The Role of Natural Polyphenols in the Prevention and Treatment of Cervical Cancer—An Overview
Abstract
:1. Introduction
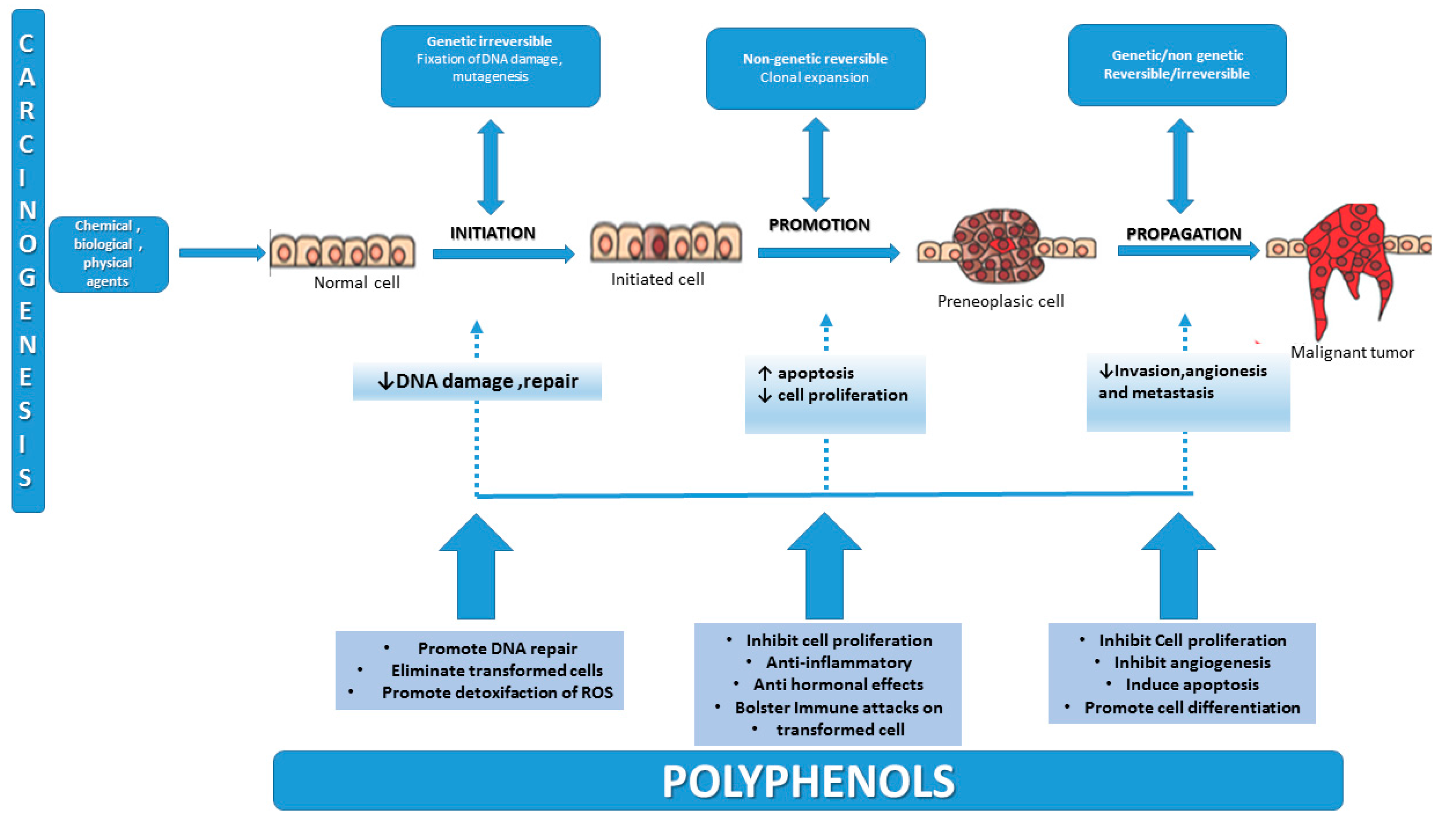
2. Carcinogenesis—An Overview of the Multifactorial Process
3. Antioxidants and Their Role in Preventing Cancer
4. Natural Sources of Polyphenols, Bioavailability and Effects on Cervical Cancer
4.1. Flavonoids
4.1.1. Flavonols
Kaempferol
Quercetin
4.1.2. Flavones
Apigenin
4.1.3. Isoflavones
4.1.4. Flavan-3-ols
Epigallocatechin-3-gallate
4.1.5. Flavanones
Naringenin
4.1.6. Anthocyanidins
4.2. Non-Flavonoids
4.2.1. Phenolic Acids
4.2.2. Curcumin
4.2.3. Stilbenes
5. Studies of Polyphenols and Cervical Cancer
6. Combinations of Polyphenols and Cervical Anti-Cancer Therapy
6.1. Polyphenols and Chemotherapy
6.2. Polyphenols and Irradiation
7. Conclusions and Future Perspectives
Acknowlegments
Author Contributions
Conflicts of Interest
Abbreviations
| 8-OhdG | 8-Oxo-2′-deoxyguanosine |
| ACE-c | Aqueous cinnamon extract |
| AKT | Protein Kinase B |
| AP-1 | Activating protein |
| Bak | Bcl-2 homologous antagonist/killer |
| Bax | Bcl-2-like protein 4 |
| Bcl-2 | B-cell lymphoma 2 |
| CaSki | Human cervical carcinoma cell line |
| Cdk1 | Cyclin-dependent kinase 1 |
| CIN | Cervical intraepithelial neoplasia |
| COX-2 | Cyclooxigenaze 2 |
| Cx43 | Connexin43 |
| DR5 | Death receptor 5 |
| EGCG | Epigallocatechin gallate |
| ERK1/2 | Extracellular signal-regulated kinases |
| ERα | Estrogen receptor alpha |
| EU | European Union |
| Fas/APO-1 | Fas receptor/Apoptosis antigen 1 |
| Her-2 | Human Epidermal Growth Factor Receptor 2 |
| HIV1 | Human Immunodeficiency Virus |
| HPV | Human Papiloma Virus |
| HSIL | High squamous intraepithelial lesions |
| JNK | c-Jun N-terminal kinase |
| LSIL | Low Squamous Intraepithelial Lesions |
| MAPK | Mitogen-Activated Protein Kinase |
| MAPKs | Mitogen-Activated Proteins |
| MMP | Metalloproteinases |
| NF-κB | Nuclear Factor-Kappab |
| p65 | Transcription factor p65 |
| ROS | Reactive oxygen species |
| SIL | Squamous Intraepithelial Lesions |
| TRAIL | Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand) |
| UV | Ultraviolet |
| WHO | World Health Organization |
References
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Antoine, J.; Mägi, M.; Smailyte, G.; Stengrevics, A.; Suteu, O.; Valerianova, Z.; Bray, F.; Weiderpass, E. Trends in cervical cancer incidence and mortality in the Baltic countries, Bulgaria and Romania. Int. J. Cancer 2011, 128, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Researvh on Cancer. Globocan 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available online: http://www.globocan.iarc.fr (accessed on 2 March 2016).
- Tomatis, L.; Huff, J.; Hertz-Picciotto, I.; Sadler, D.P.; Bucher, J.; Boffetta, P.; Axelson, O.; Blair, A.; Taylor, J.; Stayner, L.; et al. Avoided and avoidable risks of cancer. Carcinogenesis 1997, 18, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Cogliano, V.J.; Baan, R.; Straif, K.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N. Preventable exposures associated with human cancers. J. Natl. Cancer Inst. 2011, 103, 1827–1839. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, K.; Pischon, T.; Jenab, M.; Bueno-de-Mesquita, H.; Fedirko, V.; Norat, T.; Romaguera, D.; Knuppel, S.; Boutron-Ruault, M.-C.; Dossus, L.; et al. Combined impact of healthy lifestyle factors on colorectal cancer: A large European cohort study. BMC Med. 2014, 12. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.O.; Thomson, C.A. Physical activity and cancer survivorship. Nutr. Clin. Pract. 2014. [Google Scholar] [CrossRef] [PubMed]
