis of α-Oxo- or α-Hydroxy-gamma-acylaminoamides and Chromanes. Chem. Eur. J. 2013, 19, 4563–4569. [Google Scholar] [CrossRef] [PubMed]
- Banfi, L.; Basso, A.; Moni, L.; Riva, R. The Alternative Route to Enantiopure Multicomponent Reaction Products: Biocatalytic or Organocatalytic Enantioselective Production of Inputs for Multicomponent Reactions. Eur. J. Org. Chem. 2014, 2014, 2005–2015. [Google Scholar] [CrossRef]
- Moni, L.; Banfi, L.; Basso, A.; Martino, E.; Riva, R. Diastereoselective Passerini Reaction of Biobased Chiral Aldehydes: Divergent Synthesis of Various Polyfunctionalized Heterocycles. Org. Lett. 2016, 18, 1638–1641. [Google Scholar] [CrossRef] [PubMed]
- Guanti, G.; Riva, R. Synthesis of chiral non-racemic azetidines by lipase-catalysed acetylations and their transformation into amino alcohols: Precursors of chiral catalysts. Tetrahedron Asymm. 2001, 12, 605–618. [Google Scholar] [CrossRef]
- Moni, L.B.L.; Riva, R.; Basso, A. External Oxidant-based Multi Component Reactions. Synthesis 2016, in press. [Google Scholar]
- De Moliner, F.; Crosignani, S.; Banfi, L.; Riva, R.; Basso, A. Synthesis of 5-Carboxamide-oxazolines with a Passerini-Zhu/Staudinger-Aza-Wittig Two-Step Protocol. J. Comb. Chem. 2010, 12, 613–616. [Google Scholar] [CrossRef] [PubMed]
- De Moliner, F.; Crosignani, S.; Galatini, A.; Riva, R.; Basso, A. Novel Application of alpha-Azido Aldehydes in Multicomponent Reactions: Synthesis of Triazolo-Fused Dihydrooxazinones via a Passerini Reaction-Dipolar Cycloaddition Strategy. Acs Comb. Sci. 2011, 13, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Henze, M.; Kreye, O.; Brauch, S.; Nitsche, C.; Naumann, K.; Wessjohann, L.A.; Westermann, B. Photoaffinity-Labeled Peptoids and Depsipeptides by Multicomponent Reactions. Synthesis 2010. [Google Scholar] [CrossRef]
- Ngouansavanh, T.; Zhu, J.P. Alcohols in isonitrile-based multicomponent reaction: Passerini reaction of alcohols in the presence of O-iodoxybenzoic acid. Angew. Chem. Int. Ed. Engl. 2006, 45, 3495–3497. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 4a–l and 5a–l are available from the authors.

| Entry | Oxidant | Temp. | Solvent | Time 2 | Work-Up 3 | Yield 4 | Note |
|---|---|---|---|---|---|---|---|
| 1 | TEMPO-BAIB | r.t. | CH2Cl2 | 1 h | none | 58% | residual 2 |
| 2 | TEMPO-BAIB | r.t. | CH2Cl2 | 3 h | none | 37% | residual 2 |
| 3 | TEMPO-BAIB | r.t. | CH2Cl2 | 16 h | none | <15% | residual 2 |
| 4 | IBX | r.t. | THF | 24 h | none | 23% | residual 2 |
| 5 | IBX | r.t. | THF | 96 h | none | 23% | residual 2 |
| 6 | Swern (iPr2NEt) | −78 °C | CH2Cl2 | 8 h | extraction | 75% | |
| 7 | Swern (Et3N) | −78 °C | CH2Cl2 | 80 min | extraction | 82% | |
| 8 | Swern (Et3N) | −78 °C | CH2Cl2 | 30 min | extraction | 84% |
