Anti-Influenza Virus Activity and Constituents Characterization of Paeonia delavayi Extracts
Abstract
:1. Introduction
2. Results and Discussion
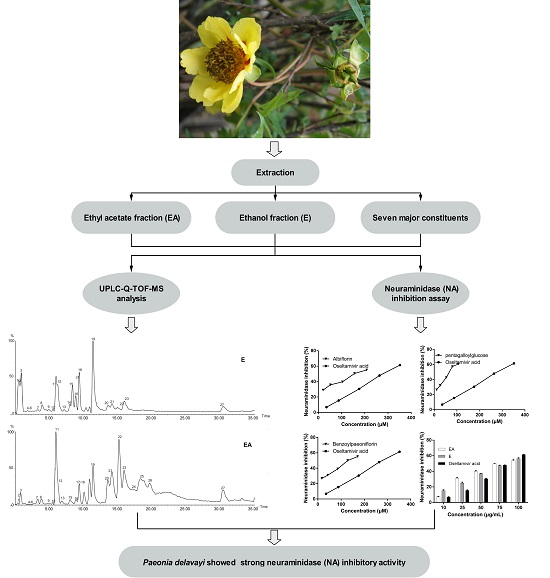
2.1. UPLC-Q-TOF-MS Analysis
2.2. Influenza Virus Neuraminidase (NA) Activity Assay
3. Experimental Section
3.1. Chemicals and Standard Substances
3.2. Neuraminidase Inhibitors Screen Kit
3.3. Plant Material Collection and Sample Preparation
3.4. UPLC-Q-TOF-MS Analysis
3.4.1. Liquid Chromatography
3.4.2. Mass Spectrometry
3.5. Neuraminidase (NA) Inhibition Assay
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- He, C.; Peng, B.; Dan, Y.; Peng, Y.; Xiao, P. Chemical taxonomy of tree peony species from China based on root cortex metabolic fingerprinting. Phytochemistry 2014, 107, 69–79. [Google Scholar] [CrossRef]
- Ho, J.Y.; Chang, H.W.; Lin, C.F.; Liu, C.J.; Hsieh, C.F.; Horng, J.T. Characterization of the anti-influenza activity of the Chinese herbal plant Paeonia lactiflora. Viruses 2014, 6, 1861–1875. [Google Scholar] [CrossRef]
- Wu, S.H.; Wu, D.G.; Chen, Y.W. Chemical constituents and bioactivities of plants from the genus Paeonia. Chem. Biodivers. 2010, 7, 90–104. [Google Scholar] [CrossRef]
- He, C.N.; Peng, Y.; Zhang, Y.C.; Xu, L.J.; Gu, J.; Xiao, P.G. Phytochemical and biological studies of Paeoniaceae. Chem. Biodivers. 2010, 7, 805–838. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, B.T. Flora of China; Science Press: Beijing, China, 1979; Volume 27, p. 47. [Google Scholar]
- Wang, D.X.; Ma, H.; Zhang, Y.L.; Duan, A.A.; Li, W.J.; Li, Z.H. Paeonia (Paeoniaceae) expressed sequence tag-derived microsatellite markers transferred to Paeonia delavayi. GMR 2013, 12, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Luo, X.D.; Ma, Y.B.; Hao, X.J.; Wu, D.G. Monoterpenoid derivatives from Paeonia delavayi. J. Asian Nat. Prod. Res. 2002, 4, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Chen, Y.W.; Yang, L.Y.; Li, S.L.; Li, Z.Y. Monoterpene glycosides from Paeonia delavayi. Fitoterapia 2007, 78, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Guo, W.; Wang, Q.; Zhang, L.; Zhu, M.; Zhu, T.; Gu, Q.; Wang, W.; Li, D. Aspulvinones from a mangrove rhizosphere soil-derived fungus aspergillus terreus Gwq-48 with anti-influenza A viral (H1N1) activity. Bioorg. Med. Chem. Lett. 2013, 23, 1776–1778. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.S.; Lee, I.K.; Choi, H.J.; Yun, B.S. Anti-influenza activities of polyphenols from the medicinal mushroom Phellinus baumii. Bioorg. Med. Chem. Lett. 2015, 25, 3256–3260. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhang, C.; Sun, H.; Liu, Q.; Huang, J.; Sheng, L.; Lin, B.; Wang, J.; Chen, L. The semi-synthesis of novel andrographolide analogues and anti-influenza virus activity evaluation of their derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Zu, M.; Yang, F.; Zhou, W.; Liu, A.; Du, G.; Zheng, L. In vitro anti-influenza virus and anti-inflammatory activities of theaflavin derivatives. Antiviral Res. 2012, 94, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). High levels of adamantane resistance among influenza A (H3N2) viruses and interim guidelines for use of antiviral agents--United States, 2005–06 influenza season. MMWR 2006, 20, 44–46. [Google Scholar]
- Pizzorno, A.; Abed, Y.; Boivin, G. Influenza drug resistance. Semin. Respir.Crit. Care Med. 2011, 32, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Pielak, R.M.; Chou, J.J. Flu channel drug resistance: A tale of two sites. Protein Cell 2010, 1, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Dolin, R. Resistance to neuraminidase inhibitors. In Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America; University of Chicago Press: Chicago, IL, USA, 2011; Volume 52, pp. 438–439. [Google Scholar]
- Stephenson, I.; Democratis, J.; Lackenby, A.; McNally, T.; Smith, J.; Pareek, M.; Ellis, J.; Bermingham, A.; Nicholson, K.; Zambon, M. Neuraminidase inhibitor resistance after oseltamivir treatment of acute influenza A and B in children. In Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America; University of Chicago Press: Chicago, IL, USA, 2009; Volume 48, pp. 389–396. [Google Scholar]