- Howes, M.J.; Simmonds, M.S. The role of phytochemicals as micronutrients in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, R.V.; Nagini, S. Cancer chemoprevention by dietary phytochemicals: Promises and pitfalls. Curr. Pharm. Biotechnol. 2012, 13, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Bode, A.M.; Dong, Z. Molecular targets of phytochemicals for cancer prevention. Nat. Rev. Cancer 2011, 11, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Loeb, L.A.; Harris, C.C. Advances in chemical carcinogenesis: A historical review and prospective. Cancer Res. 2008, 68, 6863–6872. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Broges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef] [PubMed]
- Brglez Mojzer, E.B.; Knez Hrnčič, M.; Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, P.; Blasiak, J. Role of mitochondria in carcinogenesis. Acta Biochim. Pol. 2014, 61, 671–678. [Google Scholar] [PubMed]
- Maru, G.B.; Hudlikar, R.R.; Kumar, G.; Gandhi, K.; Mahimkar, M.B. Understanding the molecular mechanisms of cancer prevention by dietary phytochemicals: From experimental models to clinical trials. World J. Biol. Chem. 2016, 7, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, R.; Takami, A.; Espinoza, J.L. Dietary phytochemicals and cancer chemoprevention: A review of the clinical evidence. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Barcellos-Hoff, M.H.; Lyden, D.; Wang, T.C. The evolution of the cancer niche during multistage carcinogenesis. Nat. Rev. Cancer 2013, 13, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Letelier, P.; Brebi, P.; Tapia, O.; Roa, J.C. DNA promoter methylation as a diagnostic and therapeutic biomarker in gallbladder cancer. Clin. Epigenet. 2012, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Aoi, J.; Endo, M.; Kadomatsu, T.; Miyata, K.; Ogata, A.; Horiguchi, H.; Odagiri, H.; Masuda, T.; Fukushima, S.; Jinnin, M.; et al. Angiopoietin-like protein 2 accelerates carcinogenesis by activating chronic inflammation and oxidative stress. Mol. Cancer Res. 2014, 12, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Solomon, H.; Brosh, R.; Buganim, Y.; Rotter, V. Inactivation of the p53 tumor suppressor gene and activation of the Ras oncogene: Cooperative events in tumorigenesis. Discov. Med. 2010, 9, 448–454. [Google Scholar] [PubMed]
- Collins, A.R.; Azqueta, A.; Langie, S.A. Effects of micronutrients on DNA repair. Eur. J. Nutr. 2012, 51, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Royston, K.J.; Tollefsbol, T.O. The epigenetic impact of cruciferous vegetables on cancer prevention. Curr. Pharmacol. Rep. 2015, 1, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.Y.; Stors, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Sgambato, A.; Zannoni, G.F.; Faraglia, B.; Camerini, A.; Tarquini, E.; Spada, D.; Cittadini, A. Decreased expression of the CDK inhibitor p27Kip1 and increased oxidative DNA damage in the multistep process of cervical carcinogenesis. Gynecol. Oncol. 