| Entry | Lewis Acid 2 | Conversion 3 | Notes | 4a:5a Ratio 3 |
|---|---|---|---|---|
| 1 | none | 100% | 57:43 | |
| 2 | FeCl3 | 100% | partially unclean | 63:37 |
| 3 | Ti(OiPr)3Cl | 98% | very unclean | 59:41 |
| 4 | TiCl4 | 100% | very unclean | 66:34 |
| 5 | SnCl4 | 100% | partially unclean | 37:63 |
| 6 | SnCl2 | 100% | 57:43 | |
| 7 | AlCl3 | 89% | 60:40 | |
| 8 | MgBr2 | 100% | 57:43 | |
| 9 | Cu(OTf)2 | <5% | - | |
| 10 | CuBr2 | 80% | very unclean | 58:42 |
| 11 | BF3·Et2O | 80% | partially unclean | 70:30 |
| 12 | ZnCl2 | 100% | 58:42 | |
| 13 | ZnCl24 | 100% | 63:37 | |
| 14 | ZnBr2 | 100% | 59:41 | |
| 15 | ZnI2 | 100% | 59:41 |
| Entry | Solvent | Temp. | Time | Yield 2 | 4a:5a Ratio 3 |
|---|---|---|---|---|---|
| 1 | iPr2O | 25 °C | 1 h | 76% | 56:44 |
| 2 | Et2O | 25 °C | 1 h | 56% | 59:41 |
| 3 | Bu2O | 25 °C | 1 h | 59% | 54:46 |
| 4 | tBuMeO | 25 °C | 1 h | 43% | 65:35 |
| 5 | MeCN | 25 °C | 1 h | 76% | 67:33 |
| 6 | THF | 25 °C | 1 h | 83% | 68:32 |
| 7 | THF | −20 °C | 2 h | 87% | 70:30 |
| 8 | THF | −20 °C | 18 h | 86% | 70:30 |
| 9 | THF | −40 °C | 18 h | 50% | 69:31 |
| Entry | Products | R 1 | R 2 | With ZnBr21 | Classic Conditions 2 | ||
|---|---|---|---|---|---|---|---|
| Yield 3 | 4:5 Ratio 4 | Yield 3 | 4:5 Ratio 4 | ||||
| 1 | 4,5a | tBu | CH3 | 86% | 70:30 | 84% | 57:43 |
| 2 | 4,5b | tBu | tBu | 75% | 70:30 | 84% | 57:43 |
| 3 | 4,5c | cyHex | tBu | 76% | 68:32 | 81% | 57:43 |
| 4 | 4,5d | tBu | nBu | 73% | 76:24 | 83% | 56:44 |
| 5 | 4,5e | cyHex | nBu | 77% | 75:25 | 85% | 58:42 |
| 6 | 4,5f | tBu | Ph | 72% | 69:31 | 81% | 56:44 |
| 7 | 4,5g | cyHex | Ph | 72% | 72:28 | 72% | 57:43 |
| 8 | 4,5h | CH2CO2Et | 3-BrC6H4 | 71% | 66:34 | - 5 | - |
| 9 | 4,5i | 2,6-diMeC6H3 | 5-Cl-2-thienyl | 67% | 69:31 | - 5 | - |
| 10 | 4,5j | nBu | (S)FmocVal | 73% | 70:30 | - 5 | - |
| 11 | 4,5k | nBu | (R)FmocVal | 69% | 73:27 | - 5 | - |
| 12 | 4,5l | Bn | CH2OMe | 64% | 70:30 | - 5 | - |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moni, L.; Banfi, L.; Basso, A.; Bozzano, A.; Spallarossa, M.; Wessjohann, L.; Riva, R. Passerini Reactions on Biocatalytically Derived Chiral Azetidines. Molecules 2016, 21, 1153. https://doi.org/10.3390/molecules21091153
Moni L, Banfi L, Basso A, Bozzano A, Spallarossa M, Wessjohann L, Riva R. Passerini Reactions on Biocatalytically Derived Chiral Azetidines. Molecules. 2016; 21(9):1153. https://doi.org/10.3390/molecules21091153
Chicago/Turabian StyleMoni, Lisa, Luca Banfi, Andrea Basso, Andrea Bozzano, Martina Spallarossa, Ludger Wessjohann, and Renata Riva. 2016. "Passerini Reactions on Biocatalytically Derived Chiral Azetidines" Molecules 21, no. 9: 1153. https://doi.org/10.3390/molecules21091153