- Moscona, A. Neuraminidase Inhibitors for Influenza. N. Engl. J. Med. 2005, 353, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Song, J.M.; Lee, K.H.; Seong, B.L. Antiviral effect of catechins in green tea on influenza virus. Antiviral Res. 2005, 68, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Kuzuhara, T.; Iwai, Y.; Takahashi, H.; Hatakeyama, D.; Echigo, N. Green tea catechins inhibit the endonuclease activity of influenza A virus RNA polymerase. PLoS Curr. 2009, 1, RRN1052. [Google Scholar] [CrossRef]
- Ma, S.C.; Du, J.; But, P.; Deng, X.L. Antiviral Chinese medicinal herbs against respiratory syncytial virus. J. Ethnopharmacol. 2002, 79, 205–211. [Google Scholar] [CrossRef]
- Xiao, C.; Wu, M.; Chen, Y.; Zhang, Y.; Zhao, X.; Zheng, X. Revealing metabolomic variations in Cortex Moutan from different root parts using HPLC-MS method. Phytochem. Anal. PCA 2015, 26, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Cui, W.; Zhou, W.; Duan, J.A.; Shang, E.; Tang, Y. Chemical fingerprinting and quantitative constituent analysis of Siwu decoction categorized formulae by UPLC-QTOF/MS/MS and HPLC-DAD. Chin. Med. 2013, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Gu, Z. Screening of bioactive compounds from moutan cortex and their anti-inflammatory activities in rat synoviocytes. eCAM 2009, 6, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Hollecker, L.; Pinna, M.; Filippino, G.; Scrugli, S.; Pinna, B.; Argiolas, F.; Murru, M. Simultaneous determination of polyphenolic compounds in red and white grapes grown in Sardinia by high performance liquid chromatography-electron spray ionisation-mass spectrometry. J. Chromatogr. A 2009, 1216, 3402–3408. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.H.; Zhu, S.; Ge, Y.W.; He, Y.M.; Kazuma, K.; Wang, Z.; Yoshimatsu, K.; Komatsu, K. Monoterpene derivatives with anti-allergic activity from red peony root, the root of Paeonia lactiflora. Fitoterapia 2016, 108, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, Z.M.; Li, T.; Zhang, Y.B.; Sze, S.C.; Wang, G.C.; Li, Y.L.; Ye, W.C. Monoterpene derivatives from the roots of Paeonia lactiflora and their anti-proliferative activity. Fitoterapia 2014, 98, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.M. Antiviral activity of compounds isolated from plants. Pharm. Biol. 2008, 41, 107–157. [Google Scholar] [CrossRef]
