4.3. Syntheses and Characterization Procedures
All organic solvents used for the synthesis were of analytical grade. All reagents used were purchased from Sigma-Aldrich (St. Louis, MO, USA), Merck (Kenilworth, NJ, USA) or AK Scientific (Union City, CA, USA) and were used as received. Melting points were determined on a Stuart Scientific SMP30 apparatus (Bibby Scientific Limited, Staffordshire, United Kingdom) and are uncorrected. NMR spectra were recorded on a Bruker Avance III HD 400 (Billerica, MA, USA) at 400 MHz for 1H and 100 MHz for 13C-NMR spectra were recorded in CDCl3 unless otherwise indicated, using the solvent signal as reference. The chemical shifts are expressed in ppm (δ scale) downfield from tetramethylsilane (TMS), and coupling constants values (J) are given in Hertz. The IR spectra were obtained on a Bruker Vector 22 spectrophotometer (Billerica, MA, USA) using KBr discs. Column chromatography was performed on Merck silica gel 60 (70–230 mesh). Thin layer chromatographic separations were performed on Merck silica gel 60 (70–230 mesh) chromatofoils. Elemental analyses were performed on a FISONS EA 1108 CHNS-O analyzer.
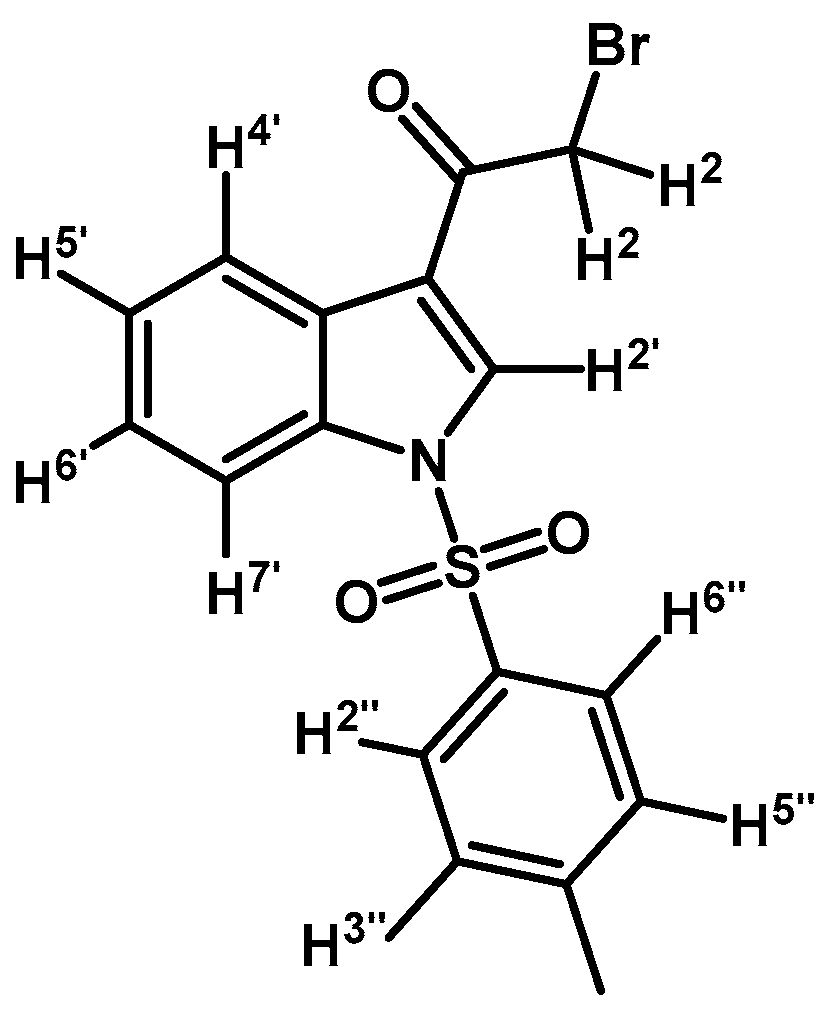
2-Bromo-1-(1H-indol-3-yl)ethanone (1)
![Molecules 21 01070 i004]()
To a solution of indole (1 g, 8.53 mmol) in dry CH2Cl2 (30 mL) was added anhydrous zinc chloride (1.7 g, 12.45 mmol) under N2 atmosphere. Immediately methylmagnesium bromide (6.1 mL, 8.53 mmol, 1.4 M in THF/toluene 1:3) was slowly added over 20 min period and the mixture was vigorously stirred for 2 h at room temperature. After this time, bromoacetyl chloride (0.84 mL, 9.65 mmol) was added in one portion and mixture was stirred until that the starting material had disappeared by checking TLC. The reaction was stopped by dilution adding an aqueous saturated solution of ammonium chloride (50 mL) and extracted with CH2Cl2 (3 × 30 mL). The combined organic layers were dried with anhydrous sodium sulfate and removal of the solvent under vacuum afforded a residue, which was further purified by column chromatography on silica gel (CH2Cl2/AcOEt 9:1) to give 1260 mg of (1) as a dark red amorphous powder. Yield: 62% m.p.: 160.3–161.0 °C; IR (KBr) cm−1: 3210, 1638, 1431, 750. 1H-NMR (acetone-d6) δ (ppm): 11.21 (s, 1H, N-H), 8.42 (d, J = 3.2 Hz, 1H, H-2′), 8.31 (dd, J = 7.8 and 4.9 Hz, 1H, H-4′), 7.59–7.53 (m, 1H, H-7′), 7.31–7.24 (m, 2H, H-5′, H-6′), 4.56 (s, 2H, H-2). 13C-NMR (acetone-d6) δ (ppm): 187.5, 138.3, 135.3, 127.3, 124.7, 123.5, 123.1, 115.6, 113.4, and 33.6. Elemental analysis for C10H8BrNO (238.08 g/mol) calcd.: C: 50.45; H: 3.39; N: 5.88. Found: C: 50.17; H: 3.73; N: 6.23.
4.3.1. General Procedure for 2-bromo-1-(arylsulfonyl-1H-3-yl)ethanone derivatives (2a–g)
2-Bromo-1-(1-tosyl-1H-indol-3-yl)ethanone (2a)
![Molecules 21 01070 i005]()
In a round bottom flask under N2, 2-bromo-1-(1H-indol-3-yl)ethanone (1) (500 mg, 2.1 mmol), p-toluenesulfonyl chloride (439 mg, 2.3 mmol), DMAP (26 mg, 0.21 mmol), and triethylamine (0.3 mL, 2.1 mmol) were dissolved in 20 mL of dry CH2Cl2, and the solution was stirred at room temperature until that the starting material had disappeared by checking TLC. The reaction mixture was quenched by dilution with CH2Cl2 (30 mL), and the organic extract was washed with 1 N HCl (20 mL). The organic layer was dried over anhydrous Na2SO4, filtered and the solvent was removed under vacuum. The product was purified by silica gel column chromatography using CH2Cl2 to give 379 mg of (2a) as brown crystalline plates. Yield: 46% m.p.: 149.5–150.2 °C; IR (KBr) cm−1: 1670, 1536, 1392, 1175, 1137, 993, 749, 569. 1H-NMR δ (ppm): 8.34 (s, 1H, H-2′), 8.29 (d, J = 7.1 Hz, 1H, H-4′), 7.93 (d, J = 7.7 Hz, 1H, H-7′), 7.83 (d, J = 8.4 Hz, 2H, H-2′′, H-6′′), 7.42–7.32 (m, 2H, H-5′, H-6′), 7.27 (d, J = 8.4 Hz, 2H, H-3′′, H-5′′), 4.59 (s, 2H, H-2), 2.37 (s, 3H, CH3). 13C-NMR δ (ppm): 187.3, 146.6, 135.1, 134.6, 132.8, 130.8 (2C), 127.9, 127.6 (2C), 126.5, 125.6, 123.2, 118.5, 113.6, 46.5 and 22.1. Elemental analysis for C17H14BrNO3S (392.27 g/mol) calcd.: C: 52.05; H: 3.60; N: 3.57; S: 8.17. Found: C: 51.75; H: 3.97; N: 3.93; S: 8.43.
2-Bromo-1-(1-(4-chlorophenylsulfonyl)-1H-indol-3-yl)ethanone (2b)
![Molecules 21 01070 i006]()
Prepared from 2-bromo-1-(1H-indol-3-yl)ethanone (1) (500 mg, 2.1 mmol), p-chlorobenzenesulfonyl chloride (486 mg, 2.3 mmol), DMAP (26 mg, 0.21 mmol) and triethylamine (0.3 mL, 2.1 mmol) to give a crude, which was purified by column chromatography on silica gel using CH2Cl2 to afford 520 mg of (2b) as brown crystalline plates. Yield: 60% m.p.: 158.1–159.6 °C; IR (KBr) cm−1: 1673, 1537, 1387, 1168, 1139, 1083, 998, 756, 569. 1H-NMR δ (ppm): 8.24 (d, J = 6.0 Hz, 1H, H-4′), 8.23 (s, 1H, H-2′), 7.85 (dd, J = 7.0, 1.7 Hz, 1H, H-7′), 7.82 (d, J = 8.8 Hz, 2H, H-2′′ and H-6′′), 7.41 (d, J = 8.8 Hz, 2H, H-3′′ and H-5′′), 7.37–7.29 (m, 2H, H-5′ and H-6′), 4.50 (s, 2H, H-2). 13C-NMR δ (ppm): 187.3, 142.2, 136.0, 135.0, 132.5, 130.5 (2C), 129.0 (2C), 127.9, 126.8, 125.9, 123.6, 119.0, 113.4 and 46.4. Elemental analysis for C16H11BrClNO3S (412.69 g/mol) calcd.: C: 46.57; H: 2.69; N: 3.39; S: 7.77. Found: C: 46.42; H: 3.02; N: 3.54; S: 8.01.
2-Bromo-1-(1-(4-fluorophenylsulfonyl)-1H-indol-3-yl)ethanone (2c)
![Molecules 21 01070 i007]()
Prepared from 2-bromo-1-(1H-indol-3-yl)ethanone (1) (500 mg, 2.1 mmol), p-fluorobenzenesulfonyl chloride (448 mg, 2.3 mmol), DMAP (26 mg, 0.21 mmol) and triethylamine (0.3 mL, 2.1 mmol) to give a crude, which was purified by column chromatography on silica gel using CH2Cl2 to give 333 mg of (2c) as brown crystalline plates. Yield: 40% m.p.: 137.3–138.6 °C; IR (KBr) cm−1: 1676, 1538, 1381, 1167, 1184, 1137, 995, 753, 570. 1H-NMR δ (ppm): 8.24 (m, 2H, H-2′ and H-4′), 7.92 (dd, J = 9.0 and 4.8 Hz, 2H, H-2′′ and H-6′′), 7.85 (dd, J = 7.0 and 1.6 Hz, 1H, H-7′), 7.37–7.29 (m, 2H, H-5′ and H-6′), 7.12 (t, J = 8.3 Hz, 2H, H-3′′ and H-5′′), 4.50 (s, 2H, H-2). 13C-NMR δ (ppm): 187.3, 166.6 (d, J = 259.2 Hz, 1C), 135.0, 133.7 (d, J= 2.7 Hz, 1C), 132.5, 130.6 (d, J= 9.9 Hz, 2C), 127.9, 126.8, 125.8, 123.6, 118.9, 117.7 (d, J= 23.0 Hz, 2C), 113.4 and 46.4. Elemental analysis for C16H11BrFNO3S (396.23 g/mol) calcd.: C: 48.50; H: 2.80; N: 3.53; S: 8.09. Found: C: 48.23; H: 3.15; N: 3.90; S: 7.79.
