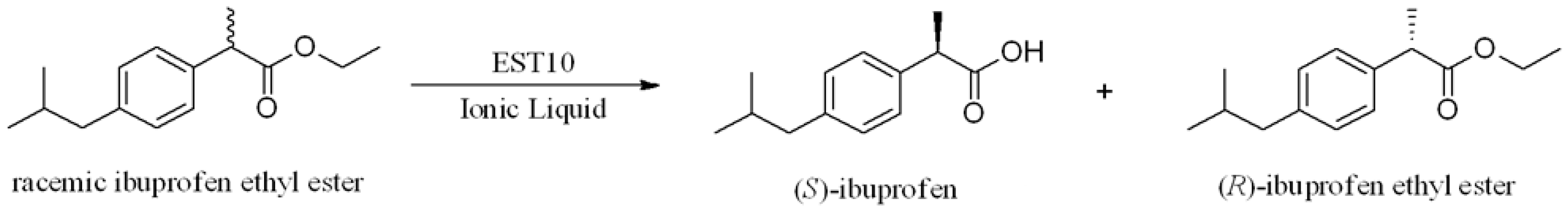
Enzymatic Hydrolytic Resolution of Racemic Ibuprofen Ethyl Ester Using an Ionic Liquid as Cosolvent
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of ILs
2.2. Conformational Studies of EST
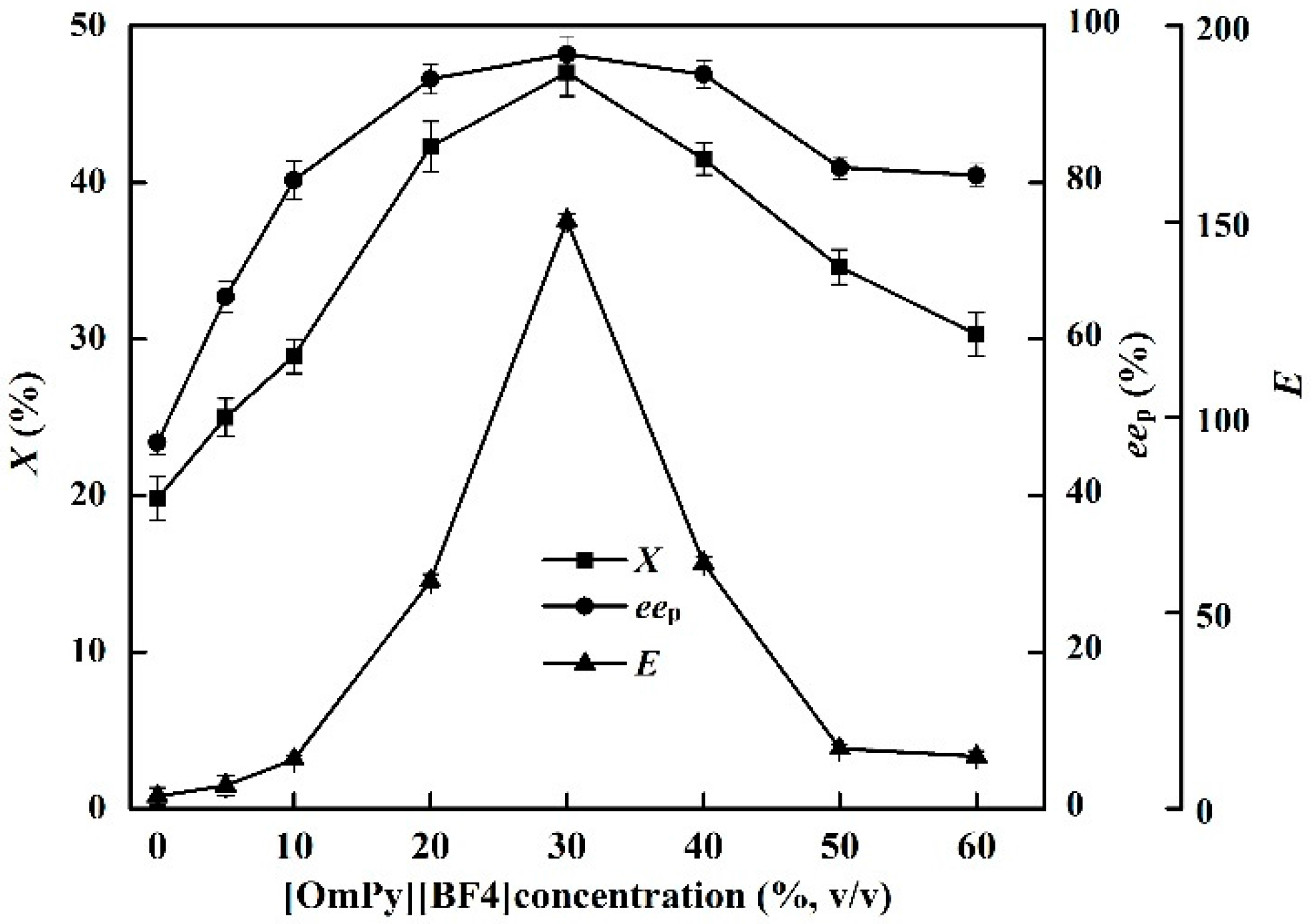
2.3. Effects of IL Concentration
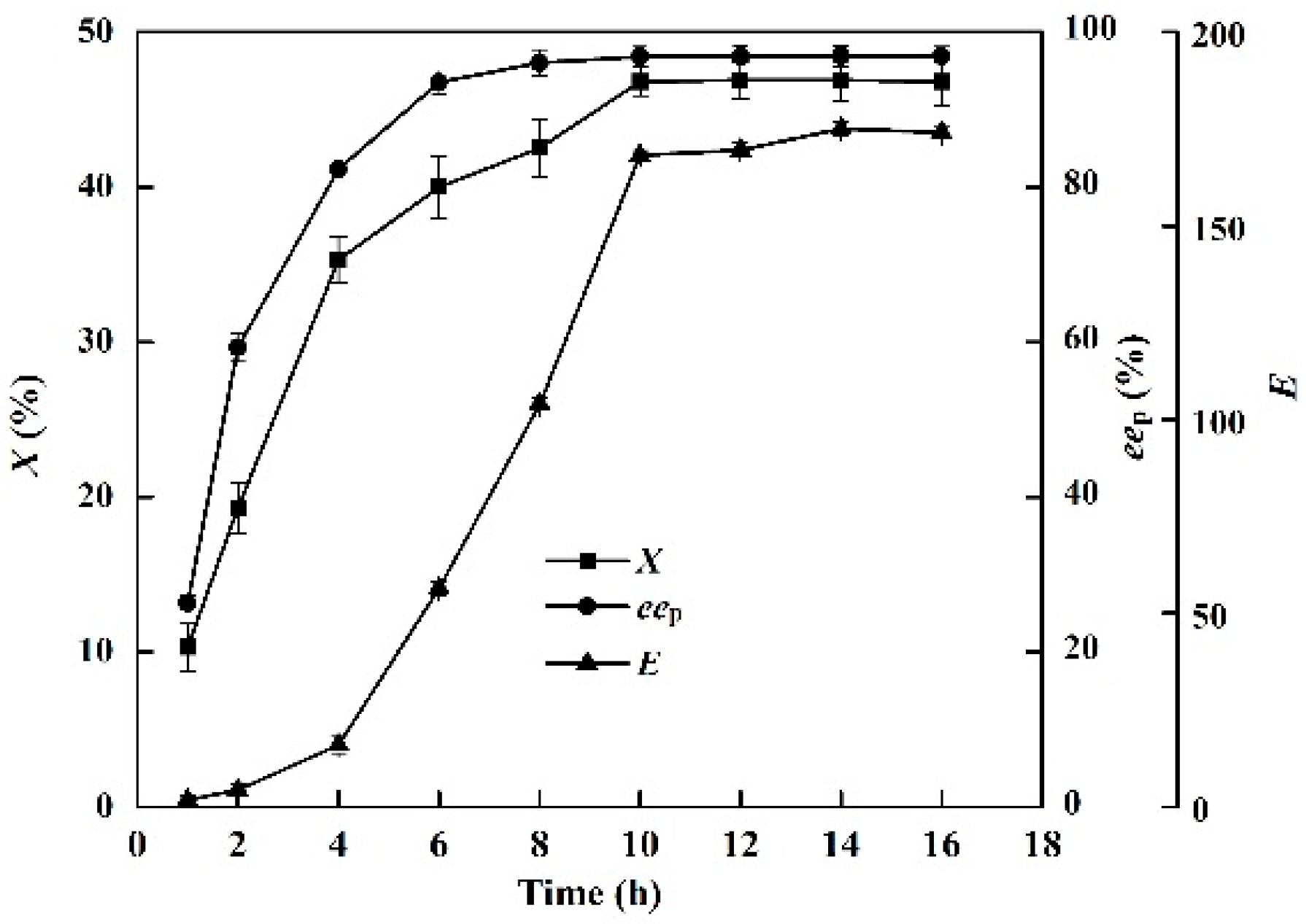
2.4. Effects of Time
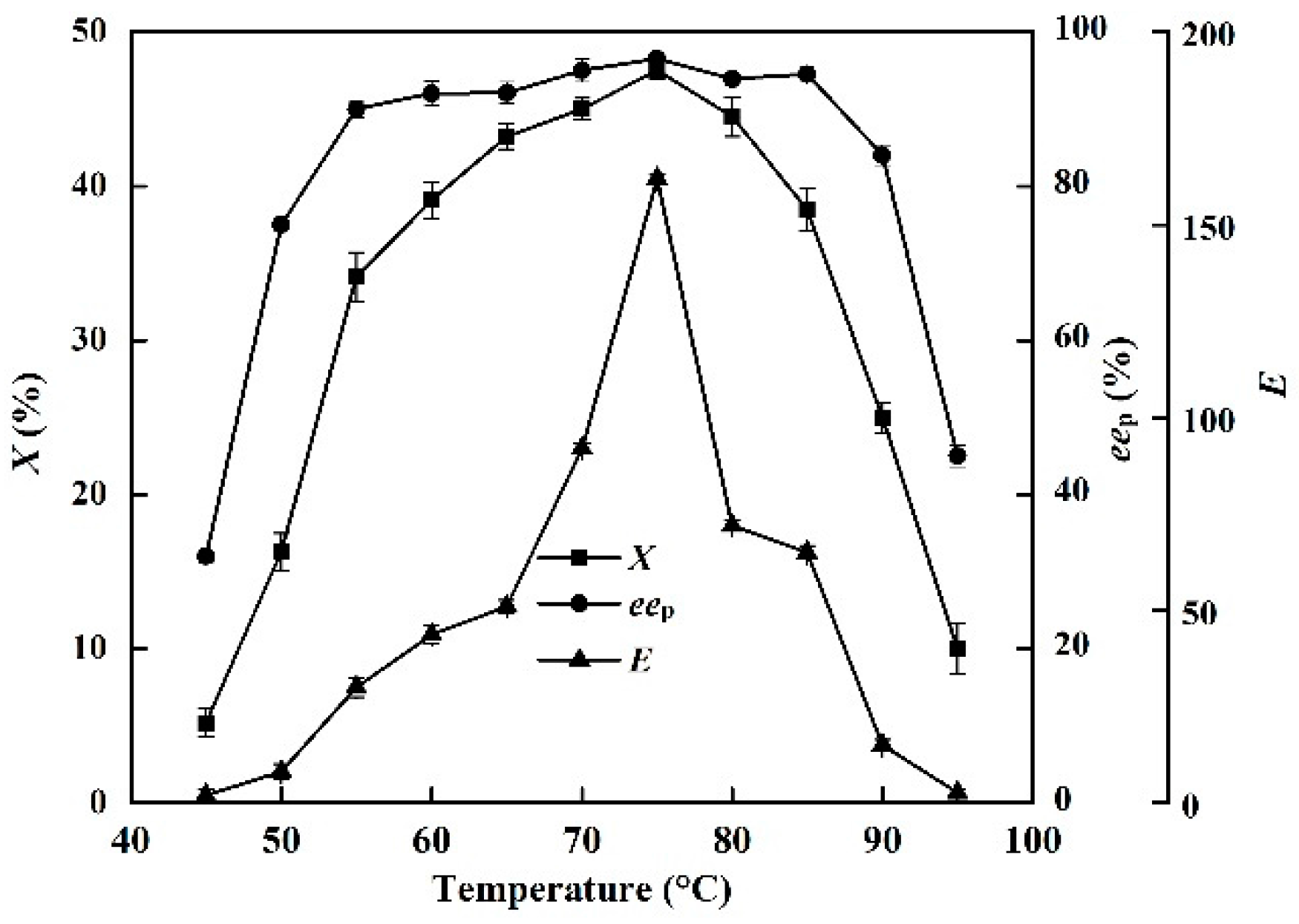
2.5. Effects of Temperature
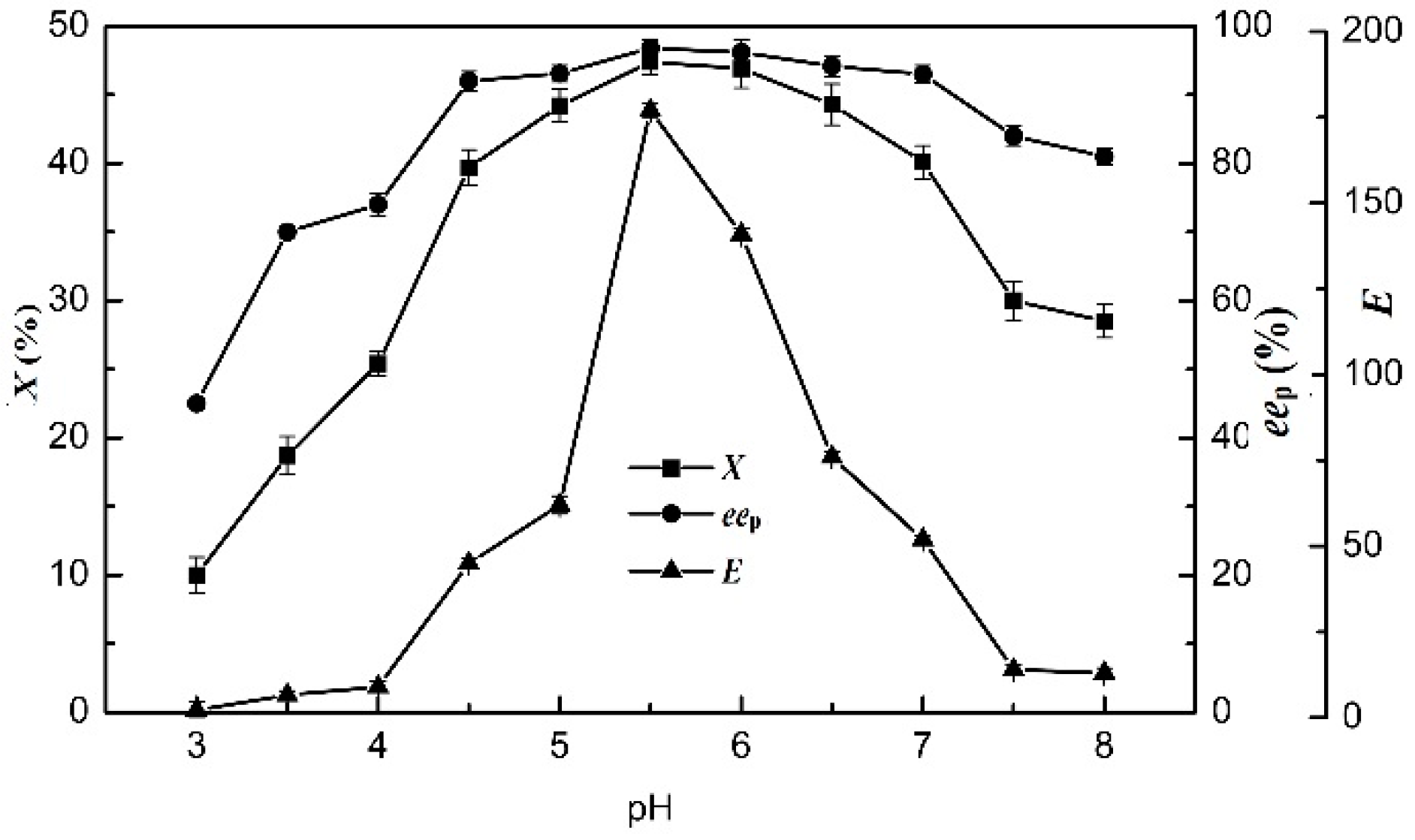
2.6. Effects of pH
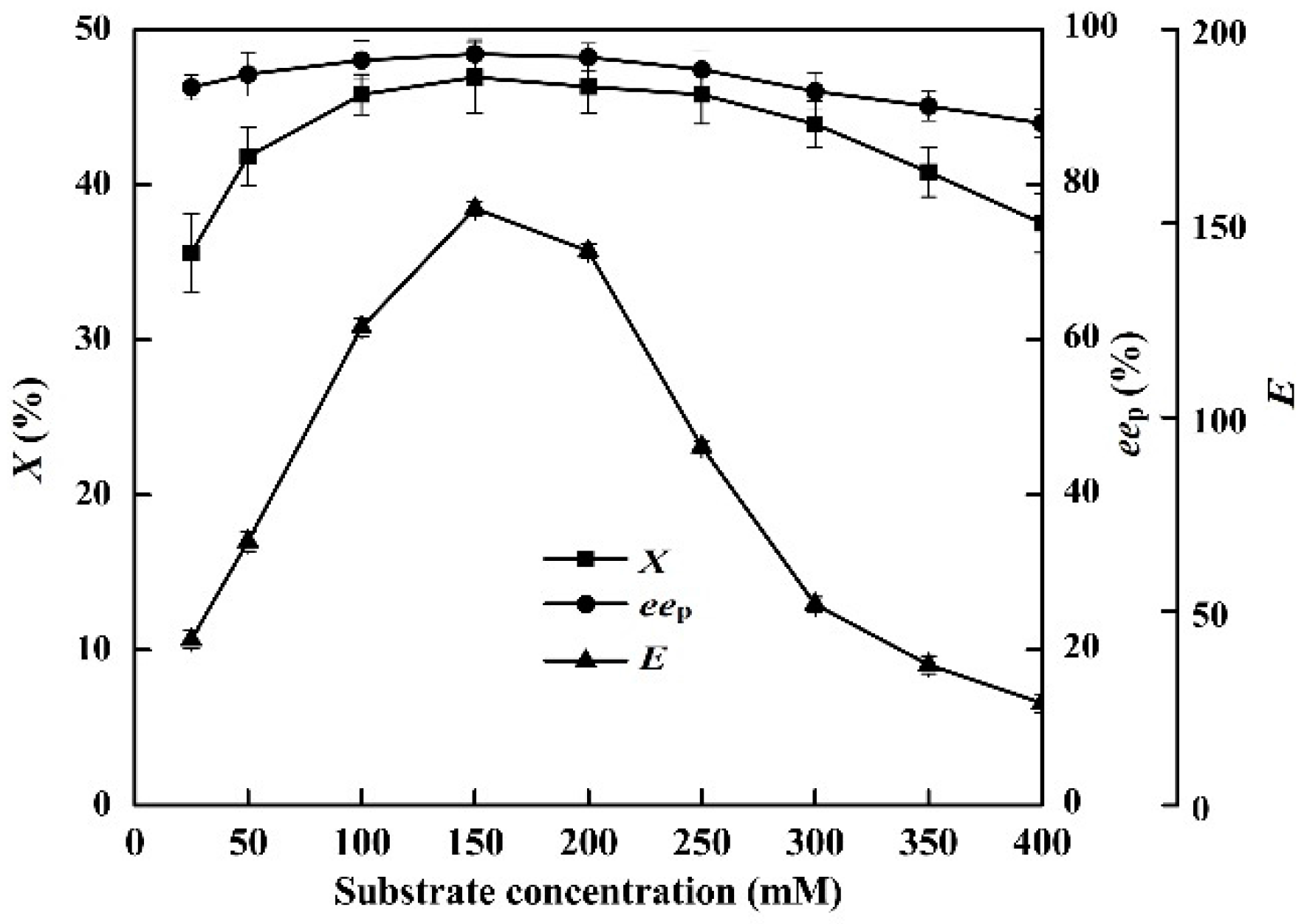
2.7. Effects of Substrate Concentration
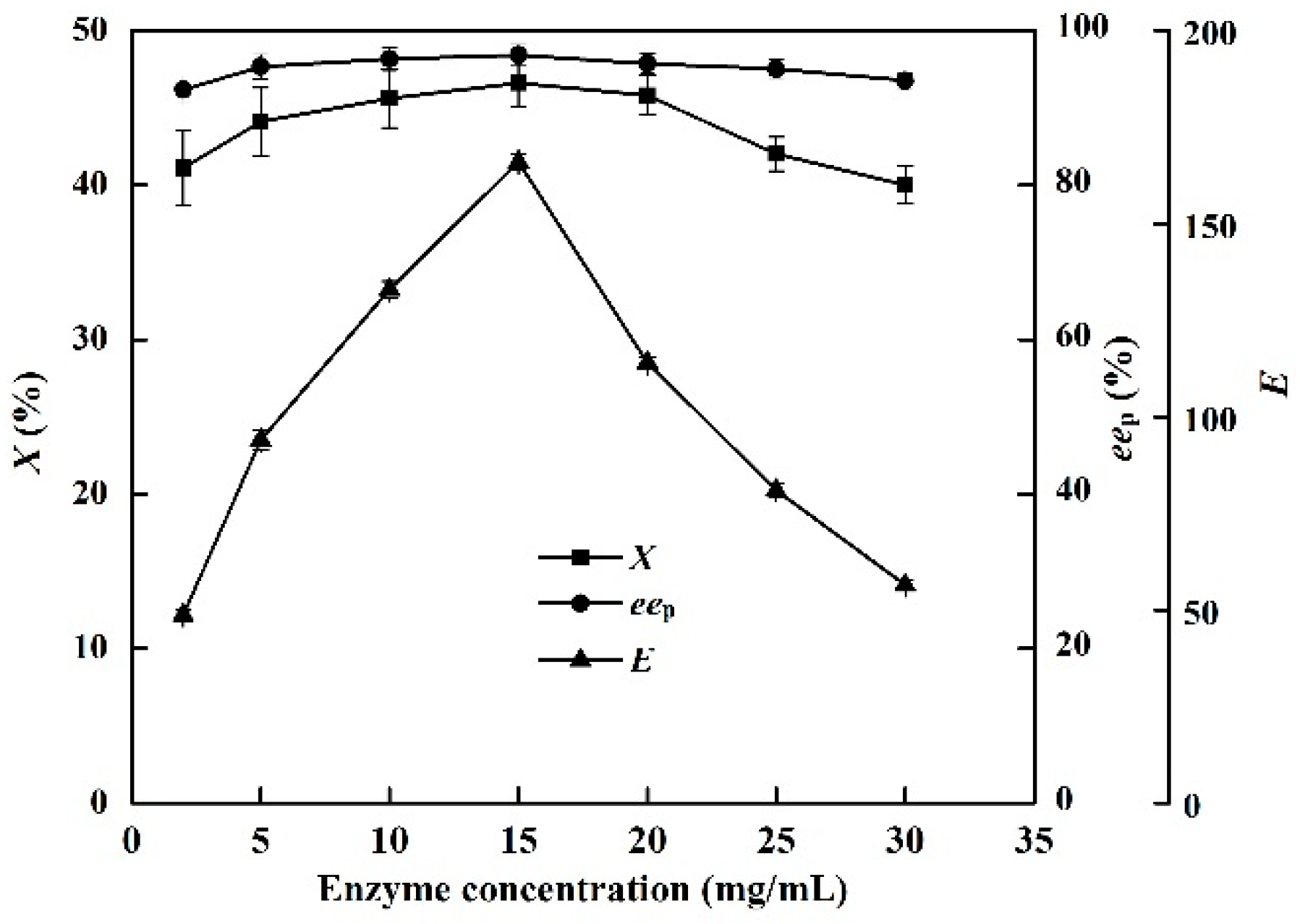
2.8. Effects of Enzyme Concentration
3. Experimental Section
3.1. Chemicals
3.2. Enzyme Assay
3.3. HPLC Analysis
3.4. CD Spectroscopy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shanbhag, V.; Crider, R.; Gokhale, A.M.; Harpalani, R.; Dick, R.M. Ester and amide prodrugs of ibuprofen and naproxen: Synthesis, anti-inflammatory activity, and gastrointestinal toxicity. J. Pharmaceut. Sci. 1992, 81, 149–154. [Google Scholar] [CrossRef]
- Sánchez, A.; Valero, F.; Lafuente, J.; Solá, C. Highly enantioselective esterification of racemic ibuprofen in a packed bed reactor using immobilized Rhizomucor miehei lipase. Enzyme Microb. Technol. 2000, 27, 157–166. [Google Scholar] [CrossRef]
- Kato, K.; Gong, Y.; Saito, T.; Kimoto, H. Efficient preparation of optically active ketoprofen by Mucor javanicus lipase immobilized on an inorganic suppor. J. Biosci. Bioeng. 2000, 90, 157–166. [Google Scholar] [CrossRef]