- Shi, L.; Lei, Y.; Srivastava, R.; Qin, W.; Chen, J.J. Gallic acid induces apoptosis in human cervical epithelial cells containing human papillomavirus type 16 episomes. J. Med. Virol. 2016, 88, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Raheel, R.; Ashraf, M.; Ejaz, S.; Javeed, A.; Altaf, I. Assessment of the cytotoxic and anti-viral potential of aqueous extracts from different parts of Acacia nilotica (Linn) Delile against Peste des petits ruminants virus. Environ. Toxicol. Pharmacol. 2013, 35, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Chamni, S.; De-Eknamkul, W. Recent progress and challenges in the discovery of new neuraminidase inhibitors. Exp. Opin. Ther. Patents 2013, 23, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Martina, R.; Lilia, S.; Elisabeth, W.; Anja, H.; Heike, B.; Ulrike, G.; Judith, M.R.; Susanne, V.G.; Klaus, R.L.; Johannes, K.; et al. Complementary assays helping to overcome challenges for identifying neuraminidase inhibitors. Future Virol. 2015, 10, 77–88. [Google Scholar]
- Bisson, J.; McAlpine, J.B.; Friesen, J.B.; Chen, S.N.; Graham, J.; Pauli, G.F. Can Invalid Bioactives Undermine Natural Product-Based Drug Discovery? J. Med. Chem. 2016, 59, 1671–1690. [Google Scholar] [CrossRef] [PubMed]
- Baell, J.B. Feeling Nature’s PAINS: Natural Products, Natural Product Drugs, and Pan Assay Interference Compounds (PAINS). J. Nat. Prod. 2016, 79, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Gubareva, L.V.; Webster, R.G.; Hayden, F.G. Detection of influenza virus resistance to neuraminidase inhibitors by an enzyme inhibition assay. Antiviral Res. 2002, 53, 47–61. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 2, 4, 9, 11, 12, 19, 27 are available from the authors.
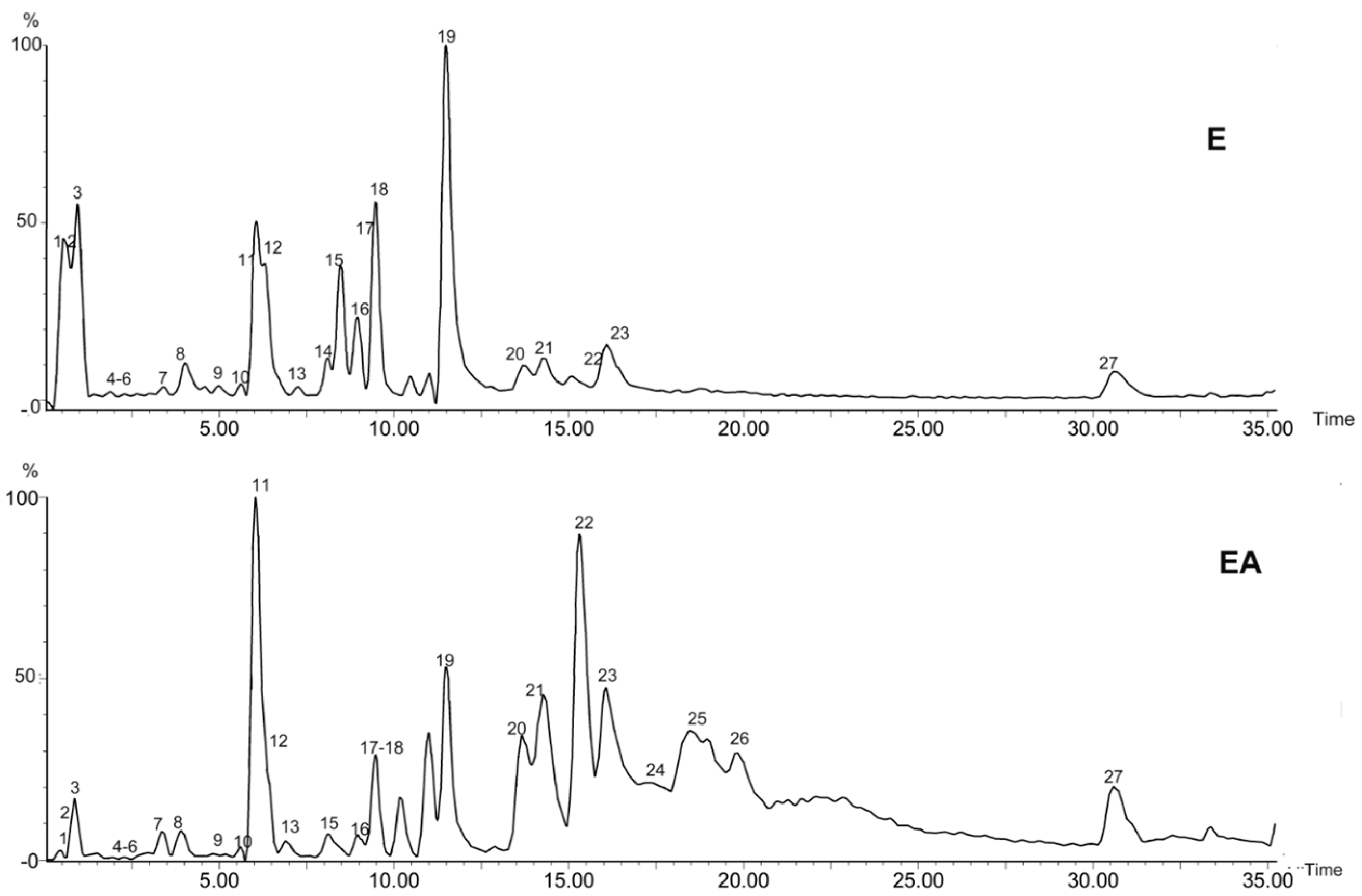
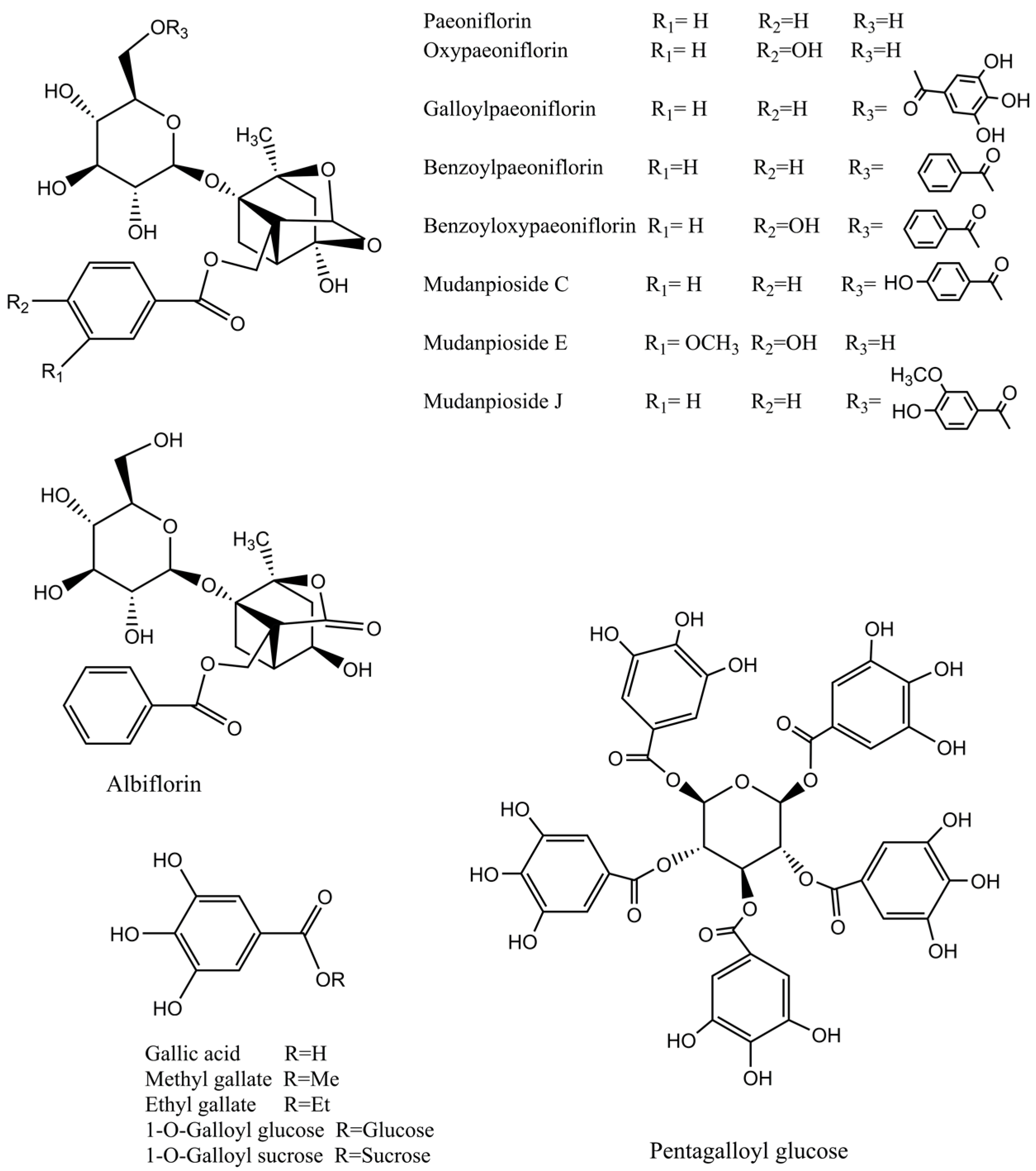

