Immobilization of Chlamydomonas reinhardtii CLH1 on APTES-Coated Magnetic Iron Oxide Nanoparticles and Its Potential in the Production of Chlorophyll Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of MIONPs and APTES-Coated MIONPs
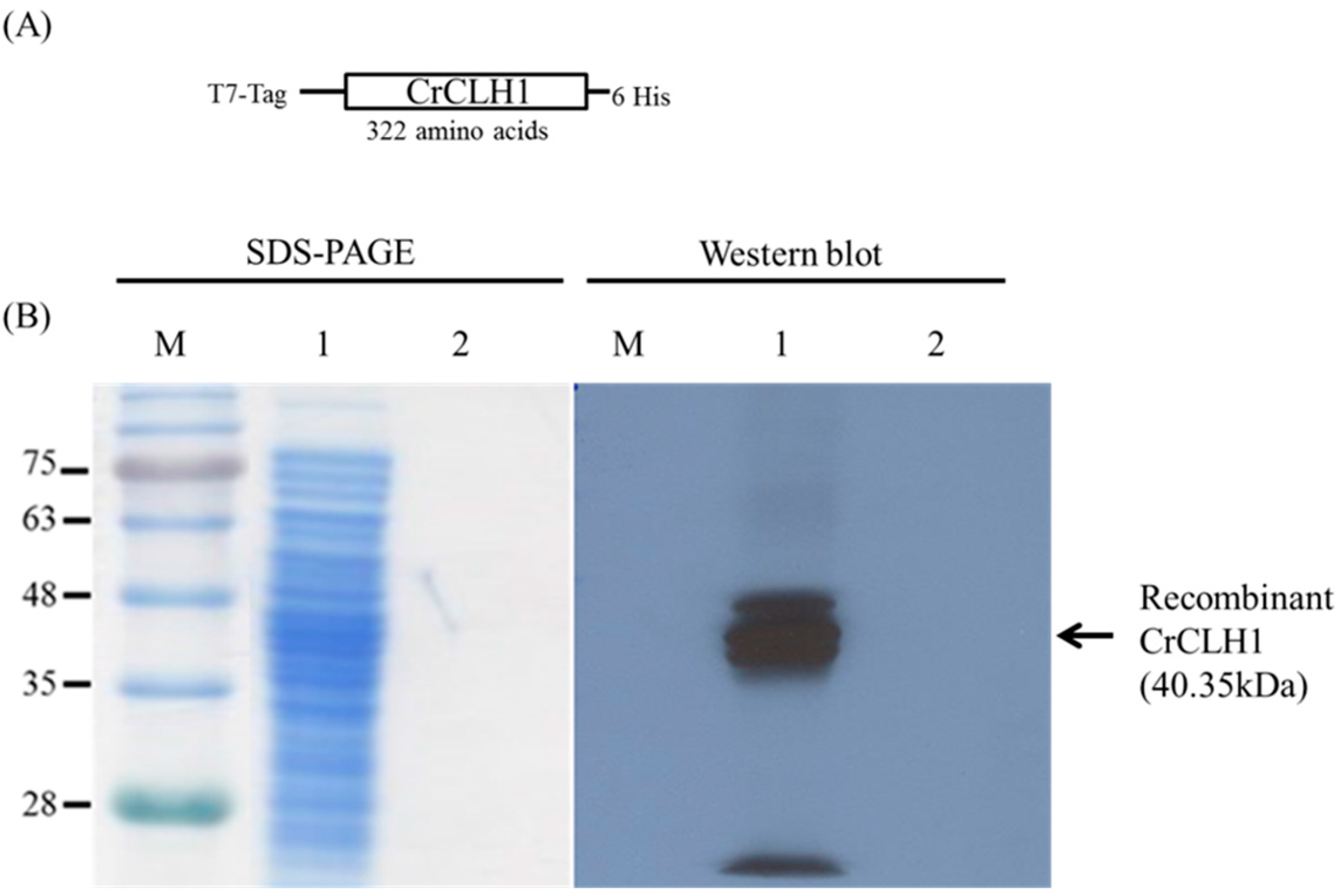
2.2. Immobilization of Recombinant CrCLH1
2.3. Immobilization Yield and Enzyme Capacity
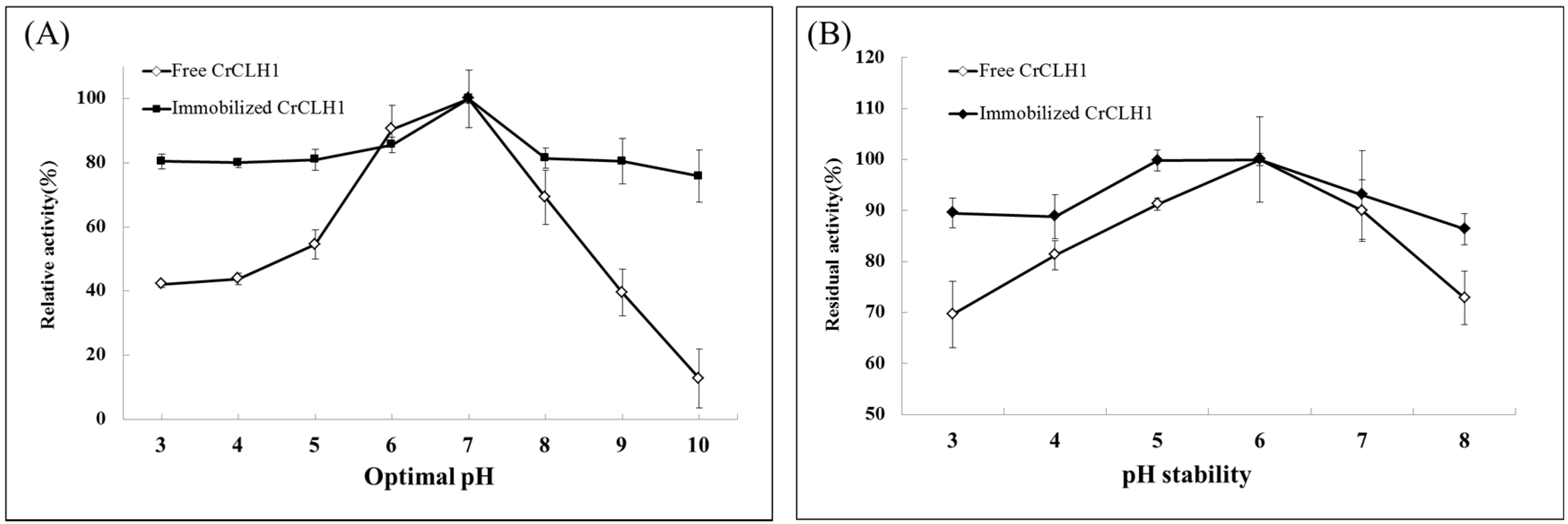
2.4. Effects of Optimal pH and pH Stability of the Free and Immobilized Enzymes
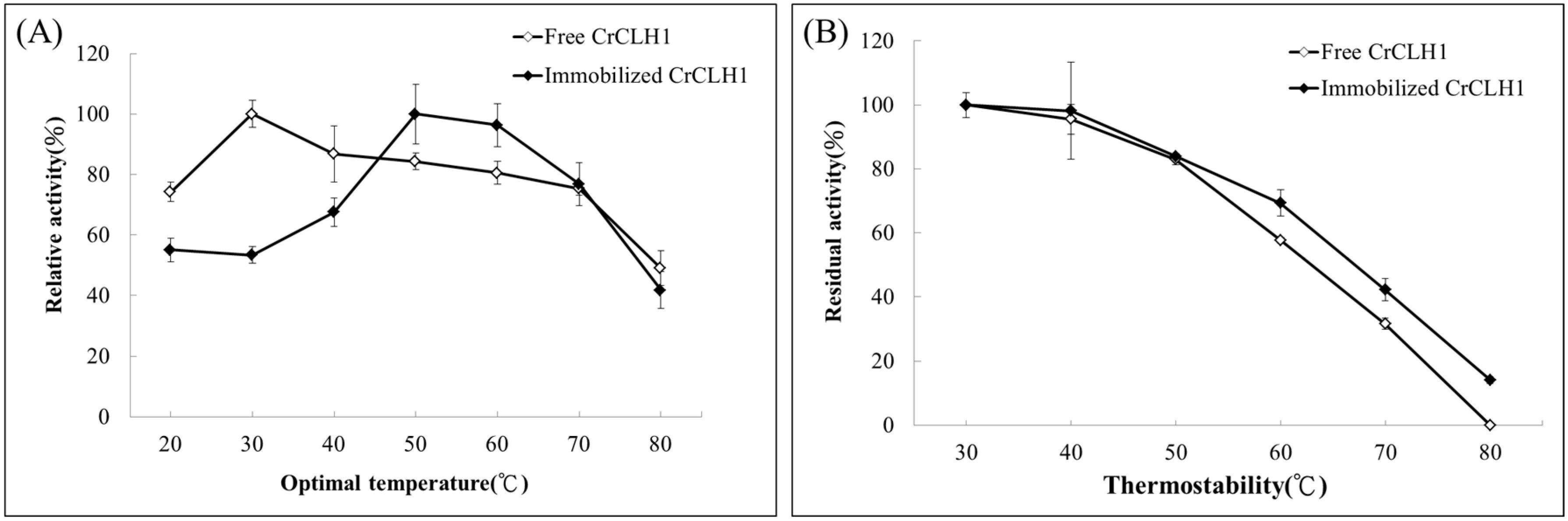
2.5. Optimal Temperature and Thermostability of Free and Immobilized Enzymes
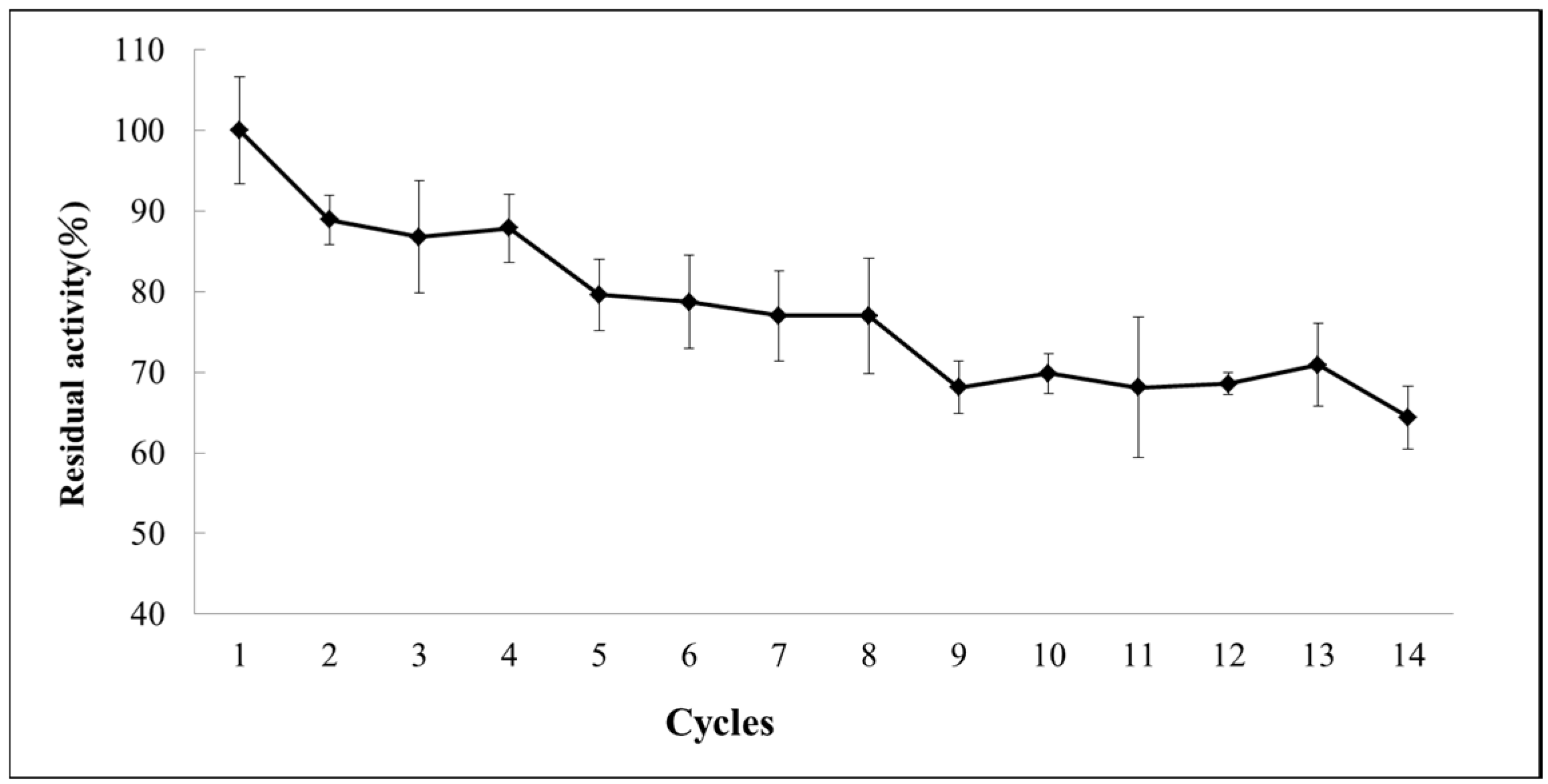
2.6. Operational Stability
3. Materials and Methods
3.1. Materials
3.2. Synthesis of MIONPs
3.3. APTES-Coated MIONPs
3.4. Characterization of MIONPs and APTES-Coated MIONPs
3.5. Synthesis of Recombinant CrCLH1-APTES-Coated MIONPs
3.6. Preparation of Enzymes
3.7. Chlase Assay of the Immobilized Enzyme
3.8. Biochemical Analyses of the Free and Immobilized Enzymes
3.9. Operational Stability
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tsuchiya, T.; Suzuki, T.; Yamada, T.; Shimada, H.; Masuda, T.; Ohta, H.; Takamiya, K. Chlorophyllase as a serine hydrolase: Identification of a putative catalytic triad. Plant Cell Physiol. 2003, 44, 96–101. [Google Scholar]
- Hörtensteiner, S.; Kräutler, B. Chlorophyll breakdown in higher plants. Biochim. Biophys. Acta Bioenergy 2011, 1807, 977–988. [Google Scholar]
- Kariola, T.; Brader, G.; Li, J.; Palva, E.T. Chlorophyllase 1, a damage control enzyme, affects the balance between defense pathways in plants. Plant Cell 2005, 17, 282–294. [Google Scholar]
- Lee, G.C.; Chepyshko, H.; Chen, H.H.; Chu, C.C.; Chou, Y.F.; Akoh, C.C.; Shaw, J.F. Genes and biochemical characterization of three novel chlorophyllaseisozymes from Brassica oleracea. J. Agric. Food Chem. 2010, 58, 8651–8657. [Google Scholar]