- Chávez-Flores, D.; Salvador, J.M. Commercially viable resolution of ibuprofen. Biotechnol. J. 2009, 4, 1222–1224. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Mohammadi, M.; Habibi, Z. Enantioselective resolution of racemic ibuprofen esters using different lipases immobilized on octyl sepharose. J. Mol. Catal. B Enzym. 2014, 104, 87–94. [Google Scholar] [CrossRef]
- Mustranta, A. Use of lipase in the resolution of racemic ibuprofen. Appl. Microbiol. Biotechnol. 1992, 38, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Siódmiak, T.; Ziegler-Borowska, M.; Marszałł, M.P. Lipase-immobilized magnetic chitosan nanoparticles for kinetic resolution of (R,S)-ibuprofen. J. Mol. Catal. B Enzym. 2013, 94, 7–14. [Google Scholar] [CrossRef]
- Quijano, G.; Couvert, A.; Amrane, A. Ionic liquids: Applications and future trends in bioreactor technology. Bioresour. Technol. 2010, 101, 8923–8930. [Google Scholar] [CrossRef] [PubMed]
- Bekhouche, M.; Blum, L.J.; Doumeche, B. Contribution of dynamic and static quenchers for the study of protein conformation in ionic liquids by steady-state fluorescence spectroscopy. J. Phys. Chem. B. 2012, 116, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Meng, X.H.; Zhou, H.; Liu, Y.; Secundo, F.; Liu, Y. Enzyme Stability and Activity in Non-Aqueous Reaction Systems: A Mini Review. Catalysts 2016, 32. [Google Scholar] [CrossRef]