| Peak | TR (min) | Formula | m/z Calculated | m/z Experimental | Assigned Identity | Fragment Ions | Extracts | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.48 | C13H16O10 | 331.0660 | 331.0826 | 1-O-Galloyl glucose | 169[M–H–glucosyl]−, 125[M–H–glucosyl–CO2]− | E,EA | [1] |
| 2 | 0.82 | C7H6O5 | 169.0132 | 169.0360 | Gallic acid * | 125[M–H–CO2]− | E,EA | [1,22] |
| 3 | 1.01 | C19H26O15 | 493.3928 | 493.1234 | 1-O-Galloyl sucrose | 457[M–H–2H2O]−, 331[M–2H–glucosyl]−, 169[M–H–2glucosyl]−, 125[M–H–2glucosyl–CO2]− | E,EA | [22,23] |
| 4 | 3.37 | C8H8O5 | 183.0288 | 183.0517 | Methyl gallate * | 169 [M–CH3]−, 168[M–H–CH3]−, 124[M–H–CH3–CO2]−, 125[M–CH3–CO2]− | E,EA | [1,22] |
| 5 | 3.93 | C20H20O14 | 483.0769 | 483.0833 | Digalloyl-hexose | 331[M–H–galloyl]−, 313[M–H–galloyl–H2O]−, 271, 169[gallic acid–H]−, 125[gallic acid–H–CO2]− | E,EA | [23] |
| 6 | 4.38 | C27H24O18 | 635.0879 | 635.0823 | Trigalloyl-glucose | 483[M–H–galloyl]−, 395[M–H–galloyl–2CO2]−, 169[gallic acid–H]−, 125[gallic acid–H–CO2]− | E,EA | [1] |
| 7 | 4.65 | C24H29O13 | 525.1595 | 525.1633 | Mudanpioside E | 363[M–H–glucosyl]−, 359[M–benzoic acid–OH–CO]−, 277, 121[benzoic acid–H]− | E,EA | [1] |
| 8 | 4.97 | C27H24O18 | 635.0879 | 635.0812 | Trigalloyl-glucose isomer | 483[M–H–galloyl]−, 465[M–H–gallic acid]−, 313[M–H–2galloyl–H2O]−, 169[gallic acid–H]−, 125[gallic acid–H–CO2]− | E,EA | [1] |
| 9 | 5.57 | C23H28O11 | 479.1548 | 479.1605 | Albiflorin * | 449[M–H–CH2O]−, 435[M–H–CO2]− , 357[M–H–benzoic acid]−, 283[M–glucose–OH]−, 121[benzoic acid–H]− | E,EA | [1,23] |
| 10 | 5.98 | C27H24O18 | 635.0879 | 635.0818 | Trigalloyl-glucose isomer | 465[M–H–gallic acid]−, 448, 197[ethyl gallate–H]−, 169[M169[gallic acid–H]−, 125[gallic acid–H–CO2]− | E,EA | [1] |
| 11 | 6.02 | C9H10O5 | 197.0445 | 197.0670 | Ethyl gallate * | 169[M–CH3CH2]−, [M–CH3CH2–OH]−, 125[M–CH3CH2–CO2]− | E,EA | [1] |
| 12 | 6.32 | C23H28O11 | 479.1548 | 479.1591 | Paeoniflorin * | 449[M–H–CH2O]−, 431[M–H–CH2O–H2O]−, 327[M–H–benzoic acid–CH2O]−, 309[M–H–benzoic acid–CH2O–H2O]−, 165[M–H–benzoic acid–CH2O–glucosyl]−, 121[benzoic acid–H]− | E,EA | [22,24] |