- Trebitsh, T.; Goldschmidt, E.E.; Riov, J. Ethylene induces de novo synthesis of chlorophyllase, a chlorophyll degrading enzyme, in Citrus fruit peel. Proc. Natl. Acad. Sci. USA 1993, 90, 9441–9445. [Google Scholar]
- Matile, P.; Schellenberg, M.; Vicentini, F. Localization of chlorophyllase in the chloroplast envelope. Planta 1997, 201, 96–99. [Google Scholar]
- Tsuchiya, T.; Ohta, H.; Okawa, K.; Iwamatsu, A.; Shimada, H.; Masuda, T.; Takamiya, K. Cloning of chlorophyllase, the key enzyme in chlorophyll degradation: finding of a lipase motif and the induction by methyl jasmonate. Proc. Natl. Acad. Sci. USA 1999, 96, 15362–15367. [Google Scholar]
- Takamiya, K.I.; Tsuchiya, T.; Ohta, H. Degradation pathway(s) of chlorophyll: What has gene cloning revealed? Trends Plant Sci. 2000, 5, 426–431. [Google Scholar]
- Arkus, K.A.; Cahoon, E.B.; Jez, J.M. Mechanistic analysis of wheat chlorophyllase. Arch. Biochem. Biophys. 2005, 438, 146–155. [Google Scholar]
- Okazawa, A.; Tango, L.; Itoh, Y.; Fukusaki, E.; Kobayashi, A. Characterization and subcellular localization of chlorophyllase from Ginkgo biloba. Z. Naturforsch. C 2006, 61, 111–117. [Google Scholar]
- Azoulay Shemer, T.; Harpaz-Saad, S.; Belausov, E.; Lovat, N.; Krokhin, O.; Spicer, V.; Standing, K.G.; Goldschmidt, E.E.; Eyal, Y. Citrus chlorophyllase dynamics at ethylene-induced fruit color-break: A study of chlorophyllase expression, posttranslational processing kinetics, and in situ intracellular localization. Plant Physiol. 2008, 148, 108–118. [Google Scholar]
- Gupta, S.; Gupta, S.M.; Sane, A.P.; Kumar, N. Chlorophyllase in Piper betle L. has a role in chlorophyll homeostasis and senescence dependent chlorophyll breakdown. Mol. Biol. Rep. 2012, 39, 7133–7142. [Google Scholar] [CrossRef]