- Patel, R.; Kumari, M.; Khan, A.B. Recent advances in the applications of ionic liquids in protein stability and activity: A review. Appl. Biochem. Biotechnol. 2014, 172, 3701–3720. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H. Methods for stabilizing and activating enzymes in ionic liquids—A review. J. Chem. Technol. Biotechnol. 2010, 85, 891–907. [Google Scholar] [CrossRef]
- Madeira Lau, R.; Van Rantwijk, F.; Seddon, K.R.; Sheldon, R.A. Lipase-catalyzed reactions in ionic liquids. Org. Lett. 2000, 2, 4189–4191. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Song, B.; Choi, M.Y.; Kim, M.J. Biocatalysis in ionic liquids: Markedly enhanced enantioselectivity of lipase. Org. Lett. 2001, 3, 1507–1509. [Google Scholar] [CrossRef] [PubMed]
- Husum, T.L.; Jorgensen, C.T.; Christensen, M.W.; Kirk, O. Enzyme catalysed synthesis in ambient temperature ionic liquids. Biocatal. Biotrans. 2001, 19, 331–338. [Google Scholar] [CrossRef]
- Park, S.; Kazlauskas, R.J. Improved preparation and use of room-temperature ionic liquids in lipase-catalyzed enantio- and regioselective acylations. J. Org. Chem. 2001, 66, 8395–8840. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, G.X.; Yu, L.; Wu, F.A.; Guo, X.J. Enhancement of the selective enzymatic biotransformation of rutin to isoquercitrin using an ionic liquid as a co-solvent. Bioresour. Technol. 