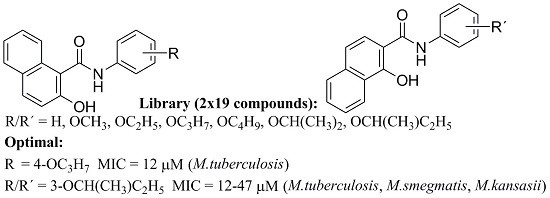
N-Alkoxyphenylhydroxynaphthalenecarboxamides and Their Antimycobacterial Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry and Physicochemical Properties
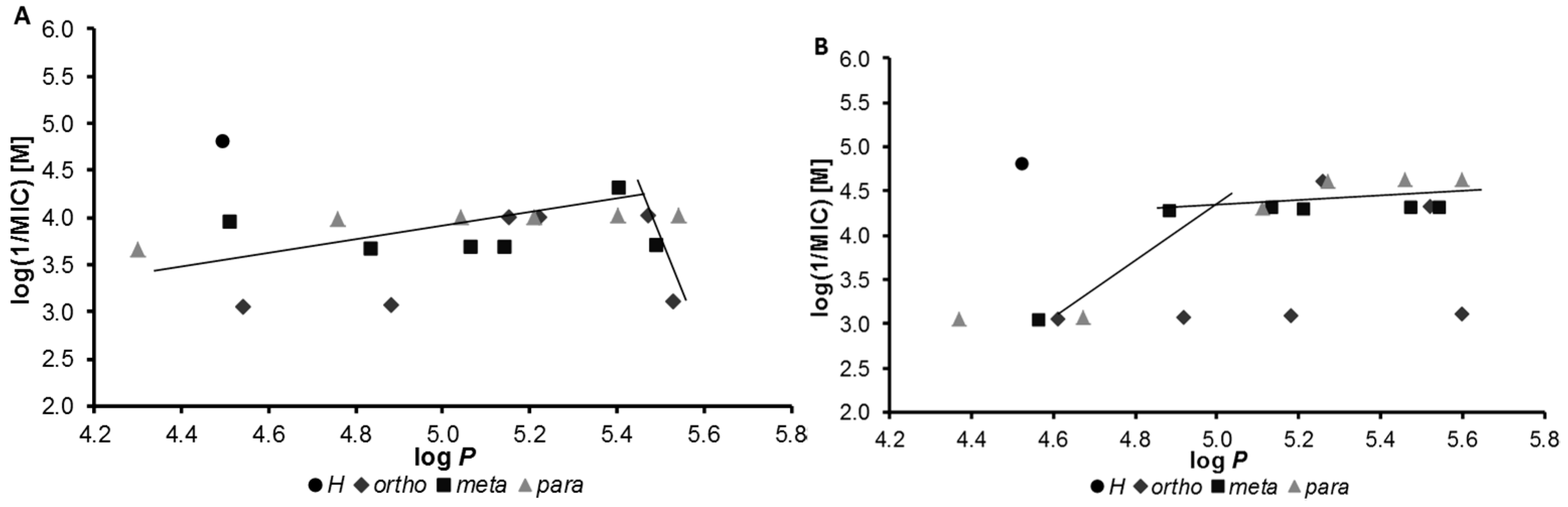
2.2. In Vitro Antimycobacterial Evaluation
2.3. In Vitro Antiproliferative Assay
3. Experimental Section
3.1. General Information
3.2. Synthesis
3.2.1. General Procedure for Synthesis of N-(Alkoxyphenyl)-2-hydroxynaphthalene-1-carboxamides 1–7c and N-(Alkoxyphenyl)-1-hydroxynaphthalene-2-carboxamides 8–14c
3.3. In Vitro Antimycobacterial Evaluation
3.4. MTT Assay
3.5. In Vitro Antiproliferative Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Working Group on New TB Drugs 2016. Available online: http://www.newtbdrugs.org/blog/category/tb-news/ (accessed on 1 July 2016).
- World Health Organization. Global Tuberculosis Report 2015; WHO Press: Geneva, Switzerland, 2015. [Google Scholar]
- Ioachimescu, O.C.; Tomford, J.W. Nontuberculous mycobacterial disorders. In Disease Management Project; Carey, W., Ed.; Cleveland Clinic—Centre for Continuing Education: Cleveland, OH, USA, 2015; Available online: http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/infectious-disease/nontuberculous-mycobacterial-disorders/Default.htm (accessed on 1 July 2016).