2004, 92, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Looi, M.L.; Dali, A.Z.H.M.; Ali, S.A.M.; Ngah, W.Z.W.; Yusof, Y.A.M. Oxidative damage and antioxidant status in patients with cervical intraepithelial neoplasia and carcinoma of the cervix. Eur. J. Cancer Prev. 2008, 17, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, J.W.; Ko, Y.S.; Koo, J.E.; Chung, H.Y.; Lee-Kim, Y.C. Changes in lipid peroxidation and antioxidant trace elements in serum of women with cervical intraepithelial neoplasia and invasive cancer. Nutr. Cancer 2007, 47, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Y.; Hail, N.; Lotan, R. Apoptosis as a novel target for cancer chemoprevention. JNCI J. Natl. Cancer Inst. 2004, 96, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Numsen, H.; Cortes, M.; Drake, E.N.; Spallholz, J.E. Cancer chemoprevention: A radical perspective. Free Radic. Biol. Med. 2008, 45, 97–110. [Google Scholar]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Lepley, D.M.; Li, B.; Birt, D.F.; Pelling, J.C. The chemopreventive flavonoid apigenin induces G2/M arrest in keratinocytes. Carcinogenesis 1996, 17, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Fresco, P.; Borges, F.; Diniz, C.; Marques, M.P.M. New insights on the anticancer properties of dietary polyphenols. Med. Res. Rev. 2006, 26, 747–766. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- D Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super. Sanita 2007, 43, 348–361. [Google Scholar]
- McNaught, A.D.; Wilkinson, A. Flavonoids (Isoflavonoids and Neoflavonoids). In IUPAC Compendium of Chemical Terminology, 2nd ed.; Blackwell Scientific: Oxford, UK, 1997. [Google Scholar]
- Xiao, Z.-P.; Peng, Z.Y.; Peng, M.J.; Yan, W.B.; Ouyang, Y.Z.; Zhu, H.L. Flavonoids health benefits and their molecular mechanism. Mini Rev. Med. Chem. 2011, 11, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Beecher, G.R. Overview of dietary flavonoids: Nomenclature, occurrence and intake. J. Nutr. 2003, 3, 3248S–3254S. [Google Scholar]
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somerset, S.M.; Johannot, L. Dietary flavonoid sources in Australian adults. Nutr. Cancer 2008, 60, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Andres-Lacueva, C.; Lamuela-Raventós, R.M.; Berenguer, T.; Jakszyn, P.; Barricarte, A.; Ardanas, E.; Amiano, P.; Dorronsoro, M.; Larranaga, N.; et al. Estimation of dietary sources and flavonoid intake in a Spanish adult population (EPIC-Spain). J. Am. Diet. Assoc. 2010, 110, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Hong, J.; Yang, G.; Liao, J.; Yang, C.S. Inhibition of carcinogenesis by polyphenols: Evidence from laboratory investigations. Am. J. Clin. Nutr. 2005, 81, 284S–291S. [Google Scholar] [PubMed]
- Tabrez, S.; Priyadarshini, M.; Urooj, M.; Shakil, S.; Ashraf, G.M.; Khan, M.S.; Kamal, M.A.; Alam, Q.; Jabir, N.R.; Abuzenadah, A.M.; et al. Cancer chemoprevention by polyphenols and their potential application as nanomedicine. J. Environ. Sci. Health 2013, 31, 67–98. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Afaw, F.; Mukhtar, H. Cancer chemoprevention through dietary antioxidants: Progress and promise. Antioxid. Redox Signal. 2008, 10, 475–510. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Lee, H.J. The roles of polyphenols in cancer chemoprevention. Biofactors 2006, 26, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Kampa, M.; Nifli, A.-P.; Notas, G.; Castanas, E. Polyphenols and cancer cell growth. Rev. Physiol. Biochem. Pharmacol. 2007, 159, 79–113. [Google Scholar] [PubMed]
- Khan, N.; Mukhtar, H. Multitargeted therapy of cancer by green tea polyphenols. Cancer Lett. 2008, 269, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Y.; Lin, S.; Kuo, G. Content and distribution of flavonoids among 91 edible plant species. Asia Pac. J. Clin. Nutr. 2008, 17, 275–279. [Google Scholar] [PubMed]