2013, 128, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Mutschler, J.; Rausis, T.; Bourgeois, J.M.; Bastian, C.; Zufferey, D.; Mohrenz, I.V.; Fischer, F. Ionic liquid-coated immobilized lipase for the synthesis of methylglucose fatty acid esters. Green Chem. 2009, 11, 1793–1800. [Google Scholar] [CrossRef]
- Zhao, D.T.; Xun, N.E.; Wang, J.X.; Wang, R.; Wei, X.F. Enantioselective esterification of ibuprofen by a novel thermophilic biocatalyst: APE1547. Biotechnol. Bioproc. E 2011, 16, 638–644. [Google Scholar] [CrossRef]
- Wei, T.; Feng, S.X.; Mao, D.B.; Yu, X.; Du, C.C.; Wang, X.H. Characterization of a new thermophilic and acid tolerant esterase from Thermotoga maritima capable of hydrolytic resolution of racemic ketoprofen ethyl ester. J. Mol. Catal. B Enzym. 2013, 85, 23–30. [Google Scholar]
- Anderson, J.L.; Ding, J.; Welton, T.; Armstrong, D.W. Characterizing ionic liquids on the basis of multiple solvation interactions. J. Am. Chem. Soc. 2002, 124, 14247–14254. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kazlauskas, R.J. Biocatalysis in ionic liquids—Advantages beyond green technology. Curr. Opin. Biotechnol. 2003, 14, 432–437. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Lau, R.M.; Sorgedrager, M.J.; van Rantwijk, F.; Seddon, K.R. Biocatalysis in ionic liquids. Green Chem. 2002, 4, 147–151. [Google Scholar] [CrossRef]