- Kratky, M.; Vinsova, J. Salicylanilide ester prodrugs as potential antimicrobial agents—A review. Curr. Pharm. Des. 2011, 17, 3494–3505. [Google Scholar] [CrossRef] [PubMed]
- Zadrazilova, I.; Pospisilova, S.; Pauk, K.; Imramovsky, A.; Vinsova, J.; Cizek, A.; Jampilek, J. In vitro bactericidal activity of 4- and 5-chloro-2-hydroxy-N-(1-oxo-1-(phenylamino)alkan-2-yl)benzamides against MRSA. BioMed. Res. Int. 2015, 2015, 349534. [Google Scholar] [CrossRef] [PubMed]
- Zadrazilova, I.; Pospisilova, S.; Masarikova, M.; Imramovsky, A.; Monreal-Ferriz, J.; Vinsova, J.; Cizek, A.; Jampilek, J. Salicylanilide carbamates: Promising antibacterial agents with high in vitro activity against methicillin-resistant Staphylococcus aureus. Eur. J. Pharm. Sci. 2015, 77, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Pattabiraman, V.R.; Bode, J.W. Rethinking amide bond synthesis. Nature 2011, 480, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Imramovsky, A.; Vinsova, J.; Monreal-Ferriz, J.; Dolezal, R.; Jampilek, J.; Kaustova, J.; Kunc, F. New antituberculotics originated from salicylanilides with promising in vitro activity against atypical mycobacterial strains. Bioorg. Med. Chem. 2009, 17, 3572–3579. [Google Scholar] [CrossRef] [PubMed]
- Zumla, A.; Nahid, P.; Cole, S.T. Advances in the development of new tuberculosis drugs and treatment regimens. Nat. Rev. Drug Discov. 2013, 12, 388–404. [Google Scholar] [CrossRef] [PubMed]
- Zucca, M.; Scutera, S.; Savoia, D. New chemotherapeutic strategies against malaria, leishmaniasis and trypanosomiases. Curr. Med. Chem. 2013, 20, 502–526. [Google Scholar] [CrossRef] [PubMed]
- Gonec, T.; Kos, J.; Zadrazilova, I.; Pesko, M.; Keltosova, S.; Tengler, J.; Bobal, P.; Kollar, P.; Cizek, A.; Kralova, K.; et al. Antimycobacterial and herbicidal activity of ring-substituted 1-hydroxynaphthalene-2-carboxanilides. Bioorg. Med. Chem. 2013, 21, 6531–6541. [Google Scholar] [CrossRef] [PubMed]
- Pauk, K.; Zadrazilova, I.; Imramovsky, A.; Vinsova, J.; Pokorna, M.; Masarikova, M.; Cizek, A.; Jampilek, J. New derivatives of salicylamides: Preparation and antimicrobial activity against various bacterial species. Bioorg. Med. Chem. 2013, 21, 6574–6581. [Google Scholar] [CrossRef] [PubMed]
- Jampilek, J. Recent advances in design of potential quinoxaline anti-infectives. Curr. Med. Chem. 2014, 21, 4347–4373. [Google Scholar] [CrossRef] [PubMed]
- Kos, J.; Nevin, E.; Soral, M.; Kushkevych, I.; Gonec, T.; Bobal, P.; Kollar, P.; Coffey, A.; O´Mahony, J.; Liptaj, T.; et al. Synthesis and antimycobacterial properties of ring-substituted 6-hydroxynaphthalene-2-carboxanilides. Bioorg. Med. Chem. 2015, 23, 2035–2043. [Google Scholar] [CrossRef] [PubMed]
- Kos, J.; Zadrazilova, I.; Nevin, E.; Soral, M.; Gonec, T.; Kollar, P.; Oravec, M.; Coffey, A.; O´Mahony, J.; Liptaj, T.; et al. Ring-substituted 8-Hydroxyquinoline-2-carboxanilides as potential antimycobacterial agents. Bioorg. Med. Chem. 2015, 23, 4188–4196. [Google Scholar] [CrossRef] [PubMed]
- Gonec, T.; Kos, J.; Zadrazilova, I.; Pesko, M.; Govender, R.; Keltosova, S.; Kollar, P.; Imramovsky, A.; O´Mahony, J.; Coffey, A.; et al. Antibacterial and herbicidal activity of ring-substituted 2-hydroxynaphthalene-1-carboxanilides. Molecules 2013, 18, 9397–9419. [Google Scholar] [CrossRef] [PubMed]