- Böger, P. Chlorophyllase of Chlorella vulgaris Beijerinck. Phytochemistry 1965, 4, 435–444. [Google Scholar]
- Tamai, H.; Shioi, Y.; Sasa, T. Studies on chlorophyllase of Chlorella protothecoides IV. Some properties of the purified enzyme. Plant Cell Physiol. 1979, 20, 1141–1145. [Google Scholar]
- Nishiyama, Y.; Kitamura, M.; Tamura, S.; Watanabe, T. Purification and substrate specificity of chlorophyllase from Chlorella regularis. Chem. Lett. 1994, 23, 69–72. [Google Scholar]
- Khalyfa, A.; Kermasha, S.; Marsot, P.; Goetghebeur, M. Purification and characterization of chlorophyllase from alga Phaeodactylumtricornutum by preparative native electrophoresis. Appl. Biochem. Biotechnol. 1995, 53, 11–27. [Google Scholar] [CrossRef]
- Gaffar, R.; Kermasha, S.; Bisakowski, B. Biocatalysis of immobilized chlorophyllase in a ternary micellar system. J. Biotechnol. 1999, 75, 45–55. [Google Scholar]
- Chou, Y.L.; Ko, C.Y.; Yen, C.C.; Chen, L.F.O.; Shaw, J.F. A novel recombinant chlorophyllase1 from Chlamydomonas reinhardtii for the production of chlorophyllide derivatives. J. Agric. Food Chem. 2015, 63, 9496–9503. [Google Scholar]
- Chou, Y.L.; Lee, Y.L.; Yen, C.C.; Chen, L.F.O.; Lee, L.C.; Shaw, J.F. A novel recombinant chlorophyllase from cyanobacterium Cyanothece sp. ATCC 51142 for the production of bacteriochlorophyllide a. Biotechnol. Appl. Biochem. 2015. [Google Scholar] [CrossRef]
- Karboune, S.; Neufeld, R.; Kermasha, S. Immobilization and biocatalysis of chlorophyllase in selected organic solvent systems. J. Biotechnol. 2005, 120, 273–283. [Google Scholar]
- Hsu, C.Y.; Chen, Y.H.; Chao, P.Y.; Chen, C.M.; Hsieh, L.L.; Hu, S.P. Naturally occurring chlorophyll derivatives inhibit aflatoxin B1-DNA adduct formation in hepatoma cells. Mutat. Res. 2008, 657, 98–104. [Google Scholar] [PubMed]