| 13 | 6.86 | C23H28O12 | 495.1497 | 495.1548 | Oxypaeoniflorin | 449[M–OH–CH2O]−, 465[M–H–CH2O]−, 327[M–(p-hydroxybenzoyl)–CO]−, 165[M–(p-hydroxybenzoyl)–CO–glucosyl]−, 137[p-hydroxybenzoyl]−, 121[benzoic acid–H]− | E,EA | [1,23] |
| 14 | 8.21 | C41H30O26 | 937.0947 | 937.0488 | Dihydroxymethyl benzoyl tetragalloyl glucose | 787[M–H–dihydroxymethylbenzoyl]−, 615[M–H–2galloyl–H2O]−, 477, 393, 183[ethyl gallate–H]−, 169[gallic acid–H]−, 125[gallic acid–H–CO2]− | E | [1] |
| 15 | 8.39 | C30H32O18 | 787.0989 | 787.0790 | Tetragalloyl glucose | 635[M–H–galloyl]−, 617[M–H–gallic acid]−, 477, 465[M–H–galloyl–gallic acid]−, 393, 301, 169[gallic acid–H]−, 125[gallic acid–H–CO2]− | E,EA | [1] |
| 16 | 8.94 | C30H32O19 | 787.0989 | 787.0802 | Tetragalloyl glucose isomer | 635[M–H–galloyl]−, 617[M–H–gallic acid]−, 477, 465[M–H–galloyl–gallic acid]−, 393 , 331 [M–H–3galloyl]−, 301, 169[gallic acid–H]−, 125[gallic acid–H–CO2]− | E,EA | [1] |
| 17 | 9.45 | C30H32O15 | 631.1658 | 631.1658 | Galloylpaeoniflorin | 449[M–H–galloyl–CH2O]−, 169[gallic acid–H]−, 125[gallic acid–H–CO2]−, 121[benzoic acid–H]− | E,EA | [22,23] |
| 18 | 9.46 | C34H28O22 | 787.0989 | 787.0787 | Tetragalloyl glucose isomer | 635[M–H–galloyl]−, 617[M–H–gallic acid]−, 477, 465[M–H–galloyl–gallic acid]−, 393 , 331[M–H–3galloyl]− 301, 169[gallic acid–H]−, 125[gallic acid–H–CO2]− | E,EA | [1] |
| 19 | 11.48 | C41H32O26 | 939.1098 | 939.0750 | Pentagalloyl glucose * | 787[M–H–galloyl]−, 769[M–H–gallic acid]−, 635[M–H–2galloyl]−, 617[M–H–galloyl–gallic acid]−, 469[M–3H–2galloyl–glucosyl]−, 393, 169[gallic acid–H]−, 125[gallic acid–H–CO2]− | E,EA | [23] |
| 20 | 13.63 | C48H34O30 | 1091.1208 | 1091.0715 | Hexagalloyl glucose | 939[M–H–galloyl]−, 769[M–H–galloyl–gallic acid]−, 469[M–3H–3galloyl–glucosyl]−, 393, 169[gallic acid–H]−, 125[gallic acid–H–CO2]− | E,EA | [1] |
| 21 | 14.25 | C48H34O31 | 1091.1208 | 1091.0712 | Hexagalloyl glucose isomer | 769[M–H–galloyl–gallic acid]−, 469[M–3H–3galloyl–glucosyl]−, 393, 197[ethyl gallate–H]−, 169[gallic acid–H]−, 125[gallic acid–H–CO2]− | E,EA | [1] |
| 22 | 16.12 | C48H34O33 | 1091.1208 | 1091.0708 | Hexagalloyl glucose isomer | 769[M–H–galloyl–gallic acid]−, 469[M–3H–3galloyl–glucosyl]−, 393, 197[ethyl gallate–H]−, 169[gallic acid–H]−, 125[gallic acid–H–CO2]− | E,EA | [1] |