2-Bromo-1-(1-(4-iodophenylsulfonyl)-1H-indol-3-yl)ethanone (2d)
Prepared from 2-bromo-1-(1H-indol-3-yl)ethanone (1) (1 g, 4.22 mmol), p-iodobenzenesulfonyl chloride (1400 mg, 4.64 mmol), DMAP (52 mg, 0.42 mmol) and triethylamine (0.6 mL, 4.2 mmol) to give a crude, which was purified by column chromatography on silica gel using CH2Cl2 to afford 1142 mg of (2d) as brown crystalline plates. Yield: 54% m.p.: 183–184 °C; IR (KBr) cm−1: 1673, 1537, 1388, 1167, 1139, 996, 749, 603, 569. 1H-NMR δ (ppm): 8.22 (m, 2H, H-2′ and H-4′), 7.82 (d, J = 7.4 Hz, 1H, H-7′), 7.75 (d, J = 8.6 Hz, 2H, H-3′′ and H-5′′), 7.55 (d, J = 8.6 Hz, 2H, H-2” and H-6”), 7.31 (m, 2H, H-5′ and H-6′), 4.50 (s, 2H, H-2). 13C-NMR δ (ppm): 187.3, 139.6, 139.3, 137.2 (2C), 135.0 (2C), 133.5, 133.23, 128.6, 127.9, 124.2, 119.0, 103.6, 100.0 and 46.5. Elemental analysis for C16H11BrINO3S (504.14 g/mol) calcd.: C: 38.12; H: 2.20; N: 2.78; S: 6.36. Found: C: 37.78; H: 2.30; N: 3.07; S: 6.59.
2-Bromo-1-(1-(naphthalen-1-ylsulfonyl)-1H-indol-3-yl)ethanone (2e)
![Molecules 21 01070 i009]()
Prepared from 2-bromo-1-(1H-indol-3-yl)ethanone (1) (320 mg, 1.35 mmol), naphthalenesulfonyl chloride (310 mg, 1.35 mmol), DMAP (17 mg, 0.14 mmol) and triethylamine (0.2 mL, 1.35 mmol) to give a crude, which was purified by column chromatography on silica gel using CH2Cl2 to afford 147 mg of (2e) as brown crystalline plates. Yield: 25% m.p.: 140–141 °C; IR (KBr) cm−1: 1678, 1536, 1385, 1164, 1132, 995, 746, 598. 1H-NMR δ (ppm): 8.61 (d, J = 8.2 Hz, 1H, H-2′′); 8.61 (s, 1H, H-2′); 8.39 (dd, J = 0.9 and 7.5 Hz, 1H, H-8′′); 8.23 (dd, J = 2.7 and 6.0 Hz, 1H, H-4′); 8.03 (d, J = 8.2 Hz, 1H, H-4′′); 7.81 (d, J = 8.1 Hz, 1H, H-5′′); 7.73 (dd, J = 2.4 and 6.4 Hz, 1H, H-7′); 7.62 (m, 1H, H-3′′); 7.46–7.56 (m, 2H, H-6′′ and H-7′′); 7.26 (m, 2H, H-5′ and H-6′); 4.59 (s, 2H, H-2). 13C-NMR δ (ppm): 187.4, 137.1, 135.0, 134.7, 133.4, 132.5, 131.2, 130.0, 129.8, 128.2, 128.0, 127.7, 126.5, 125.5, 124.6, 123.5, 123.4, 117.9, 113.4 and 46.5. Elemental analysis for C20H14BrNO3S (428.30 g/mol) calcd.: C: 56.09; H: 3.29; N: 3.27; S: 7.49. Found: C: 55.82; H: 3.59; N: 3.44; S: 7.88.
2-Bromo-1-(1-(4-methoxyphenylsulfonyl)-1H-indol-3-yl)ethanone (2f)
![Molecules 21 01070 i010]()
Prepared from 2-bromo-1-(1H-indol-3-yl)ethanone (1) (894 mg, 3.77 mmol), p-methoxybenzenesulfonyl chloride (800 mg, 3.77 mmol), DMAP (52 mg, 0.42 mmol) and triethylamine (0.63 mL, 4.52 mmol) to give a crude, which was purified by column chromatography on silica gel using CH2Cl2 to afford 1.35 g of (2f) as red-brown crystalline plates. Yield: 88% m.p.: 189–190 °C; IR (KBr) cm−1: 1672, 1537, 1381, 1170, 1141, 996, 747, 575. 1H-NMR δ (ppm): 8.18 (s, 1H, H-2′); 8.15 (d, J = 7.8 Hz, 1H, H-4′); 7.78 (d, J = 7.8 Hz, 1H, H-7′); 7.75 (d, J = 9.0 Hz, 2H, H-2” and H-6”); 7.23 (m, 2H, H-5′ and H-6′); 6.79 (d, J = 9.0 Hz, 2H, H-3′′ and H-5′′); 4.43 (s, 2H, H-2); 3.66 (s, 2H, OCH3). 13C-NMR δ (ppm): 186.9; 164.5; 134.7; 132.4; 129.6 (2C); 128.4; 127.5; 126.1; 125.1; 123.0; 118.0; 114.9 (2C); 113.1; 55.8 and 46.0. Elemental analysis for C17H14BrNO4S (408.27 g/mol) calcd.: C: 50.01; H: 3.46; N: 3.43; S: 7.85. Found: C: 49.85; H: 3.61; N: 3.57; S: 7.59.
2-Bromo-1-(1-(3,5-difluorophenylsulfonyl)-1H-indol-3-yl)ethanone (2g)
Prepared from 2-bromo-1-(1H-indol-3-yl)ethanone (1) (1 g, 2.41 mmol), 3,5-difluorobenzenesulfonyl chloride (1.07 g, 5.03 mmol), DMAP (50 mg, 0.41 mmol) and triethylamine (1 mL, 7.0 mmol) to give a crude, which was purified by column chromatography on silica gel using CH2Cl2 to afford 182 mg of (2g) as a pale brown gel. Yield: 18%; m.p.: product in gel state; IR (KBr) cm−1: 1692, 1392, 1190, 1134, 1005, 576. 1H-NMR δ (ppm): 8.29 (bs, 2H, H-2′ and H-4′); 7.90 (d, J = 8.2 Hz, 1H, H-7′); 7.48 (bs, 2H, H-2′′ and H-6′′); 7.41 (m, 2H, H-5′ and H-6′); 7.03 (t, J = 8.3 Hz, 1H, H-4′′); 4.60 (s, 2H, H-2). 13C-NMR δ (ppm): 187.3;163.3 (dd, J = 257.1 and 11.7 Hz, 2C); 140.5 (t, J = 8.6 Hz, 1C); 135.0; 132.4; 127.9; 127.1; 126.1; 123.7; 119.5; 113.4; 111.0 (m, 3C); 46.5. Elemental analysis for C16H10BrF2NO3S (414.22 g/mol) calcd.: C: 46.39; H: 2.43; N: 3.38; S: 7.74. Found: C: 46.27; H: 2.61; N: 3.53; S: 8.03.
4.3.2. General Procedure for 2-(4-(Aryl)piperazin-1-yl)-1-(1-arylsulfonyl-1H-indol-3-yl)ethanone Derivatives (3a–m)
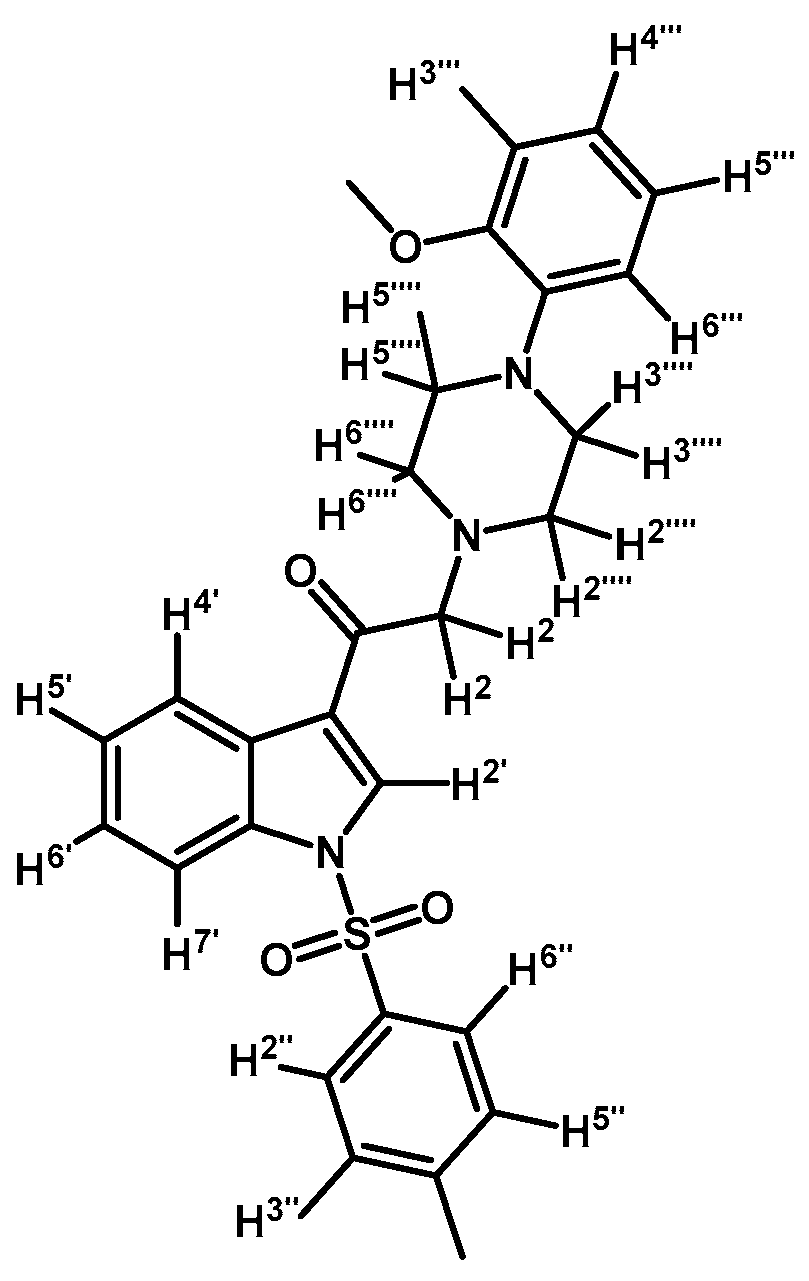
2-(4-(Pyridyl-2-yl)piperazin-1-yl)-1-(1-tosyl-1H-indol-3-yl)ethanone (3a)
![Molecules 21 01070 i012]()
To a solution of 1-(2-pyridyl)-piperazine (125 mg, 0.765 mmol) and potassium carbonate (106 mg, 0.765 mmol) in acetone (30 mL) 2-bromo-1-(1-tosyl-1H-indol-3-yl)ethanone (2a) (300 mg, 0.765 mmol) was added and the mixture was stirred for 24 h at room temperature. The reaction was stopped by dilution with water (30 mL) and the organic layer was extracted with CH2Cl2 (3 × 30 mL). The combined organic layers were dried with anhydrous sodium sulfate and removal of the solvent under vacuum afforded a crude residue. The solid was purified by column chromatography on silica gel (CH2Cl2/acetone 9:1) to yield 165 mg of (3a) as orange crystalline plates. Yield: 46% m.p.: 74.9–75.7 °C; IR (KBr) cm−1: 1664, 1594, 1437, 1379, 1173, 980, 661. 1H-NMR δ (ppm): 8.62 (s, 1H, H-2′), 8.28 (d, J = 6.9 Hz, 1H, H-4′), 8.14 (d, J = 4.8 Hz, 1H, H-3′′′′), 7.88 (d, J = 7.2 Hz, 1H, H-7′), 7.8 (d, J = 8.3 Hz, 2H, H-2′′and H-6′′), 7.46–7.39 (m, 1H, H-5′′′′), 7.33–7.24 (m, 2H, H-5′ and H-6′), 7.18 (d, J = 7.8 Hz, 2H, H-3′′and H-5′′), 6.62–6.54 (m, 2H, H-4′′′′ and H-6′′′′), 3.64 (s, 2H, H-2), 3.54 (t, J = 4.9 Hz, 4H, H-3′′′ and H-5′′′), 2.65 (t, J = 4.9 Hz, 4H, H-2′′′ and H-6′′′), 2.27 (s, 3H, CH3). 13C-NMR δ (ppm): 193.6, 159.9, 148.4, 146.4, 138.0, 134.9, 133.3, 130.7 (2C), 128.6, 128.3, 127.6 (2C), 126.2, 125.3, 123.5, 119.7, 113.9, 113.5, 107.6, 67.0, 53.7 (2C), 45.6 (2C) and 22.0. Elemental analysis for C26H26N4O3S (474.57 g/mol) calcd.: C: 65.80; H: 5.52; N: 11.81; S: 6.76. Found: C: 65.94; H: 5.33; N: 11.58; S: 6.63.