- Hsu, C.Y.; Yang, C.M.; Chen, C.M.; Chao, P.Y.; Hu, S.P. Effects of chlorophyll-related compounds on hydrogen peroxide induced DNA damage within human lymphocytes. J. Agric. Food Chem. 2005, 53, 2746–2750. [Google Scholar]
- Guo, H.; Pan, X.; Mao, R.; Zhang, X.; Wang, L.; Lu, X.; Chang, J.; Guo, J.T.; Passic, S.; Krebs, F.C.; et al. Alkylated porphyrins have broad antiviral activity against hepadnaviruses, flaviviruses, filoviruses, and arenaviruses. Antimicrob. Agents Chemother. 2011, 55, 478–486. [Google Scholar]
- You, H.; Yoon, H.E.; Jeong, P.H.; Ko, H.; Yoon, J.H.; Kim, Y.C. Pheophorbide-a conjugates with cancer-targeting moieties for targeted photodynamic cancer therapy. Bioorg. Med. Chem. 2015, 23, 1453–1462. [Google Scholar]
- Della Pietra, E.; Simonella, F.; Bonavida, B.; Xodo, L.E.; Rapozzi, V. Repeated sub-optimal photodynamic treatments with pheophorbidea induce an epithelial mesenchymal transition in prostate cancer cells via nitric oxide. Nitric Oxide 2015, 45, 43–53. [Google Scholar]
- Azizullah, A.; Rehman, Z.U.; Ali, I.; Murad, W.; Muhammad, N.; Ullah, W.; Häder, D.P. Chlorophyll derivatives can be an efficient weapon in the fight against dengue. Parasitol. Res. 2014, 113, 4321–4326. [Google Scholar]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar]
- Kouassi, G.K.; Irudayaraj, J.; McCarty, G. Examination of Cholesterol oxidase attachment to magnetic nanoparticles. J. Nanobiotechnol. 2005, 20, 1–9. [Google Scholar]
- Johnson, A.K.; Zawadzka, A.M.; Deobald, L.A.; Crawford, R.L.; Paszczynski, A.J. Novel method for immobilization of enzymes to magnetic nanoparticles. J. Nanopart. Res. 2008, 10, 1009–1025. [Google Scholar]