- De Marco, A.; de Candia, M.; Carotti, A.; Cellamare, S.; de Candia, E.; Altomare, C. Lipophilicity-related inhibition of blood platelet aggregation by nipecotic acid anilides. Eur. J. Pharm. Sci. 2004, 22, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Gonec, T.; Zadrazilova, I.; Nevin, E.; Kauerova, T.; Pesko, M.; Kos, J.; Oravec, M.; Kollar, P.; Coffey, A.; O´Mahony, J.; et al. Synthesis and biological evaluation of N-alkoxyphenyl-3-hydroxynaphthalene-2-carboxanilides. Molecules 2015, 20, 9767–9787. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Lu, L.; Wang, B.; Pu, S.; Zhang, X.; Zhu, G.; Shi, W.; Zhang, L.; Wang, H.; Wang, S.; et al. Genetic basis of virulence attenuation revealed by comparative genomic analysis of Mycobacterium tuberculosis strain H37Ra versus H37Rv. PLoS ONE 2008, 3, e2375. [Google Scholar] [CrossRef] [PubMed]
- Matveychuk, A.; Fuks, L.; Priess, R.; Hahim, I.; Shitrit, D. Clinical and radiological features of Mycobacterium kansasii and other NTM infections. Resp. Med. 2012, 106, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Acharya, N.; Varshney, U. Biochemical properties of single-stranded DNA-binding protein from Mycobacterium smegmatis, a fast-growing Mycobacterium and its physical and functional interaction with uracil DNA glycosylases. J. Mol. Biol. 2002, 318, 1251–1264. [Google Scholar] [CrossRef]
- Bueno, J. Antitubercular in vitro drug discovery: Tools for begin the search. In Understanding Tuberculosis-New Approaches to Fighting against Drug Resistance; Cardona, P.J., Ed.; InTech: Rijeka, Croatia, 2012; pp. 147–168. [Google Scholar]
- Terada, H. Uncouplers of oxidative phosphorylation. Environ. Health Perspect. 1990, 87, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhu, W.; Schurig-Briccio, L.A.; Lindert, S.; Shoen, C.; Hitchings, R.; Li, J.; Wang, Y.; Baig, N.; Zhou, T.; et al. Antiinfectives targeting enzymes and the proton motive force. Proc. Natl. Acad. Sci. USA 2015, 112, E7073–E7082. [Google Scholar] [CrossRef] [PubMed]
- Imramovsky, A.; Pesko, M.; Monreal-Ferriz, J.; Kralova, K.; Vinsova, J.; Jampilek, J. Photosynthesis-inhibiting efficiency of 4-chloro-2-(chlorophenylcarbamoyl)phenyl alkyl-carbamates. Bioorg. Med. Chem. Lett. 2011, 21, 4564–4567. [Google Scholar] [CrossRef] [PubMed]
- Kralova, K.; Perina, M.; Waisser, K.; Jampilek, J. Structure-activity relationships of N-benzylsalicylamides for inhibition of photosynthetic electron transport. Med. Chem. 2015, 11, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Terada, H.; Goto, S.; Yamamoto, K.; Takeuchi, I.; Hamada, Y.; Miyake, K. Structural requirements of salicylanilides for uncoupling activity in mitochondria: Quantitative analysis of structure-uncoupling relationships. Biochim. Biophys. Acta. 1988, 936, 504–512. [Google Scholar] [CrossRef]
- Parasitipedia.net. Salicylanilides: Anthelmintics for Veterinary Use on Cattle, Sheep, Goats, Pigs, Poultry, Horses, Dogs and Cats against Parasitic Worms. Available online: http://parasitipedia.net/index.php?option=com_content&view=article&id=2447&Itemid=2714 (accessed on 4 August 2016).