2-(4-(2-Methoxyphenyl)piperazin-1-yl)-1-(1-tosyl-1H-indol-3-yl)ethanone (3b)
![Molecules 21 01070 i013]()
Prepared from 1-(2-methoxyphenyl)-piperazine (147 mg, 0.765 mmol), potassium carbonate (106 mg, 0.765 mmol) and 2-bromo-1-(1-tosyl-1H-indol-3-yl)ethanone (2a) (300 mg, 0.765 mmol) to give a solid, which was purified by column chromatography on silica gel using CH2Cl2/acetone 9:1 to obtain 255 mg of pure product (3b) as white crystalline plates. Yield: 66% m.p.: 132.3–133.6 °C; IR (KBr) cm−1: 1655, 1374 and 1171, 1244, 755, 742. 1H-NMR δ (ppm): 8.70 (s, 1H, H-2′), 8.31 (d, J = 7.3 Hz, 1H, H-4′), 7.92 (d, J = 7.5 Hz, 1H, H-7′), 7.81 (d, J = 8.1 Hz, 2H, H-2′′ and H-6′′), 7.36–7.29 (m, 2H, H-5′ and H-6′), 7.22 (d, J = 8.1 Hz, 2H, H-3′′ and H-5′′), 7.01–6.90 (m, 3H, H-3′′′, H-4′′′ and H-5′′′), 6.84 (d, J = 7.7 Hz, 1H, H-6′′′), 3.84 (s, 3H, O-CH3), 3.71 (s, 2H, H-2), 3.13 (bs, 4H, H-3′′′′ and H-5′′′′), 2.80 (bs, 4H, H-2′′′′ and H-6′′′′), 2.31 (s, 3H, CH3). 13C-NMR δ (ppm): 193.2, 152.3, 145.9, 141.1, 134.6, 134.5, 133.0, 130.3 (2C), 127.9, 127.2 (2C), 125.7, 124.9, 123.1, 123.0, 121.0, 119.3, 118.3, 113.1, 111.3, 66.6, 55.4, 53.7 (2C), 50.5 (2C) and 21.6. Elemental analysis for C28H29N3O4S (503.61 g/mol) calcd.: C: 66.78; H: 5.80; N: 8.34; S: 6.37. Found: C: 66.55; H: 5.97; N: 8.21; S: 6.75.
2-(4-(Pyrimidin-2-yl)piperazin-1-yl)-1-(1-tosyl-1H-indol-3-yl)ethanone (3c)
![Molecules 21 01070 i014]()
Prepared from 1-(2-pyrimidyl)-piperazine (84 mg, 0.512 mmol), potassium carbonate (70 mg, 0.512 mmol) and 2-bromo-1-(1-tosyl-1H-indol-3-yl)ethanone (2a) (200 mg, 0.512 mmol) to give a solid, which was purified by column chromatography on silica gel using CH2Cl2/acetone 9:1 to obtain 221 mg of pure product (3c) as white crystalline plates. Yield: 91%; m.p.: 133–134 °C; IR (KBr) cm−1: 1651, 1586, 1378, 1173, 571. 1H-NMR δ (ppm): 8.72 (s, 1H, H-2′); 8.35 (d, J = 7.1 Hz, 1H, H-4′); 8.31 (d, J = 4.8 Hz, 2H, H-4′′′ and H-6′′′); 7.95 (d, J = 7.6 Hz, 1H, H-7′); 7.84 (d, J = 8.3 Hz, 2H, H-2′′ and H-6′′); 7.35 (m, 2H, H-5′ and H-6′); 7.25 (d, J = 8.2 Hz, 2H, H-3′′ and H-5′′); 6.49 (t, J = 4.7 Hz, 1H, H-5′′′); 3.90 (t, J = 4.9 Hz, 4H, H-3′′′′ and H-5′′′′); 3.71 (s, 2H, H-2); 2.66 (t, J = 5.0 Hz, 4H, H-2′′′′ and H-6′′′′); 2.33 (s, 3H, CH3). 13C-NMR δ (ppm): 193.5, 162.0, 158.1 (2C), 146.4, 135.0, 134.9, 133.3, 130.7 (2C), 128.3, 127.5 (2C), 126.1, 125.3, 123.5, 119.8, 113.5, 110.4, 67.0, 53.8 (2C), 44.0 (2C) and 22.0. Elemental analysis for C25H25N5O3S (475.56 g/mol) calcd.: C: 63.14; H: 5.30; N: 14.73; S: 6.74. Found: C: 62.75; H: 5.11; N: 14.35; S: 6.66.
1-(1-(4-Chlorophenylsulfonyl)-1H-indol-3-yl)-2-(4-(pyrimidin-2-yl)piperazin-1-yl)ethanone (3d)
![Molecules 21 01070 i015]()
Prepared from 1-(2-pyrimidyl)-piperazine (100 mg, 0.640 mmol), potassium carbonate (90 mg, 0.640 mmol) and 2-bromo-1-(1-(4-chlorophenylsulfonyl)-1H-indol-3-yl)ethanone (2b) (263 mg, 0.640 mmol) to give a solid, which was purified by column chromatography on silica gel using CH2Cl2/acetone 4:1 to obtain 281 mg of pure product (3d) as white crystalline plates. Yield: 89% m.p.: 168–169 °C; IR (KBr) cm−1: 1666, 1587, 1387, 1168, 760, 569. 1H-NMR δ (ppm): 8.72 (s, 1H, H-2′); 8.36 (dd, J = 6.7 and 2.1 Hz, 1H, H-4′); 8.31 (d, J = 4.8, 2H, H-4′′′ and H-6′′′); 7.92 (dd, J = 7.0 and 1.6 Hz, 1H, H-7′); 7.87 (d, J = 8.7 Hz, 2H, H-2′′ and H-6′′); 7.41 (d, J = 8.7 Hz, 2H, H-3′′ and H-5′′); 7.39–7.34 (m, 2H, H-5′ and H-6′); 6.49 (t, J = 4.7 Hz, 1H, H-5′′′); 3.91 (t, J = 4.9 Hz, 4H, H-3′′′′ and H-5′′′′); 3.72 (s, 2H, H-2); 2.67 (t, J = 5.0 Hz, 4H, H-2′′′′ and H-6′′′′). 13C-NMR δ (ppm): 193.5, 162.0, 158.1 (2C), 141.8, 136.2, 134.8, 133.0, 130.4 (2C), 128.9 (2C), 128.4, 126.4, 125.6, 123.6, 120.2, 113.3, 110.4, 67.1, 53.8 (2C) and 44.1 (2C). Elemental analysis for C24H22ClN5O3S (495.98 g/mol) calcd.: C: 58.12; H: 4.47; N: 14.12; S: 6.46. Found: C: 58.07; H: 4.40; N: 14.27; S: 6.59.
1-(1-(4-Fluorophenylsulfonyl)-1H-indol-3-yl)-2-(4-(pyrimidin-2-yl)piperazin-1-yl)ethanone (3e)
![Molecules 21 01070 i016]()
Prepared from 1-(2-pyrimidyl)-piperazine (83 mg, 0.506 mmol), potassium carbonate (70 mg, 0.506 mmol) and 2-bromo-1-(1-(4-fluorophenylsulfonyl)-1H-indol-3-yl)ethanone (2c) (200 mg, 0.506 mmol) to give a solid, which was purified by column chromatography on silica gel using CH2Cl2/acetone 9:1 to obtain 187 mg of pure product (3e) as white crystalline plates. Yield: 77% m.p.: 145–146 °C; IR (KBr) cm−1: 1666, 1587, 1386, 1172, 1186, 571. 1H-NMR δ (ppm): 8.71 (s, 1H, H-2′), 8.37 (d, J = 7.2 Hz, 1H, H-4′); 8.32 (d, J = 4.8 Hz, 2H, H-4′′′ and H-6′′′); 7.98 (dd, J = 8.7 and 4.9 Hz, 2H, H-2′′ and H-6′′); 7.93 (d, J = 7.6, 1H, H-7′); 7.42–7.33 (m, 2H, H-5′ and H-6′); 7.16 (t, J = 8.4 Hz, 2H, H-3′′ and H-5′′); 6.50 (t, J = 4.7 Hz, 1H, H-5′′′); 3.91 (t, J = 4.9 Hz, 4H, H-3′′′′ and H-5′′′′); 3.72 (s, 2H, H-2); 2.67 (t, J = 4.9 Hz, 4H, H-2′′′′ and H-6′′′′). 13C-NMR δ (ppm): 193.5, 166.5 (d, J = 258.8 Hz, 1C); 162.0, 158.1 (2C), 134.8, 133.9 (d, J = 3.2 Hz, 1C), 133.0, 130.4 (d, J = 9.8 Hz, 2C), 128.3, 126.4, 125.5, 123.6, 120.1, 117.6 (d, J = 23.0 Hz, 2C), 113.3, 110.5, 67.1, 53.8 (2C) and 44.1 (2C). Elemental analysis for C24H22FN5O3S (479.53 g/mol) calcd.: C: 60.11; H: 4.62; N: 14.60; S: 6.69. Found: C: 59.92; H: 4.45; N: 14.80; S: 6.83.