- Solanki, K.; Gupta, M.N. Simultaneous purification and immobilization of Candida rugosa lipase on superparamagnetic Fe3O4 nanoparticles for catalyzing transesterification reactions. New J. Chem. 2011, 35, 2551–2556. [Google Scholar]
- Chen, Y.Y.; Tsai, M.G.; Chi, M.C.; Wang, T.F.; Lin, L.L. Covalent immobilization of Bacillus licheniformis γ-Glutamyltranspeptidase on aldehyde-functionalized magnetic nanoparticles. Int. J. Mol. Sci. 2013, 14, 4613–4628. [Google Scholar]
- Yang, C.H.; Yen, C.C.; Jheng, J.J.; Wang, C.Y.; Chen, S.S.; Huang, P.Y.; Huang, K.S.; Shaw, J.F. Immobilization of Brassica oleracea chlorophyllase 1 (BoCLH1) and Candida rugosa lipase (CRL) in magnetic alginate beads: An enzymatic evaluation in the corresponding proteins. Molecules 2014, 19, 11800–11815. [Google Scholar] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernández-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar]
- Ma, M.; Zhang, Y.; Yu, W.; Shen, H.Y.; Zhang, H.Q.; Gu, N. Preparation and characterization of magnetite nanoparticles coated by amino silane. Colloid Surf. A Physicochem. Eng. Asp. 2003, 212, 219–226. [Google Scholar]
- Chen, Y.; Xin, Y.; Yang, H.; Zhang, L.; Zhang, Y.; Xia, X.; Tong, Y.; Wang, W. Immobilization and stabilization of cholesterol oxidase on modified sepharose particles. Int. J. Biol. Macromol. 2013, 56, 6–13. [Google Scholar] [PubMed]
- Ariga, K.; Kawakami, K.; Ebara, M.; Kotsuchibashi, Y.; Ji, Q.; Hill, J.P. Bioinspired nanoarchitectonics as emerging drug delivery systems. New J. Chem. 2014, 38, 5149–5163. [Google Scholar]
- Nakanishi, W.; Minami, K.; Shrestha, L.K.; Ji, Q.; Hill, J.P.; Ariga, K. Bioactive nanocarbon assemblies: Nanoarchitectonics and applications. Nano Today 2014, 9, 378–394. [Google Scholar]