- Zhang, D.H.; Bai, S.; Ren, M.Y.; Sun, Y. Optimization of lipase-catalyzed enantioselective esterification of (±)-menthol in ionic liquid. Food Chem. 2008, 109, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.D.; Qin, F.; Bi, Y.L.; Chen, J.N.; Yang, G.L.; Liu, W. Enhanced transesterification of ethyl ferulate with glycerol for preparing glyceryl diferulate using a lipase in ionic liquids as reaction medium. Biotechnol. Lett. 2013, 35, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, D.W.; Yan, Y.J.; Peng, C.; Xu, L. Biodiesel synthesis and conformation of lipase from Burkholderia cepacia in room temperature ionic liquids and organic solvents. Bioresour. Technol. 2011, 102, 10414–10418. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, D.W.; Yan, Y.J. Effect of ionic liquids, organic solvents and supercritical CO2 pretreatment on the conformation and catalytic properties of Candida rugosa lipase. J. Mol. Catal. B Enzym. 2013, 90, 123–127. [Google Scholar] [CrossRef]
- Wei, T.; Jia, W.W.; Yu, X.; Mao, D.B. Enhancement of enzymatic synthesis of sucrose 6-acetate with Aspergillus oryzae fructosyltransferase using ionic liquid as a cosolvent. J. Mol. Catal. B Enzym. 2016, 123, 100–106. [Google Scholar] [CrossRef]
- Silva, W.S.D.; Lapis, A.A.M.; Suarez, P.A.Z. Enzyme-mediated epoxidation of methyl oleate supported by imidazolium-based ionic liquids. J. Mol. Catal. B Enzym. 2011, 68, 98–103. [Google Scholar] [CrossRef]
- Pavlidis, I.V.; Gournis, D.; Papadopoulos, G.K. Lipases in water-in-ionic liquid microemulsions: Structural and activity studies. J. Mol. Catal. B Enzym. 2009, 60, 50–56. [Google Scholar] [CrossRef]