1-(1-(4-Iodophenylsulfonyl)-1H-indol-3-yl)-2-(4-(pyridin-2-yl)piperazin-1-yl)ethanone (3f)
![Molecules 21 01070 i017]()
Prepared from 1-(2-pyridyl)-piperazine (97 mg, 0.595 mmol), potassium carbonate (83 mg, 0.595 mmol) and 2-bromo-1-(1-(4-iodophenylsulfonyl)-1H-indol-3-yl)ethanone (2d) (300 mg, 0.595 mmol) to obtain a crude which was purified by column chromatography employing CH2Cl2/acetone 9:1 to yield 324 mg of (3f) as light yellow crystalline plates. Yield: 93% m.p.: 179–180 °C; IR (KBr) cm−1: 1671, 1593, 1479, 1434, 1396, 1163, 1136, 982, 604, 568. 1H-NMR δ (ppm): 8.68 (s, 1H, H-2′); 8.36 (dd, J = 6.1 and 2.7 Hz, 1H, H-4′); 8.22 (dd, J = 4.9 and 1.2 Hz, 1H, H-3′′′); 7.93 (dd, J = 6.4 and 2.4, 1H, H-7′); 7.84 (d, J = 8.7 Hz, 2H, H-2′′ and H-6′′); 7.64 (d, J = 8.7 Hz, 2H, H-3′′ and H-5′′); 7.51 (td, J = 8.9, 7.2 and 2.0 Hz, 1H, H-5′′′); 7.35–7.43 (m, 2H, H-5′ and H-6′); 6.63–6.70 (m, 2H, H-4′′′ and H-6′′′); 3.70 (s, 2H, H-2); 3.62 (t, J = 5.0 Hz, 4H, H-3′′′′ and H-5′′′′); 2.72 (t, J = 5.0 Hz, 4H, H-2′′′′ and H-6′′′′). 13C-NMR δ (ppm): 193.5, 159.9, 148.4, 139.4 (2C), 138.0, 137.5, 134.8, 133.0, 128.6 (2C), 128.4, 126.4, 125.6, 123.7, 120.2, 114.0, 113.4, 107.6, 103.2, 67.2, 53.8 (2C) and 45.7 (2C). Elemental analysis for C25H23IN4O3S (586.44 g/mol) calcd.: C: 51.20; H: 3.95; N: 9.55; S: 5.47. Found: C: 51.32; H: 3.82; N: 9.92; S: 5.20.
1-(1-(4-Iodophenylsulfonyl)-1H-indol-3-yl)-2-(4-(2-methoxyphenyl)piperazin-1-yl)ethanone (3g)
![Molecules 21 01070 i018]()
Prepared from 1-(2-methoxyphenyl)-piperazine (114 mg, 0.595 mmol), potassium carbonate (82 mg, 0.595 mmol) and 2-bromo-1-(1-(4-iodophenylsulfonyl)-1H-indol-3-yl)ethanone (2d) (300 mg, 0.595 mmol) to give a solid, which was purified by column chromatography on silica gel using CH2Cl2/acetone 9:1 to give 358 mg of (3g) as orange crystalline plates. Yield: 98% m.p.: 182–183 °C; IR (KBr) cm−1: 1655, 1385, 1172, 741, 603, 570. 1H-NMR δ (ppm): 8.72 (s, 1H, H-2′); 8.36 (dd, J = 6.4 and 2.6 Hz, 1H, H-4′); 7.93 (dd, J = 6.6 and 2.2 Hz, 1H, H-7′); 7.82 (d, J = 8.6 Hz, 2H, H-2′′ and H-6′′); 7.64 (d, J = 8.6 Hz, 2H, H-3′′ and H-5′′); 7.35–7.41 (m, 2H, H-5′ and H-6′); 7.05–7.00 (m, 1H, H-5′′′); 6.99–6.94 (m, 2H, H-3′′′ and H-4′′′); 6.88 (d, J = 7.7 Hz, 1H, H-6′′′); 3.87 (s, 3H, OCH3); 3.71 (s, 2H, H-2); 3.15 (bs, 4H, H-3′′′′ and H-5′′′′); 2.81 (bs, 4H, H-2′′′′ and H-6′′′′). 13C-NMR δ (ppm): 193.8, 152.7, 139.4 (2C), 137.6, 134.9, 133.2, 128.6 (2C), 128.4, 126.4, 125.6, 123.7, 123.6, 121.5, 120.3, 118.7, 113.4, 111.8, 103.2, 67.3, 55.8, 54.2 (2C) and 51.1 (2C). Elemental analysis for C27H26IN3O4S (615.48 g/mol) calcd.: C: 52.69; H: 4.26; N: 6.83; S: 5.21. Found: C: 52.60; H: 4.21; N: 6.80; S: 4.97.
1-(1-(4-Iodophenylsulfonyl)-1H-indol-3-yl)-2-(4-(pyrimidin-2-yl)piperazin-1-yl)ethanone (3h)
![Molecules 21 01070 i019]()
Prepared from 1-(2-pyrimidyl)-piperazine (100 mg, 0.595 mmol), potassium carbonate (82 mg, 0.595 mmol) and 2-bromo-1-(1-(4-iodophenylsulfonyl)-1H-indol-3-yl)ethanone (2d) (300 mg, 0.595 mmol) to give a solid, which was purified by column chromatography on silica gel using CH2Cl2/acetone 9:1 to obtain 334 mg of (3h) as white crystalline plates. Yield: 96% m.p.: 184–185 °C; IR (KBr) cm−1: 1667, 1587, 1386, 1169, 981, 740, 569. 1H-NMR δ (ppm): 8.68 (s, 1H, H-2′); 8.36 (dd, J = 6.3 and 2.5 Hz, 1H, H-4′); 8.2 (d, J =, 4.7 Hz, 2H, H-4′′′ and H-6′′′); 7.91, (dd, J = 6.6 and 2.2 Hz, 1H, H-7′); 7.82 (d, J = 8.7 Hz, 2H, H-2′′ and H-6′′); 7.63 (d, J = 8.7 Hz, 2H, H-3′′ and H-5′′); 7.38 (m, 2H, H-5′ and H-6′); 6.50 (t, J = 4.7 Hz, 1H, H-5′′′); 3.91 (t, J = 4.9 Hz, 4H, H-3′′′′ and H-5′′′′); 3.70 (s, 2H, H-2); 2.67 (t, J = 5.0 Hz, 4H, H-2′′′′ and H-6′′′′). 13C-NMR δ (ppm): 193.5, 162.0, 158.2 (2C), 139.4 (2C), 137.5, 134.8, 132.9, 128.6 (2C), 128.4, 126.4, 125.6, 123.7, 120.3, 113.3, 110.5, 103.3, 67.1, 53.8 (2C) and 44.1 (2C). Elemental analysis for C24H22IN5O3S (587.43 g/mol) calcd.: C: 49.07; H: 3.77; N: 11.92; S: 5.46. Found: C: 49.11; H: 3.71; N: 12.09; S: 5.50.