- Ariga, K.; Minami, K.; Ebara, M.; Nakanishi, J. What are the emerging concepts and challenges in NANO? Nanoarchitectonics, hand-operating nanotechnology and mechanobiology. Polym. J. 2016, 48, 371–389. [Google Scholar]
- Babazadeh, M.; Hosseinzadeh Khanmiri, R.; Abolhasani, J.; Ghorbani-Kalhor, E.; Hassanpour, A. Synthesis and application of a novel functionalized magnetic metal-organic framework sorbent for determination of heavy metal ions in fish samples. Bull. Chem. Soc. Jpn. 2015, 88, 871–879. [Google Scholar]
- Ariga, K.; Ji, Q.; Mori, T.; Naito, M.; Yamauchi, Y.; Abe, H.; Hill, J.P. Enzyme nanoarchitectonics: Organization and device application. Chem. Soc. Rev. 2013, 7, 6322–6345. [Google Scholar]
- Chou, Y.L.; Ko, C.Y.; Chen, L.F.; Yen, C.C.; Shaw, J.F. Purification and immobilization of the recombinant Brassica oleracea Chlorophyllase 1 (BoCLH1) on DIAION®CR11 as potential biocatalyst for the production of chlorophyllide and phytol. Molecules 2015, 20, 3744–3757. [Google Scholar]
- Brady, D.; Jordaan, J. Advances in enzyme immobilisation. Biotechnol. Lett. 2009, 31, 1639–1650. [Google Scholar] [PubMed]
- Hwang, E.T.; Gu, M.B. Enzyme stabilization by nano/microsized hybrid materials. Eng. Life Sci. 2013, 13, 49–61. [Google Scholar]
- Eş, I.; Vieira, J.D.; Amaral, A.C. Principles, techniques, and applications of biocatalyst immobilization for industrial application. Appl. Microbiol. Biotechnol. 2015, 99, 2065–2082. [Google Scholar]
- Pitcher, W.H., Jr. Design and operation of immobilized enzyme reactors. Adv. Biochem. Eng. Biotechnol. 1978, 10, 1–26. [Google Scholar]
- Kang, Y.S.; Risbud, S.; Rabolt, J.F.; Stroeve, P. Synthesis and characterization of nanometer-size Fe3O4 and γ-Fe2O3 particles. Chem. Mater. 1996, 8, 2209–2211. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.