- Arjunan, V.; Santhanam, R.; Rani, T.; Rosi, H.; Mohan, S. Conformational, vibrational, NMR and DFT studies of N-methylacetanilide. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 104, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Malik, I.; Bukovsky, M.; Andriamainty, F.; Galisinova, J. Anti-microbial activity of meta-alkoxyphenylcarbamates containing substituted N-phenylpiperazine fragment. Braz. J. Microbiol. 2012, 43, 959–965. [Google Scholar] [PubMed]
- Sindt, A.; Mackay, M. Flukicides. IV. Crystal and molecular structure of 3′-chloro-4′-(p-chlorophenoxy)-3,5-diiodosalicylanilide (rafoxanide). Aust. J. Chem. 1980, 33, 203–207. [Google Scholar] [CrossRef]
- Li, D.D.; Qin, Y.J.; Sun, J.; Li, J.R.; Fang, F.; Du, Q.R.; Qian, Y.; Gong, H.B.; Zhu, H.L. Optimization of substituted 6-salicyl-4-anilinoquinazoline derivatives as dual EGFR/HER2 tyrosine kinase inhibitors. PLoS ONE 2013, 8, e69427. [Google Scholar] [CrossRef] [PubMed]
- Musiol, R.; Jampilek, J.; Buchta, V.; Niedbala, H.; Podeszwa, B.; Palka, A.; Majerz-Maniecka, K.; Oleksyn, B.; Polanski, J. Antifungal properties of new series of quinoline derivatives. Bioorg. Med. Chem. 2006, 14, 3592–3598. [Google Scholar] [CrossRef] [PubMed]
- Bobal, P.; Sujan, J.; Otevrel, J.; Imramovsky, A.; Padelkova, Z.; Jampilek, J. Microwave-assisted synthesis of new substituted anilides of quinaldic acid. Molecules 2012, 17, 1292–1306. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Schurig-Briccio, L.A.; Feng, X.; Upadhyay, A.; Pujari, V.; Lechartier, B.; Fontes, F.L.; Yang, H.; Rao, G.; Zhu, W.; et al. Multitarget drug discovery for tuberculosis and other infectious diseases. J. Med. Chem. 2014, 57, 3126–3139. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.T.; Liu, J.; Lee, R.B.; Lee, R.E. New agents for the treatment of drug-resistant Mycobacterium tuberculosis. Adv. Drug Deliv. Rev. 2016, 102, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Thannaa, S.; Sucheck, S.J. Targeting the trehalose utilization pathways of Mycobacterium tuberculosis. Med. Chem. Commun. 2016, 7, 69–85. [Google Scholar] [CrossRef] [PubMed]
- ROCHE. Cell Proliferation Reagent WST-1. Roche Diagnostics GmbH: Mannheim, Germany, 2011. Available online: http://www.molecularinfo.com/MTM/J/J2/J2–2/J2–2.pdf (accessed on 1 July 2016).
- Suffness, M.; Douros, J. Current status of the NCI plant and animal product program. J. Nat. Prod. 1982, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.H.; Wintringham, A.C.; American Cyanamid Co. N-Substituted Amides of Aromatic ortho-Hydroxy Carboxylic Acids. US 2,410,397, 29 October 1946. [Google Scholar]
- Jadhav, G.V.; Rao, S.N.; Hirwe, N.W. Derivatives of 1-hydroxy-2-naphthoic acid. Part III. Arylamides and their bromination products. J. Univ. Bombay B 1936, 5, 137–141. [Google Scholar]
- Schwalbe, R.; Steele-Moore, L.; Goodwin, A.C. Antimicrobial Susceptibility Testing Protocols; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Sample Availability: Samples of compounds are available from authors T. Gonec, J. Kos, J. Jampilek.


 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound | R | log P a | MV a | σ a | MIC (µM) | IC50 (µM) | ||
| MT | MS | MK | ||||||
| 1 | H | 4.49 | 0 | 0 | 94 | 486 | 15 | >30 |
| 2a | 2-OCH3 | 4.54 | 37.15 | −0.28 | 852 | 873 | 873 | - |
| 2b | 3-OCH3 | 4.51 | 37.15 | 0.11 | 852 | 218 | 109 | - |
| 2c | 4-OCH3 | 4.30 | 37.15 | −0.27 | 852 | 436 | 218 | - |