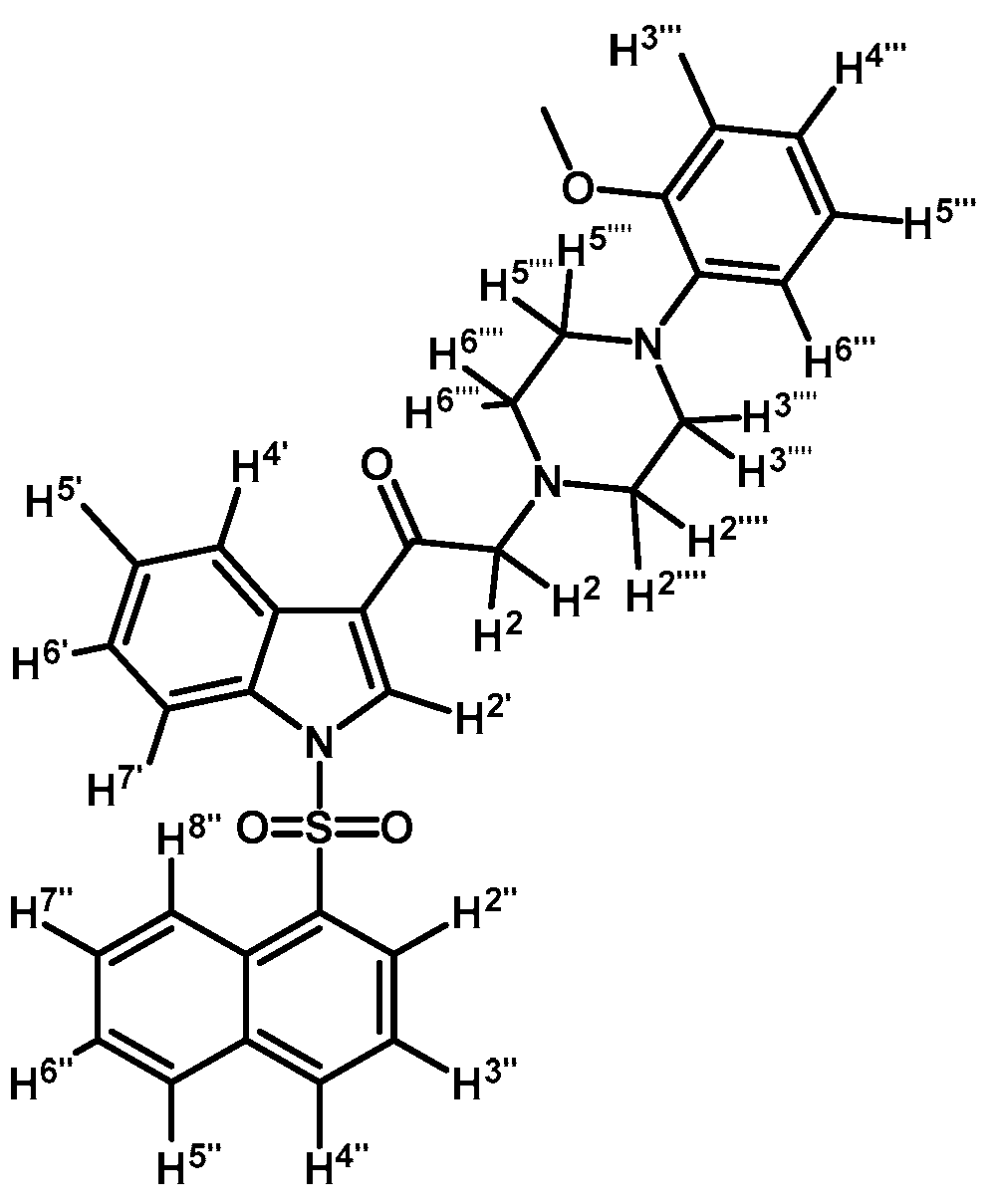
1-(1-(Naphthalen-1-ylsulfonyl)-1H-indol-3-yl)-2-(4-(pyridin-2-yl)piperazin-1-yl)ethanone (3i)
![Molecules 21 01070 i020]()
Prepared from 1-(2-pyridyl)-piperazine (27 mg, 0.163 mmol), potassium carbonate (23 mg, 0.163 mmol) and 2-bromo-1-(1-(naphthalen-1-ylsulfonyl)-1H-indol-3-yl)ethanone (2e) (70 mg, 0.163 mmol) to give a solid, which was purified by column chromatography on silica gel using CH2Cl2/acetone 9:1 to obtain 71 mg of (3i) as white crystalline plates. Yield: 86%; m.p.: 76–77 °C; IR (KBr) cm−1: 1664, 1593, 1437, 1371, 1169, 1133, 769. 1H-NMR δ (ppm): 8.96 (s, 1H, H-2′); 8.65 (d, J = 8.6 Hz, 1H, H-2′′); 8.38 (dd, J = 7.5 and 1.0 Hz, 1H, H-8′′); 8.34 (dd, J = 6.2 and 2.9 Hz, 1H, H-4′); 8.22 (dd, J = 4.8 and 1.0 Hz, 1H, H-3′′′); 8.04 (d, J = 8.2 Hz, 1H, H-4′′); 7.84 (d, J = 8.1 Hz, 1H, H-5′′); 7.81 (dd, J = 6.3 and 3.1 Hz, 1H, H-7′); 7.60 (m, 1H, H-3′′); 7.56 (t, J = 7.8 Hz, 1H, H-5′′′); 7.53–747 (m, 2H, H-6′′ and H-7′′); 7.31 (dd, J = 6.1 and 3.2 Hz, 2H, H-5′ and H-6′); 6.68–6.62 (m, 2H, H-4′′′ and H-6′′′); 3.69 (s, 2H, H-2); 3.55 (t, J = 4.9 Hz, 4H, H-3′′′′ and H-5′′′′); 2.69 (t, J = 5.0 Hz, 4H, H-2′′′′ and H-6′′′′). 13C-NMR δ (ppm): 193.6, 159.8, 148.3, 138.0, 136.9, 134.9, 134.7, 133.7, 133.0, 130.9, 129.9, 129.7, 128.4, 128.1, 127.9, 126.1, 125.3, 124.6, 123.7, 123.5, 119.3, 114.0, 113.4, 107.6, 67.3, 53.7 (2C) and 45.6 (2C). Elemental analysis for C29H26N4O3S (510.61 g/mol) calcd.: C: 68.21; H: 5.13; N: 10.97; S: 6.28. Found: C: 67.89; H: 5.40; N: 11.08; S: 6.48.
2-(4-(2-Methoxyphenyl)piperazin-1-yl)-1-(1-(naphthalen-1-ylsulfonyl)-1H-indol-3-yl)ethanone (3j)
![Molecules 21 01070 i021]()
Prepared from 1-(2-methoxyphenyl)-piperazine (31 mg, 0.163 mmol), potassium carbonate (23 mg, 0.163 mmol) and 2-bromo-1-(1-(naphthalen-1-ylsulfonyl)-1H-indol-3-yl)ethanone (2e) (70 mg, 0.163 mmol) to give a solid, which was purified by column chromatography on silica gel using CH2Cl2/AcOEt 9:1 to give 65 mg of (3j) as white crystalline plates. Yield: 74%; m.p.: 74–75 °C; IR (KBr) cm−1: 1663, 1372, 1172, 1133, 745. 1H-NMR δ (ppm): 8.99 (s, 1H, H-2′); 8.67 (d, J = 8.7 Hz, 1H, H-2′′); 8.38 (dd, J = 7.5 and 0.9 Hz, 1H, H-8′′); 8.34 (m, 1H, H-4′); 8.07 (d, J = 8.2 Hz, 1H, H-4′′); 7.87 (d, J = 8.1 Hz, 1H, H-5′′); 7.80 (m, 1H, H-7′); 7.66–7.60 (m, 1H, H-3′′); 7.59–7.51 (m, 2H, H-6′′ and H-7′′); 7.33–7.27 (m, 2H, H-5′ and H-6′); 6.99–7.06 (m, 1H, H-5′′′); 6.98–6.94 (m, 2H, H-3′′′ and H-4′′′); 6.88 (d, J = 7.7 Hz, H-6′′′); 3.87 (s, OCH3); 3.73 (s, 2H, H-2); 3.15 (bs, 4H, H-3′′′′ and H-5′′′′); 2.82 (bs, 4H, H-2′′′′ and H-6′′′′). 13C-NMR δ (ppm): 193.8, 152.7, 141.5, 136.9, 134.9, 134.7, 133.8, 133.1, 130.9, 129.9, 129.7, 128.4, 128.2, 127.9, 126.0, 125.2, 124.6, 123.8, 123.6, 123.5, 121.4, 119.3, 118.8, 113.4, 111.7, 67.5, 55.8, 54.3(2C) and 51.0(2C). Elemental analysis for C31H29N3O4S (539.64 g/mol) calcd.: C: 69.00; H: 5.42; N: 7.79; S: 5.94. Found: C: 68.81; H: 5.34; N: 7.71; S: 6.06.
1-(1-(Naphthalen-1-ylsulfonyl)-1H-indol-3-yl)-2-(4-(pyrimidin-2-yl)piperazin-1-yl)ethanone (3k)
![Molecules 21 01070 i022]()
Prepared from 1-(2-pyrimidyl)-piperazine (96 mg, 0.583 mmol), potassium carbonate (80 mg, 0.583 mmol) and 2-bromo-1-(1-(naphthalen-1-ylsulfonyl)-1H-indol-3-yl)ethanone (2e) (250 mg, 0.583 mmol) to give a solid, which was purified by column chromatography on silica gel using CH2Cl2/acetone 9:1 to give 136 mg of (3k) as white crystalline plates. Yield: 46% m.p.: 88–89 °C; IR (KBr) cm−1: 1664, 1585, 1360, 1168, 1133. 1H-NMR δ (ppm): 8.98 (s, 1H, H-2′); 8.67 (d, J = 8.7 Hz, 1H, H-2′′); 8.38 (d, J = 7.4 Hz, 1H, H-8′′); 8.34 (dd, J = 3.2, 1H, H-4′); 8.32 (d, J = 4.8, 2H, H-4′′′ and H-6′′′); 8.04 (d, J = 8.2 Hz, 1H, H-4′′); 7.84 (d, J = 8.1 Hz, 1H, H-5′′); 7.79 (dd, J = 6.1 and 3.2 Hz, 1H, H-7′); 7.61 (t, J = 7.6 Hz, 1H, H-3′′); 7.57–7.47 (m, 2H, H-6′′ and H-7′′); 7.32–7.26 (m, 2H, H-5′ and H-6′); 6.49 (t, J = 4.7 Hz, 1H, H-5′′′); 3.87 (t, J = 4.9 Hz, 4H, H-3′′′′ and H-5′′′′); 3.69 (s, 2H, H-2); 2.64 (t, J = 4,9 Hz, 4H, H-2′′′′ and H-6′′′′). 13C-NMR δ (ppm): 193.6, 162.1, 158.1(2C), 136.9, 134.9, 134.7, 133.7, 133.1, 130.9, 129.9, 129.6, 128.4, 128.1, 127.9, 126.1, 125.2, 124.6, 123.7, 123.5, 119.3, 113.4, 110.5, 67.3, 53.8(2C) and 44.1(2C). Elemental analysis for C28H25N5O3S (511.59 g/mol) calcd.: C: 65.74; H: 4.93; N: 13.69; S: 6.27. Found: C: 65.61; H: 5.16; N: 13.52; S: 6.49.
1-(1-(4-Methoxyphenylsulfonyl)-1H-indol-3-yl)-2-morpholinoethanone (3l)
![Molecules 21 01070 i023]()
Prepared from morpholine (35 mg, 0.40 mmol), potassium carbonate (46 mg, 0.33 mmol) and 2-bromo-1-(1-(4-methoxyphenylsulfonyl)-1H-indol-3-yl)ethanone (2f) (136 mg, 0.33 mmol) to give a gel, which was purified by column chromatography on silica gel using CH2Cl2/acetone 9:1 to give 138 mg of product (3l) as a yellow gel. Yield: 81%; m.p.: product in gel state; IR (KBr) cm−1: 1665, 1378, 1169, 573. 1H-NMR δ (ppm): 8.66 (s, 1H, H-2′); 8.33 (dd, J = 6.5 and 1.3 Hz, 1H, H-4′); 7.93 (dd, J = 7.0 and 1.2 Hz, 1H, H-7′); 7.9 (d, J = 9.1 Hz, 2H, H-2′′ and H-6′′); 7.30–7.39 (m, 2H, H-5′ and H-6′); 6.92 (d, J = 9.1 Hz, 2H, H-3′′ and H-5′′); 3.76–3.79 (m, 7H, H-3′′′′, H-5′′′′ and OCH3); 3.68 (s, 2H, H-2); 2.61 (bs, 4H, H-2′′′′, H-6′′′′). 13C-NMR δ (ppm): 193.3, 164.8, 134.9, 133.2, 129.9 (2C), 129.2, 128.3, 126.1, 125.2, 123.4, 119.6, 115.3 (2C), 113.4, 67.3 (2C), 67.1, 56.2, 54.2 (2C). Elemental analysis for C21H22N2O5S (414.47 g/mol) calcd.: C: 60.85; H: 5.35; N: 6.76; S: 7.74. Found: C: 60.70; H: 5.53; N: 6.85; S: 7.80.