| 23 | 16.98 | C30H32O13 | 599.1765 | 599.1752 | Mudanpioside C | 477[M–benzoic acid]−, 257 , 137[p-hydroxybenzoyl]−, 121[benzoic acid–H]− | E,EA | [1] |
| 24 | 17.42 | C31H34O14 | 629.1870 | 629.1805 | Mudanpioside J | 599[M–H–CH2O]−, 257, 121[benzoic acid–H]− | EA | [1,22] |
| 25 | 19.34 | C25H32O11 | 507.1861 | 507.1892 | 4-O-Ethylpaeoniflorin | 385[M–benzoic acid]−, 121[benzoic acid–H]−, 103[benzoic acid–H–H2O]− | EA | [4,7] |
| 26 | 20.18 | C30H32O13 | 599.1765 | 599.1741 | Benzoyloxypaeoniflorin | 522, 447[M–H–CH2O–benzoic acid]−, 137[p-hydroxybenzoyl]−, 121[benzoic acid–H]− | EA | [1,24] |
| 27 | 30.63 | C30H31O12 | 583.1816 | 583.1792 | Benzoylpaeoniflorin * | 553[M–H–CH2O]−, 431[M–H–benzoic acid–CH2O]− 165[M–H–benzoic acid–benzoyl–CH2O–glucosyl]−, 121[benzoic acid–H]− | E,EA | [24] |
| Compounds | IC50 | Compound Classified |
|---|---|---|
| Paeoniflorin | 210.786 µM | Monoterpene glycosides |
| Albiflorin | 167.115 µM | Monoterpene glycosides |
| Benzoylpaeoniflorin | 143.701 µM | Monoterpene glycosides |
| Gallic acid | 373.289 µM | Phenols |
| pentagalloylglucose | 62.671 µM | Tannins |
| Methyl gallate | 338.285 µM | Phenols |
| Ethyl gallate | 274.195 µM | Phenols |
| the ethyl acetate fraction (EA) | 75.932 µg/mL | |
| the ethanol fraction (E) | 83.550 µg/mL | |
| Oseltamivir acid (Postive control) | 281.308 µM (79.990 µg/mL) |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Yang, X.; Huang, L. Anti-Influenza Virus Activity and Constituents Characterization of Paeonia delavayi Extracts. Molecules 2016, 21, 1133. https://doi.org/10.3390/molecules21091133
Li J, Yang X, Huang L. Anti-Influenza Virus Activity and Constituents Characterization of Paeonia delavayi Extracts. Molecules. 2016; 21(9):1133. https://doi.org/10.3390/molecules21091133
Chicago/Turabian StyleLi, Jinhua, Xianying Yang, and Linfang Huang. 2016. "Anti-Influenza Virus Activity and Constituents Characterization of Paeonia delavayi Extracts" Molecules 21, no. 9: 1133. https://doi.org/10.3390/molecules21091133