- Lou, W.; Zong, M.; Liu, Y.; Wang, J. Efficient enantioselective hydrolysis of d,l-phenylglycine methyl ester catalyzed by immobilized Candida Antarctica lipase B in ionic liquid containing systems. J. Biotechnol. 2006, 125, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Manimaran, T.; Harkins, A.E. Process for Producing High Purity Ketones by Friedel-Crafts Acylation at Low Temperature. U.S. W.O. 2007044270, 19 April 2004. [Google Scholar]
- Chaudhari, RV.; Majeed, S.A.; Seayad, J. Process for the Preparation of Ibuprofen. U.S. 60933847, 25 July 2000. [Google Scholar]
- Marszałł, M.P.; Siódmiak, T. Immobilization of Candida rugosa lipase onto magnetic beads for kinetic resolution of (R,S)-ibuprofen. Catal. Commun. 2012, 24, 80–84. [Google Scholar] [CrossRef]
- Habibi, Z.; Mohammadi, M.; Yousefi, M. Enzymatic hydrolysis of racemic ibuprofen esters using Rhizomucor miehei lipase immobilized on different supports. Process Biochem. 2013, 48, 669–676. [Google Scholar] [CrossRef]
- Yang, Z.; Pan, W.B. Ionic liquids: Green solvents for nonaqueous biocatalysis. Enzyme Microb. Technol. 2005, 37, 19–28. [Google Scholar] [CrossRef]
- Chen, C.S.; Fujimoto, Y.; Girdaukas, G.; Sih, C.J. Quantitative analyses of biochemical kinetic resolutions of enantiomers. J. Am. Chem. Soc. 1982, 104, 7294–7299. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
| Ionic Liquids | X (%) | eep (%) | E |
|---|---|---|---|