1-(1-(3,5-Difluorophenylsulfonyl)-1H-indol-3-yl)-2-morpholinoethanone (3m)
![Molecules 21 01070 i024]()
Prepared from morpholine (37 mg, 0.42 mmol), potassium carbonate (70 mg, 0.54 mmol) and 2-bromo-1-(1-(3,5-difluorophenylsulfonyl)-1H-indol-3-yl)ethanone (2g) (174 mg, 0.42 mmol) to give a gel, which was purified by column chromatography on silica gel using CH2Cl2/acetone 9:1 to give 142 mg of product (3m) as an orange gel. Yield: 80%; m.p.: product in gel state; IR (KBr) cm−1: 1685, 1607, 1444, 1300, 1389, 1173, 1132, 992, 614. 1H-NMR δ (ppm): 8.63 (s, 1H, H-2′); 8.35 (d, J = 7.4 Hz, 1H, H-4′); 7.92 (d, J = 7.9 Hz, 1H, H-7′); 7.49 (bs, 2H, H-2′′ and H-6′′); 7.40 (m, 2H, H-5′ and H-6′); 7.06 (t, J = 7.7 Hz, 1H, H-4′′); 3.78 (bs, 4H, H-3′′′ and H-5′′′); 3.67 (s, 1H, H-2); 2.61 (bs, 4H, H-2′′′ and H-6′′′). 13C-NMR δ (ppm): 193.3, 163.3 (dd, J = 256.9 and 11.7 Hz, 2C), 140.8 (t, J = 8.9 Hz, 1C), 134.8, 132.8, 128.4, 126.7, 125.8, 123.8, 120.7, 113.3, 111.1 (m, 3C), 67.5, 67.3 (2C), 54.3 (2C). Elemental analysis for C20H18F2N2O4S (420.43 g/mol) calcd.: C: 57.14; H: 4.32; N: 6.66; S: 7.63. Found: C: 57.27; H: 4.53; N: 6.81; S: 7.77.
4.3.3. General Procedure for 2-(4-(Aryl)piperazin-1-yl)-1-(1-arylsulfonyl-1H-indol-3-yl)ethanol Derivatives (4a–m)
2-(4-(Pyridin-2-yl)piperazin-1-yl)-1-(1-tosyl-1H-indol-3-yl)ethanol (4a)
![Molecules 21 01070 i025]()
To a solution of (3a) (200 mg, 0.419 mmol) in ethanol (30 mL) was added sodium borohydride (17 mg, 0.450 mmol) in one portion and mixture was vigorously stirred at room temperature until the starting material had disappeared by checking TLC. Adding water (30 mL) stopped the reaction. The organic phase was extracted with CH2Cl2 (3 × 30 mL). The combined organic layers were dried with anhydrous sodium sulfate and removal of the solvent under vacuum afforded a residue, which was further purified by column chromatography on silica gel using CH2Cl2/acetone 9:1 to give 200 mg of (4a) as white crystalline plates. Yield: 98%; m.p.: 72.4–73.9 °C; IR (KBr) cm−1: 3422, 1595, 1438, 1369, 1174, 574. 1H-NMR δ (ppm): 8.13 (dd, J = 4.7 and 1.2 Hz, 1H, H-3′′′′); 7.91 (d, J = 8.3 Hz, 1H, H-4′); 7.69 (d, J = 8.3 Hz, 2H, H-2′′ and H-6′′); 7.55 (d, J = 7.8 Hz, 1H, H-7′); 7.51 (s, 1H, H-2′); 7.45–7.38 (m, 1H, H-5′′′′); 7.23 (t, J = 7.4 Hz, 1H, H-6′); 7.19–7.14 (m, 1H, H-5′); 7.13 (d, J = 8.1 Hz, 2H, H-3′′ and H-5′′); 6.61–6.53 (m, 2H, H-4′′′′ and H-6′′′′); 4.98 (dd, J = 10.1 and 3.1 Hz, 1H, H-1); 3.59–3.44 (m, 4H, H-3′′′′ and H-5′′′′); 2.82–2.75 (m, 2H, H-2a′′′′ and H-6a′′′′); 2.70 (d, J = 10.3 Hz, 1H, H-2a); 2.63 (dd, J = 12.5 and 3.4 Hz, 1H, H-2b); 2.55–2.48 (m, 2H, H-2b′′′′ and H-6b′′′′); 2.25 (s, 3H, CH3). 13C-NMR δ (ppm): 159.4, 148.0, 145.0, 137.6, 135.5, 135.3, 129.9 (2C), 128.9, 126.9 (2C), 124.8, 123.2, 123.1, 123.0, 120.3, 113.8, 113.6, 107.2, 64.0, 63.2, 53.0 (2C), 45.4 (2C) and 22.7. Elemental analysis for C26H28N4O3S (476.59 g/mol) calcd.: C: 65.52; H: 5.92; N: 11.76; S: 6.73. Found: C: 65.37; H: 5.83; N: 11.50; S: 6.79.
2-(4-(2-Methoxyphenyl)piperazin-1-yl)-1-(1-tosyl-1H-indol-3-yl)ethanol (4b)
![Molecules 21 01070 i026]()
Prepared from (3b) (200 mg, 0.395 mmol) and sodium borohydride (17 mg, 0.450 mmol) to give a solid, which was purified by column chromatography on silica gel using AcOEt/hexane 9:1 to obtain 197 mg of product (4b) as white crystalline plates. Yield: 99%; m.p.: 86.3–86.9 °C; IR (KBr) cm−1: 3446, 1371, 1174, 1241, 1121, 748, 574. 1H-NMR δ (ppm): 8.00 (d, J = 8.3 Hz, 1H, H-4′), 7.80 (d, J = 8.2 Hz, 2H, H-2′′, H-6′′), 7.65 (d, J = 7.8 Hz, 1H, H-7′), 7.60 (s, 1H, H-2′), 7.34 (t, J = 7.6 Hz, 1H, H-6′), 7.22–7.27 (m, 3H, H-5′, H-3′′ and H-5′′), 7.08–7.01 (m, 1H, H-5′′′′), 7.01–6.93 (m, 2H, H-3′′′′ and H-4′′′′), 6.90 (d, J = 7.9 Hz, 1H, H-6′′′′), 5.06 (dd, J = 9.6, 3.9 Hz, 1H, H-1), 3.90 (s, 3H, OCH3), 3.16 (bs, 4H, H-3′′′ and H-5′′′), 2.99 (bs, 2H, H-2′′′), 2.85–2.75 (m, 2H, H-2), 2.71 (bs, 2H, H-6′′′), 2.36 (s, 3H, CH3), 1.70 (bs, 1H, OH). 13C-NMR δ (ppm): 152.6, 145.4, 141.4, 135.9, 135.6, 130.3 (2C), 129.4, 127.3 (2C), 125.2, 123.6, 123.5, 123.5, 123.4, 121.4, 120.7, 118.6, 114.2, 111.6, 64.3, 63.4, 55.8, 53.8, 51.2, 31.4 (2C) and 22.0. Elemental analysis for C28H31N3O4S (505.63 g/mol) Calcd.: C: 66.51; H: 6.18; N: 8.31; S: 6.34. Found: C: 66.21; H: 6.00; N: 8.09; S: 6.63.
2-(4-(Pyrimidin-2-yl)piperazin-1-yl)-1-(1-tosyl-1H-indol-3-yl)ethanol (4c)
![Molecules 21 01070 i027]()
Prepared from (3c) (75 mg, 0.157 mmol) and sodium borohydride (10 mg, 0.264 mmol) to give a solid, which was purified by column chromatography on silica gel using AcOEt/hexane 9:1 to obtain 51 mg of (4c) as white crystalline plates. Yield: 68%; m.p.: 81–82 °C; IR (KBr) cm−1: 3423, 1586, 1360, 1174, 574. 1H-NMR δ (ppm): 8.32 (d, J = 4.6 Hz, 2H, H-4′′′ and H-6′′′); 7.98 (d, J = 8.3 Hz, 1H, H-4′); 7.77 (d, J = 8.0 Hz, 2H, H-2′′ and H-6′′); 7.62 (d, J = 7.8 Hz, 1H, H-7′); 7.58 (s, 1H, H-2′); 7.31 (t, J = 7.7 Hz, 1H, H-6′); 7.27–7.18 (m, 3H, H-5′, H-3′′ and H-5′′); 6.51 (t, J = 4.6 Hz, 1H, H-5′′′); 5.1 (dd, J = 10.2 and 2.8 Hz, 1H, H-1); 3.82–3.95 (m, 4H, H-3′′′′ and H-5′′′′); 2.75–2.86 (m, 3H, H-2a′′′′, H-6a′′′′ and H-2a); 2.70 (dd, J = 12.5 and 3.0 Hz, 1H, H-2b); 2.50–2.59 (m, 2H, H-2b′′′′ and H-6b′′′′); 2.34 (s, 3H, CH3). 13C-NMR δ (ppm): 162.0, 158.2 (2C), 145.4, 135.9, 135.7, 130.3 (2C), 129.4, 127.3 (2C), 125.2, 123.5 (2C), 123.4, 120.7, 114.2, 110.5, 64.5, 63.6, 53.5 (2C), 44.2 (2C) and 22.0. Elemental analysis for C25H27N5O3S (477.58 g/mol) calcd.: C: 62.87; H: 5.70; N: 14.66; S: 6.71. Found: C: 62.67; H: 5.93; N: 14.82; S: 6.76.
1-(1-(4-Chlorophenylsulfonyl)-1H-indol-3-yl)-2-(4-(pyrimidin-2-yl)piperazin-1-yl)ethanol (4d)
![Molecules 21 01070 i028]()
Prepared from (3d) (170 mg, 0.343 mmol) and sodium borohydride (16 mg, 0.422 mmol) to give a solid, which was purified by column chromatography on silica gel using AcOEt/hexane 9:1 to yield 126 mg of compound (4d) as pale brown crystalline plates. Yield: 75%; m.p.: 81–82 °C; IR (KBr) cm−1: 3423, 1586, 1360, 1176, 570. 1H-NMR δ (ppm): 8.32 (d, J = 4.7 Hz, 2H, H-4′′′ and H-6′′′); 7.96 (d, J = 8.3 Hz, 1H, H-4′); 7.81 (d, J = 8.6 Hz, 2H, H-2′′ and H-6′′); 7.62 (d, J = 7.8 Hz, 1H, H-7′); 7.56 (s, 1H, H-2′); 7.39 (d, J = 8.6 Hz, 2H, H-3′′ and H-5′′); 7.33 (t, J = 7.7, 1H, H-6′); 7.25 (t, J = 7.5 Hz, 1H, H-5′); 6.51 (t, J = 4.7 Hz, 1H, H-5′′′); 5.08 (dd, J = 10.0 and 3.2 Hz, 1H, H-1); 3.83-3.92 (m, 4H, H-3′′′′ and H-5′′′′); 3.37 (bs, 1H, OH); 2.79–2.87 (m, 2H, H-2a′′′′ and H-6a′′′′); 2.77 (d, J = 10.3 Hz, 1H, H-2a); 2.72 (dd, J = 12.5 and 3.4 Hz, 1H, H-2b); 2.52–2.61 (m, 2H, H-2b′′′′ and H-6b′′′′). 13C-NMR δ (ppm): 162.0, 158.2 (2C), 141.0, 136.9, 135.8, 130.1 (2C), 129.4, 128.6 (2C), 125.5, 124.3, 123.9, 123.2, 120.9, 114.1, 110.6, 64.5, 63.5, 53.5 (2C), 44.1 (2C). Elemental analysis for C24H24ClN5O3S (498.00 g/mol) calcd.: C: 57.88; H: 4.86; N: 14.06; S: 6.44. Found: C: 57.97; H: 4.88; N: 13.69; S: 6.78.