| Samples | Amount of Protein Binding (mg/g Gel) | Immobilization Yield (% b) | Enzymatic Activity (U c/g Gel) | Specific Activity of Immobilized Enzymes (U/mg Protein) | Specific Activity of Non-Immobilized Enzymes (U/mg Protein) |
|---|---|---|---|---|---|
| Free CrCLH1 | 0 | ND a | ND | ND | 186 ± 22 |
| Immobilized CrCLH1 | 32.1 ± 0.3 | 98.99 ± 0.91 | 722.3 ± 50.3 | 131.0 ± 9.1 | 4.1 ± 0.3 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, C.-C.; Chuang, Y.-C.; Ko, C.-Y.; Chen, L.-F.O.; Chen, S.-S.; Lin, C.-J.; Chou, Y.-L.; Shaw, J.-F. Immobilization of Chlamydomonas reinhardtii CLH1 on APTES-Coated Magnetic Iron Oxide Nanoparticles and Its Potential in the Production of Chlorophyll Derivatives. Molecules 2016, 21, 972. https://doi.org/10.3390/molecules21080972
Yen C-C, Chuang Y-C, Ko C-Y, Chen L-FO, Chen S-S, Lin C-J, Chou Y-L, Shaw J-F. Immobilization of Chlamydomonas reinhardtii CLH1 on APTES-Coated Magnetic Iron Oxide Nanoparticles and Its Potential in the Production of Chlorophyll Derivatives. Molecules. 2016; 21(8):972. https://doi.org/10.3390/molecules21080972
Chicago/Turabian StyleYen, Chih-Chung, Yao-Chen Chuang, Chia-Yun Ko, Long-Fang O. Chen, Sheau-Shyang Chen, Chia-Jung Lin, Yi-Li Chou, and Jei-Fu Shaw. 2016. "Immobilization of Chlamydomonas reinhardtii CLH1 on APTES-Coated Magnetic Iron Oxide Nanoparticles and Its Potential in the Production of Chlorophyll Derivatives" Molecules 21, no. 8: 972. https://doi.org/10.3390/molecules21080972
APA StyleYen, C.-C., Chuang, Y.-C., Ko, C.-Y., Chen, L.-F. O., Chen, S.-S., Lin, C.-J., Chou, Y.-L., & Shaw, J.-F. (2016). Immobilization of Chlamydomonas reinhardtii CLH1 on APTES-Coated Magnetic Iron Oxide Nanoparticles and Its Potential in the Production of Chlorophyll Derivatives. Molecules, 21(8), 972. https://doi.org/10.3390/molecules21080972