| Control | 19.5 | 45.2 | 2.95 |
| [Emim][PF6] | 25.3 | 75.4 | 9.1 |
| [Bmim][PF6] | 39.2 | 85.6 | 22.4 |
| [Hmim][PF6] | 42.1 | 91.2 | 43.3 |
| [Omim][PF6] | 43.2 | 93.5 | 63.5 |
| [Dmim][PF6] | 44.2 | 94.8 | 84.9 |
| [Emim][BF4] | 34.5 | 76.9 | 11.3 |
| [Bmim][BF4] | 40.6 | 87.6 | 27.8 |
| [Omim][BF4] | 44.9 | 92.3 | 56.9 |
| [OmPy][BF4] | 46.5 | 96.2 | 134.7 |
| [Bmim][Tf2N] | 44.6 | 95.2 | 94.5 |
| [Emim][TfO] | 10.2 | 35.0 | 2.2 |
| [Bmim][TfO] | 8.3 | 19.5 | 1.5 |
| [Bmim][CH3SO] | 4.3 | 1.4 | 1.0 |
| [Omim][CH3SO] | 5.8 | 4.5 | 1.1 |
| [Bmim][Cl] | 6.8 | 9.5 | 1.2 |
| [Emim][Cl] | 2.3 | 0.6 | 1.0 |
| [Omim][Cl] | 3.5 | 3.8 | 1.1 |
| [Bmim][NO3] | 34.1 | 82.2 | 15.5 |
| [Bmim][CH3COO] | 32.1 | 66.9 | 6.8 |
| Ionic Liquids | α-Helix (%) | β-Sheet (%) | β-Turn (%) | Random Coil (%) |
|---|---|---|---|---|
| Control | 21.5 ± 0.3 | 26.5 ± 0.5 | 35.2 ± 0.2 | 16.8 ± 0.2 |
| [Emim][PF6] | 21.3 ± 0.2 | 26.0 ± 0.4 | 36.3 ± 0.2 | 16.4 ± 0.1 |
| [Bmim][PF6] | 20.5 ± 0.2 | 27.8 ± 0.3 | 34.7 ± 0.1 | 17.0 ± 0.3 |
| [Hmim][PF6] | 19.2 ± 0.5 | 28.9 ± 0.4 | 33.7 ± 0.2 | 17.8 ± 0.1 |
| [Omim][PF6] | 18.2 ± 0.2 | 30.7 ± 0.3 | 33.1 ± 0.3 | 18.0 ± 0.2 |
| [Dmim][PF6] | 17.9 ± 0.3 | 31.3 ± 0.2 | 32.5 ± 0.4 | 18.3 ± 0.1 |
| [Emim][BF4] | 20.5 ± 0.3 | 28.2 ± 0.5 | 34.9 ± 0.1 | 16.4 ± 0.4 |
| [Bmim][BF4] | 18.8 ± 0.1 | 30.1 ± 0.3 | 33.7 ± 0.2 | 17.4 ± 0.1 |
| [Omim][BF4] | 16.4 ± 0.1 | 31.2 ± 0.5 | 32.2 ± 0.3 | 20.2 ± 0.4 |
| [OmPy][BF4] | 15.6 ± 0.2 | 32.3 ± 0.3 | 32.0 ± 0.3 | 20.1 ± 0.4 |