1-(1-(4-Fluorophenylsulfonyl)-1H-indol-3-yl)-2-(4-(pyrimidin-2-yl)piperazin-1-yl)ethanol (4e)
![Molecules 21 01070 i029]()
Prepared from (3e) (140 mg, 0.292 mmol) and sodium borohydride (13 mg, 0.340 mmol) to give a solid, which was purified by column chromatography on silica gel using AcOEt/hexane 9:1 to obtain 97 mg of compound (4e) as pale yellow crystalline plates. Yield: 69%; m.p.: 72–73 °C; IR (KBr) cm−1: 3422, 1587, 1360, 1180, 982, 573. 1H-NMR δ (ppm): 8.32 (d, J = 4.7 Hz, 2H, H-4′′′ and H-6′′′); 7.97 (d, J = 8.3 Hz, 1H, H-4′); 7.90 (dd, J = 8.9 and 4.9, 2H, H-2′′ and H-6′′); 7.63 (d, J = 7.8 Hz, 1H, H-7′); 7.57 (s, 1H, H-2′); 7.19–7.35 (m, 2H, H-5′ and H-6′); 7.09 (t, J = 8.5 Hz, 2H, H-3′′ and H-5′′); 6.50 (t, J = 4.7 Hz, 1H, H-5′′′); 5.07 (dd, J = 10.2 and 3.3 Hz, 1H, H-1); 3.84–3.95 (m, 4H, H-3′′′′ and H-5′′′′); 3.46 (bs, 1H, OH); 2.77–2.86 (m, 2H, H-2a′′′′ and H-6a′′′′); 2.77 (d, J = 10.2 Hz, 1H, H-2a); 2.71 (dd, J = 12.6 and 3.5 Hz, 1H, H-2b); 2.52–2.59 (m, 2H, H-2b′′′′ and H-6b′′′′). 13C-NMR δ (ppm): 166.1 (d, J = 257.2 Hz, 1C); 162.0; 158.2 (2C); 135.8; 134.6 (d, J = 3.1 Hz, 1C); 130.1 (d, J = 9.7 Hz, 2C); 125.4; 124.2; 123.8; 123.3; 120.8; 117.1 (d, J = 22.9 Hz, 2C); 115.2; 114.1; 110.5; 64.5; 63.6; 53.5 (2C); 44.2 (2C). Elemental analysis for C24H24FN5O3S (481.54 g/mol) calcd.: C: 59.86; H: 5.02; N: 14.54; S: 6.66. Found: C: 59.82; H: 5.13; N: 14.29; S: 6.99.
1-(1-(4-Iodophenylsulfonyl)-1H-indol-3-yl)-2-(4-(pyridin-2-yl)piperazin-1-yl)ethanol (4f)
![Molecules 21 01070 i030]()
Prepared from (3f) (130 mg, 0.222 mmol) and sodium borohydride (10 mg, 0.269 mmol) to give a solid, which was purified by column chromatography on silica gel using AcOEt/hexane 9:1 to obtain 72 mg of compound (4f) as pale brown crystalline plates. Yield: 56%; m.p.: 157–158 °C; IR (KBr) cm−1: 3382, 1592, 1384, 1174, 1122, 1098, 607, 568. 1H-NMR δ (ppm): 8.20 (dd, J = 4.8 and 1.2 Hz, 1H, H-3′′′); 7.96 (d, J = 8.3 Hz, 1H, H-4′); 7.76 (d, J = 8.6 Hz, 2H, H-2′′ and H-6′′); 7.62 (d, J = 7.8 Hz, 1H, H-7′); 7.57 (d, J = 8.7 Hz, 2H, H-3′′ and H-5′′); 7.55 (s, 1H, H-2′); 7.45–7.53 (m, 1H, H-5′′′); 7.33 (t, J = 7.3 Hz, 1H, H-5′); 7.25 (t, J = 7.2 Hz, 1H, H-6′); 6.62–6.68 (m, 2H, H-4′′′ and H-6′′′), 5.06 (dd, J = 9.6 and 3.7 Hz, 1H, H-1); 3.52–3.66 (m, 4H, H-3′′′′ and H-5′′′′); 2.81–2.91 (m, 2H, H-2a′′′′ and H-6a′′′′); 2.69–2.80 (m, 2H, H-2a and H-2b); 2.56–2.63 (m, 2H, H-2b′′′′ and H-6b′′′′). 13C-NMR δ (ppm): 159.8; 148.4; 139.0 (2C); 138.1; 138.0; 135.8; 129.4; 128.4 (2C); 125.5; 124.4; 123.9; 123.2; 120.9; 114.1; 114.0; 107.6; 102.2; 64.4; 63.6; 53.4 (2C); 45.8 (2C). Elemental analysis for C25H25IN4O3S (588.46 g/mol) calcd.: C: 51.03; H: 4.28; N: 9.52; S: 5.45. Found: C: 50.85; H: 4.44; N: 9.43; S: 5.51.
1-(1-(4-Iodophenylsulfonyl)-1H-indol-3-yl)-2-(4-(2-methoxyphenyl)piperazin-1-yl)ethanol (4g)
![Molecules 21 01070 i031]()
Prepared from (3g) (138 mg, 0.224 mmol) and sodium borohydride (10 mg, 0.269 mmol) to give a solid, which was purified by column chromatography on silica gel using AcOEt/hexane 9:1 to obtain 86 mg of compound (4g) as pale brown crystalline plates. Yield: 62%; m.p.: 109–110 °C; IR (KBr) cm−1: 3423, 1500, 1447, 1385, 1175, 1241, 1120, 748, 734, 608, 568. 1H-NMR δ (ppm): 7.96 (d, J = 8.2 Hz, 1H, H-4′); 7.75 (d, J = 8.4 Hz, 2H, H-2′′ and H-6′′); 7.63 (d, J = 7.8 Hz, 1H, H-7′); 7.56 (d, J = 8.3 Hz, 3H, H-2′, H-3′′ and H-5′′); 7.33 (t, J = 7.7 Hz, 1H, H-6′); 7.25 (t, J = 7.4 Hz, 1H, H-5′); 6.98-7.06 (m, 1H, H-5′′′); 6.90–6.97 (m, 2H, H-3′′′ and H-4′′′); 6.87 (d, J = 7.9 Hz, 1H, H-6′′′); 5.05 (t, J = 6.7 Hz, 1H, H-1); 3.87 (s, 3H, OCH3); 3.14 (bs, 4H, H-3′′′′ and H-5′′′′); 2.97 (bs, 2H, H-2a′′′′ and H-6a′′′′); 2.77 (d, J = 6.5 Hz, 2H, H-2); 2.70 (bs, 2H, H-2b′′′′ and H-6b′′′′). 13C-NMR δ (ppm): 152.7; 141.5; 139.0 (2C); 138.1; 135.8; 129.5; 128.4 (2C); 125.5; 124.6; 123.9; 123.6; 123.2; 121.5; 120.9; 118.7; 114.1; 111.7; 102.2; 64.4; 63.5; 55.8 (2C); 53.8; 51.1 (2C). Elemental analysis for C27H28IN3O4S (617.50 g/mol) calcd.: C: 52.52; H: 4.57; N: 6.80; S: 5.19 Found: C: 52.40; H: 4.75; N: 6.65; S: 5.55.
1-(1-(4-Iodophenylsulfonyl)-1H-indol-3-yl)-2-(4-(pyrimidin-2-yl)piperazin-1-yl)ethanol (4h)
![Molecules 21 01070 i032]()
Prepared from (3h) (147 mg, 0.25 mmol) and sodium borohydride (12 mg, 0.3 mmol) to give a solid, which was purified by column chromatography on silica gel using AcOEt/hexane 9:1 to yield 98 mg of (4h) as brown crystalline plates. Yield: 67%; m.p.: 171–172 °C; IR (KBr) cm−1: 3451, 1585, 1485, 1449, 1359, 1174, 1125, 982, 609, 569. 1H-NMR δ (ppm): 8.31 (d, J = 4.8 Hz, 2H, H-4′′′ and H-6′′′); 7.96 (d, J = 8.3 Hz, 1H, H-4′); 7.74 (d, J = 8.6 Hz, 2H, H-2′′ and H-6′′); 7.62 (d, J = 7.8 Hz, 1H, H-7′); 7.56 (d, J = 8.5 Hz, 2H, H-3′′ and H-5′′); 7.55 (s, 1H, H-2′); 7.32 (t, J = 7.4 Hz, 1H, H-6′); 7.24 (t, J = 7.3 Hz, 1H, H-5′); 6.50 (t, J = 4.7, 1H, H-5′′′); 5.06 (dd, J = 10.0 and 3.3 Hz, 1H, H-1); 3.82–3.94 (m, 4H, H-3′′′′ and H-5′′′′); 2.76–2.84 (m, 2H, H-2a′′′′ and H-6a′′′′); 2.75 (d, J = 10.1 Hz, 1H, H-2a); 2.70 (dd, J = 12.6 and 3.7 Hz, 1H, H-2b); 2.51–2.57 (m, 2H, H-2b′′′′ and H-6b′′′′). 13C-NMR δ (ppm): 162.0; 158.2 (2C); 139.0 (2C); 138.1; 135.8; 129.4; 128.4 (2C); 125.5; 124.4; 123.9; 123.2; 120.9; 114.1; 110.5; 102.2; 64.5; 63.6; 53.5 (2C); 44.2 (2C). Elemental analysis for C24H24IN5O3S (589.45 g/mol) calcd.: C: 48.90; H: 4.10; N: 11.88; S: 5.44. Found: C: 48.71; H: 4.28; N: 11.67; S: 5.58.