| 3a | 2-OC2H5 | 4.88 | 53.66 | −0.29 | 26 | 832 | 832 | >30 |
| 3b | 3-OC2H5 | 4.83 | 53.66 | 0.10 | 813 | 416 | 208 | - |
| 3c | 4-OC2H5 | 4.76 | 53.66 | −0.29 | 26 | 832 | 104 | >30 |
| 4a | 2-OC3H7 | 5.22 | 70.16 | −0.29 | 37 | 796 | 99 | >30 |
| 4b | 3-OC3H7 | 5.14 | 70.16 | 0.14 | 46 | 398 | 199 | 8.97 ± 0.34 |
| 4c | 4-OC3H7 | 5.21 | 70.16 | −0.28 | 12 | 398 | 99 | >30 |
| 5a | 2-OC4H9 | 5.53 | 86.67 | −0.28 | 745 | 763 | 763 | >30 |
| 5b | 3-OC4H9 | 5.49 | 86.67 | 0.14 | 23 | 381 | 190 | >30 |
| 5c | 4-OC4H9 | 5.54 | 86.67 | −0.28 | 745 | 763 | 95 | >30 |
| 6a | 2-OCH(CH3)2 | 5.15 | 70.54 | −0.47 | 31 | 796 | 99 | >30 |
| 6b | 3-OCH(CH3)2 | 5.06 | 70.54 | 0.10 | 778 | 398 | 199 | 8.84 ± 0.22 |
| 6c | 4-OCH(CH3)2 | 5.04 | 70.54 | −0.45 | 25 | 796 | 99 | >30 |
| 7a | 2-OCH(CH3)CH2CH3 | 5.47 | 87.05 | −0.27 | 44 | 763 | 95 | 8.35 ± 1.25 |
| 7b | 3-OCH(CH3)CH2CH3 | 5.40 | 87.05 | 0.14 | 23 | 12 | 47 | >30 |
| 7c | 4-OCH(CH3)CH2CH3 | 5.40 | 87.05 | −0.27 | 23 | 12 | 95 | >30 |
 | ||||||||
| Compound | R | log P a | MV a | σ a | MIC (µM) | IC50 (µM) | ||
| MT | MS | MK | ||||||
| 8 | H | 4.52 | 0 | 0 | 97 | 243 | 15 | >30 |
| 9a | 2-OCH3 | 4.61 | 37.15 | −0.28 | 852 | 873 | 873 | - |
| 9b | 3-OCH3 | 4.56 | 37.15 | 0.11 | 852 | 873 | 873 | - |
| 9c | 4-OCH3 | 4.37 | 37.15 | −0.27 | 852 | 873 | 873 | - |
| 10a | 2-OC2H5 | 4.92 | 53.66 | −0.29 | 813 | 832 | 832 | - |
| 10b | 3-OC2H5 | 4.88 | 53.66 | 0.10 | 813 | 832 | 52 | 3.02 ± 0.96 |
| 10c | 4-OC2H5 | 4.67 | 53.66 | −0.29 | 813 | 832 | 832 | - |
| 11a | 2-OC3H7 | 5.26 | 70.16 | −0.29 | 778 | 796 | 24 | - |
| 11b | 3-OC3H7 | 5.21 | 70.16 | 0.14 | 24 | 199 | 49 | 1.37 ± 0.72 |
| 11c | 4-OC3H7 | 5.27 | 70.16 | −0.28 | 778 | 796 | 24 | >30 |
| 12a | 2-OC4H9 | 5.60 | 86.67 | −0.28 | 745 | 763 | 763 | - |
| 12b | 3-OC4H9 | 5.54 | 86.67 | 0.14 | 23 | 190 | 47 | 1.80 ± 0.32 |
| 12c | 4-OC4H9 | 5.60 | 86.67 | −0.28 | 745 | 763 | 23 | >30 |
| 13a | 2-OCH(CH3)2 | 5.18 | 70.54 | −0.47 | 778 | 796 | 796 | - |
| 13b | 3-OCH(CH3)2 | 5.13 | 70.54 | 0.10 | 389 | 796 | 796 | 4.32 ± 0.74 |
| 13c | 4-OCH(CH3)2 | 5.11 | 70.54 | −0.45 | 778 | 796 | 49 | 9.39 ± 1.39 |
| 14a | 2-OCH(CH3)CH2CH3 | 5.52 | 87.05 | −0.27 | 745 | 763 | 47 | 9.03 ± 1.18 |
| 14b | 3-OCH(CH3)CH2CH3 | 5.47 | 87.05 | 0.14 | 23 | 12 | 47 | 1.17 ± 0.38 |
| 14c | 4-OCH(CH3)CH2CH3 | 5.46 | 87.05 | −0.27 | 745 | 12 | 23 | 3.41 ± 0.41 |
| INH | - | −0.63 | - | - | 58 | 117 | 29 | >30 |
| RIF | - | 2.24 | - | - | 10 | 19 | 0.15 | >30 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonec, T.; Pospisilova, S.; Kauerova, T.; Kos, J.; Dohanosova, J.; Oravec, M.; Kollar, P.; Coffey, A.; Liptaj, T.; Cizek, A.; et al. N-Alkoxyphenylhydroxynaphthalenecarboxamides and Their Antimycobacterial Activity. Molecules 2016, 21, 1068. https://doi.org/10.3390/molecules21081068
Gonec T, Pospisilova S, Kauerova T, Kos J, Dohanosova J, Oravec M, Kollar P, Coffey A, Liptaj T, Cizek A, et al. N-Alkoxyphenylhydroxynaphthalenecarboxamides and Their Antimycobacterial Activity. Molecules. 2016; 21(8):1068. https://doi.org/10.3390/molecules21081068
Chicago/Turabian StyleGonec, Tomas, Sarka Pospisilova, Tereza Kauerova, Jiri Kos, Jana Dohanosova, Michal Oravec, Peter Kollar, Aidan Coffey, Tibor Liptaj, Alois Cizek, and et al. 2016. "N-Alkoxyphenylhydroxynaphthalenecarboxamides and Their Antimycobacterial Activity" Molecules 21, no. 8: 1068. https://doi.org/10.3390/molecules21081068