| [Bmim][Tf2N] | 16.5 ± 0.3 | 33.0 ± 0.2 | 32.3 ± 0.2 | 18.2 ± 0.3 |
| [Emim][TfO] | 22.3 ± 0.6 | 28.2 ± 0.2 | 37.8 ± 0.1 | 11.7 ± 0.3 |
| [Bmim][TfO] | 24.9 ± 0.4 | 26.3 ± 0.3 | 39.3 ± 0.2 | 9.5 ± 0.1 |
| [Bmim][CH3SO3] | 25.3 ± 0.2 | 25.9 ± 0.4 | 40.3 ± 0.3 | 8.5 ± 0.2 |
| [Omim][CH3SO3] | 26.8 ± 0.4 | 26.0 ± 0.2 | 40.1 ± 0.2 | 7.1 ± 0.3 |
| [Bmim][Cl] | 25.3 ± 0.2 | 27.2 ± 0.3 | 39.0 ± 0.5 | 8.5 ± 0.2 |
| [Emim][Cl] | 25.7 ± 0.1 | 28.3 ± 0.3 | 38.3 ± 0.4 | 7.7 ± 0.5 |
| [Omim][Cl] | 26.3 ± 0.2 | 28.5 ± 0.4 | 38.2 ± 0.5 | 7.0 ± 0.3 |
| [Bmim][NO3] | 26.5 ± 0.3 | 28.3 ± 0.3 | 39.3 ± 0.1 | 5.9 ± 0.5 |
| [Bmim][CH3COO] | 26.3 ± 0.4 | 28.1 ± 0.3 | 39.1 ± 0.2 | 6.5 ± 0.1 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, T.; Yang, K.; Bai, B.; Zang, J.; Yu, X.; Mao, D. Enzymatic Hydrolytic Resolution of Racemic Ibuprofen Ethyl Ester Using an Ionic Liquid as Cosolvent. Molecules 2016, 21, 905. https://doi.org/10.3390/molecules21070905
Wei T, Yang K, Bai B, Zang J, Yu X, Mao D. Enzymatic Hydrolytic Resolution of Racemic Ibuprofen Ethyl Ester Using an Ionic Liquid as Cosolvent. Molecules. 2016; 21(7):905. https://doi.org/10.3390/molecules21070905
Chicago/Turabian StyleWei, Tao, Kunpeng Yang, Bing Bai, Jie Zang, Xuan Yu, and Duobin Mao. 2016. "Enzymatic Hydrolytic Resolution of Racemic Ibuprofen Ethyl Ester Using an Ionic Liquid as Cosolvent" Molecules 21, no. 7: 905. https://doi.org/10.3390/molecules21070905