1-(1-(Naphthalen-1-ylsulfonyl)-1H-indol-3-yl)-2-(4-(pyridin-2-yl)piperazin-1-yl)ethanol (4i)
![Molecules 21 01070 i033]()
Prepared from (3i) (110 mg, 0.216 mmol) and sodium borohydride (10 mg, 0.269 mmol) to give a solid, which was purified by column chromatography on silica gel using AcOEt/hexane 9:1 to obtain 80 mg of compound (4i) as pale brown crystalline plates. Yield: 72%; m.p.: 80–81°C; IR (KBr) cm−1: 3423, 1594, 1437, 1361, 1171, 1122, 981, 769, 600. 1H-NMR δ (ppm): 8.72 (d, J = 8.6 Hz, 1H, H-2′′); 8.20 (d, J = 3.8 Hz, 1H, H-3′′′); 8.10 (d, J = 7.4 Hz, 1H, H-4′′); 8.02 (d, J = 8.2 Hz, 1H, H-4′); 7.85 (d, J = 9.3 Hz, 1H, H-7′); 7.83 (d, J = 9.1 Hz, 1H, H-8′′); 7.78 (s, 1H, H-2′); 7.60–7.65 (m, 2H, H-3′′ and H-5′′); 7.53 (t, J = 7.6 Hz, 1H, H-6′); 7.44–7.50 (m, 2H, H-5′ and H-5′′′); 7.16–7.27 (m, 2H, H-6′′ and H-7′′); 6.61–6.67 (m, 2H, H-4′′′ and H-6′′′); 5.08 (dd, J = 10.1 and 3.0 Hz, 1H, H-1); 3.51–3.64 (m, 4H, H-3′′′′ and H-5′′′′); 2.80–2.88 (m, 2H, H-2a′′′′ and H-6a′′′′); 2.77 (d, J = 10.3 Hz, 1H, H-2a); 2.69 (dd, J = 12.5 and 3.4 Hz, H-2b); 2.55–2.63 (m, 2H, H-2b′′′′ and H-6b′′′′). 13C-NMR δ (ppm): 159.8; 148.4; 137.9; 135.9; 135.8; 134.7; 134.5; 129.6; 129.5; 129.2; 129.0; 128.6; 127.6; 125.2; 124.5; 124.4; 123.9; 123.5; 122.9; 120.8; 114.0; 113.9; 107.6; 64.5; 63.7; 53.4 (2C); 45.8 (2C).Elemental analysis for C29H28N4O3S (512.62 g/mol) calcd.: C: 67.95; H: 5.51; N: 10.93; S: 6.26 Found: C: 68.10; H: 5.73; N: 11.16; S: 6.02.
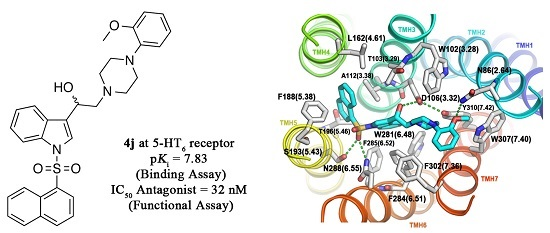
2-(4-(2-Methoxyphenyl)piperazin-1-yl)-1-(1-(naphthalen-1-ylsulfonyl)-1H-indol-3-yl)ethanol (4j)
![Molecules 21 01070 i034]()
Prepared from (3j) (82 mg, 0.15 mmol) and sodium borohydride (7 mg, 0.19 mmol) to give a solid, which was purified by column chromatography on silica gel using AcOEt/hexane 9:1 to obtain 80 mg of compound (4j) as light brown crystalline plates. Yield: 61%; m.p.: 89–90 °C; IR (KBr) cm−1: 3423, 1500, 1448, 1361, 1172, 1241, 1121, 747. 1H-NMR δ (ppm): 8.73 (d, J = 8.6 Hz, 1H, H-2′′); 8.08 (d, J = 7.4 Hz, 1H, H-4′′); 8.04 (d, J = 8.2 Hz, 1H, H-4′); 7.87 (d, J = 8.1 Hz, 1H, H-7′); 7.83 (d, J = 8.2 Hz, 1H, H-8′′); 7.78 (s, 1H, H-2′); 7.61–7.67 (m, 2H, H-3′′ and H-5′′); 7.55 (t, J = 7.5 Hz, 1H, H-6′); 7.49 (t, J = 7.9 Hz, 1H, H-5′); 7.18–7.28 (m, 2H, H-6′′ and H-7′′); 7.00–7.06 (m, 1H, H-5′′′); 6.92–6.96 (m, 2H, H-3′′′ and H-4′′′); 6.88 (d, J = 7.9 Hz, 1H, H-6′′′); 5.1 (dd, J = 9.7 and 3.2 Hz, 1H, H-1); 3.87 (s, 3H, OCH3); 3.15 (bs, 4H, H-3′′′′ and H-5′′′′); 2.98 (bs, 2H, H-2a′′′′ and H-6a′′′′); 2.71–2.85 (m, 4H, H-2a, H-2b, H-2b′′′′ and H-6b′′′′). 13C-NMR δ (ppm): 152.7; 141.5; 135.9; 135.8; 134.7; 134.5; 129.5 (2C); 129.1; 129.0; 128.6; 127.6; 125.1 (2C); 124.5; 123.9; 123.6; 123.5; 123.0; 121.5; 120.8; 118.7; 114.0; 111.7; 64.4; 63.5; 55.8 (2C); 53.8; 51.1 (2C). Elemental analysis for C31H31N3O4S (541.66 g/mol) calcd.: C: 68.74; H: 5.77; N: 7.76; S: 5.92 Found: C: 68.58; H: 5.92; N: 7.62; S: 5.72.
1-(1-(Naphthalen-1-ylsulfonyl)-1H-indol-3-yl)-2-(4-(pyrimidin-2-yl)piperazin-1-yl)ethanol (4k)
![Molecules 21 01070 i035]()
Prepared from (3k) (73 mg, 0,143 mmol) and sodium borohydride (10 mg, 0.26 mmol) to give a solid, which was purified by column chromatography on silica gel using AcOEt/hexane 9:1 to obtain 45 mg of compound (4k) as pale brown crystalline plates. Yield: 62%; m.p.: 82–83 °C; IR (KBr) cm−1: 3424, 1586, 1447, 1360, 1171, 1122, 983, 599. 1H-NMR δ (ppm): 8.72 (d, J = 8.7 Hz, 1H, H-2′′); 8.31 (d, J = 4.7 Hz, 2H, H-4′′′ and H-6′′′); 8.09 (d, J = 7.4 Hz, 1H, H-4′′); 8.02 (d, J = 8.2 Hz, 1H, H-4′); 7.85 (d, J = 8.2 Hz, 1H, H-7′); 7.82 (d, J = 8.4 Hz, 1H, H-8′′); 7.78 (s, 1H, H-2′); 7.60–7.65 (m, 2H, H-3′′ and H-5′′); 7.54 (t, J = 7.5 Hz, 1H, H-6′); 7.47 (t, J = 7.9 Hz, 1H, H-5′); 7.17–7.27 (m, 2H, H-6′′ and H-7′′), 6.50 (t, J = 4.7 Hz, 1H, H-5′′′), 5.09 (dd, J = 10.2 and 2.8 Hz, 1H, H-1); 3.83–3.93 (m, 4H, H-3′′′′ and H-5′′′′); 2.75 (m, 3H, H-2a′′′′, H-6a′′′′ and H-2a); 2.69 (dd, J = 12.5 and 3.1 Hz, H-1, H-2b); 2.51–2.58 (m, 2H, H-2b′′′′, H-6b′′′′). 13C-NMR δ (ppm): 162.0; 158.1 (2C), 135.9; 135.8; 134.7; 134.5; 129.6; 129.5; 129.1; 129.0; 128.6; 127.6; 125.2; 124.5; 124.4; 123.9; 123.5; 122.8; 120.8; 114.0; 110.5; 64.6; 63.6; 53.5 (2C); 44.2 (2C). Elemental analysis for C28H27N5O3S (513.61 g/mol) calcd.: C: 65.48; H: 5.30; N: 13.64; S: 6.24 Found: C: 65.57; H: 5.11; N: 13.49; S: 6.03.
1-(1-(4-Methoxyphenylsulfonyl)-1H-indol-3-yl)-2-morpholinoethanol (4l)
![Molecules 21 01070 i036]()
Prepared from (3l) (73 mg, 0.18 mmol) and sodium borohydride (67 mg, 1.77 mmol) to give a solid, which was purified by column chromatography on silica gel using AcOEt to yield 39 mg of compound (4l) as yellow crystalline plates. Yield: 53%; m.p.: 149–152 °C; IR (KBr) cm−1: 3454, 1595, 1364, 1270, 1166, 1117, 573. 1H-NMR δ (ppm): 7.97 (d, J = 8.3 Hz, 1H, H-4′); 7.81 (d, J = 9.0 Hz, 2H, H-2′′ and H-6′′); 7.60 (d, J = 7.8 Hz, 1H, H-7′); 7.57 (s, 1H, H-2′); 7.31 (t, J = 7.5 Hz, 1H, H-6′); 7.22 (t, J = 7.5 Hz, 1H, H-5′); 6.85 (d, J = 9.0 Hz, 2H, H-3′′ and H-5′′); 5.02 (dd, J = 9.1 and 4.4 Hz, 1H, H-1); 3.69–3.80 (m, 7H, H-3′′′′, H-5′′′′ and OCH3); 2.68–2.77 (m, 4H, H-2a′′′′, H-6a′′′′, H-2a and H-2b); 2.45–2.51 (m, 2H, H-2b′′′′ and H-6b′′′′). 13C-NMR δ (ppm): 164.2, 135.8, 130.1, 129.5 (2C), 129.3, 125.2, 123.5, 123.4 (2C), 120.6, 114.9 (2C), 114.2, 67.4 (2C), 64.9, 63.4, 56.1 (2C), 54.0. Elemental analysis for C21H24N2O5S (416.49 g/mol) calcd.: C: 60.56; H: 5.81; N: 6.73; S: 7.70 Found: C: 60.42; H: 5.88; N: 6.79; S: 7.57.
1-(1-(3,5-Difluorophenylsulfonyl)-1H-indol-3-yl)-2-morpholinoethanol (4m)
![Molecules 21 01070 i037]()
Prepared from (3m) (70 mg, 0.167 mmol) and sodium borohydride (10 mg, 0.26 mmol) to give an oil, which was purified by column chromatography on silica gel using AcOEt to obtain 46 mg of (4m) as a yellow oil. Yield: 66%; m.p.: product is an oil; IR (KBr) cm−1: 3381 (OH), 1606, 1444, 1384 and 1179, 1298, 1115, 617. 1H-NMR δ (ppm): 7.96 (d, J = 8.3 Hz, 1H, H-4′); 7.63 (d, J = 7.8 Hz, 1H, H-7′); 7.52 (d, J = 0.6 Hz, 1H, H-2′); 7.41 (m, 2H, H-2′′ and H-6′′); 7.36 (m, 1H, H-6′); 7.27 (m, 1H, H-5′); 6.98 (tt, J = 8.4 and 2.3 Hz, 1H, H-4′′); 5.03 (ddd, J = 8.6, 5.1 and 0.7 Hz, 1H, H-1); 3.77 (m, 4H, H-3′′′′ and H-5′′′′); 2.76 (m, 2H, H-2a′′′′ and H-6a′′′′); 2.71 (s, 1H, H-2a); 2.70 (d, J= 3.9 Hz, 1H, H-2b); 2.50 (m, 2H, H-2b′′′′ and H-6b′′′′). 13C-NMR δ (ppm): 163.1 (dd, J = 255.8 and 11.7 Hz, 2C); 141.4 (t, J = 8.5 Hz, 1C), 135.8, 129.4, 125.8, 124.9, 124.2, 123.0, 121.0, 114.1, 111.0 (m, 2C), 110.0 (t, J = 25.0 Hz, 1C), 67.4 (2C), 64.8, 63.3, 54.0 (2C). Elemental analysis for C20H20F2N2O4S (422.45 g/mol) calcd.: C: 56.86; H: 4.77; N: 6.63; S: 7.59 Found: C: 56.69; H: 4.93; N: 6.74; S: 7.52.