- Calderon-Montaño, J.M.; Burgos-Moron, E.; Perez-Guerrero, C.; Lopez-Lazaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef] [PubMed]
- Aniya, Y.; Koyama, T.; Miyagi, C.; Miyahira, M.; Inomata, C.; Kinoshita, S.; Ichiba, T. Free radical scavenging and hepatoprotective actions of the medicinal herb, Crassocephalum crepidioides from the Okinawa Islands. Biol. Pharm. Bull. 2005, 28, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, H.M.; Lehtinen, O.; Suomela, J.P.; Viitanen, M.; Kallio, H. Flavonol glycosides of sea buckthorn (Hippophae rhamnoides ssp. sinensis) and lingonberry (Vaccinium vitis-idaea) are bioavailable in humans and monoglucuronidated for excretion. J. Agric. Food Chem. 2010, 58, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, A.; Marotti, I.; Dinelli, G. Urinary excretion of kaempferol from common beans (Phaseolus vulgaris L.) in humans. Int. J. Food Sci. Nutr. 2007, 58, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Barve, A.; Chen, C.; Hebbar, V.; Desiderio, J.; Saw, C.L.; Kong, A.N. Metabolism, oral bioavailability and pharmacokinetics of chemopreventive kaempferol in rats. Biopharm. Drug Dispos. 2009, 30, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Radtke, J.; Linseisen, J.; Wolfram, G. Fasting plasma concentrations of selected flavonoids as markers of their ordinary dietary intake. Eur. J. Nutr. 2002, 41, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhang, Y.; Chen, W.; Zhao, X. The relationship between fasting plasma concentrations of selected flavonoids and their ordinary dietary intake. Br. J. Nutr. 2010, 103, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.J.; Ferrandiz, M.L.; Cejudo, M.; Terencio, M.C.; Gil, B.; Bustos, G.; Ubeda, A.; Gunasegaran, R.; Alcaraz, M.J. Influence of a series of natural flavonoids on free radical generating systems and oxidative stress. Xenobiotica 1994, 24, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.R.; Vijayakumar, M.; Mathela, C.S.; Rao, C.V. In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem. Toxicol. 2009, 47, 2196–2201. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Hibatallah, J.; Carduner, C.; Poelman, M.C. In Vivo and in vitro assessment of the free-radical-scavenger activity of Ginkgo flavone glycosides at high concentration. J. Pharm. Pharmacol. 1999, 51, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Bonina, F.; Puglia, C.; Ventura, D.; Aquino, R.; Tortora, S.; Sacchi, A.; Saija, A.; Tomaino, A.; Pellegrino, M.L.; de Caprariis, P. In vitro antioxidant and in vivo photoprotective effects of a lyophilized extract of Capparis spinosa L buds. J. Cosmet. Sci. 2002, 53, 321–335. [Google Scholar] [PubMed]
- Kampkotter, A.; Gombitang, N.C.; Zurawski, R.F.; Timpel, C.; Chovolou, Y.; Watjen, W.; Kahl, R. Effects of the flavonoids kaempferol and fisetin on thermotolerance, oxidative stress and FoxO transcription factor DAF-16 in the model organism Caenorhabditis elegans. Arch. Toxicol. 2007, 81, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Russo, G.L. The flavonoid quercetin in disease prevention and therapy: Facts and fancies. Biochem. Pharmacol. 2012, 83, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Graefe, E.U.; Wittig, J.; Mueller, S.; Riethling, A.K.; Uehleke, B.; Drewelow, B.; Pforte, H.; Jacobasch, G.; Derendorf, H.; Veit, M. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J. Clin. Pharmacol. 2001, 41, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Olthof, M.R.; Hollman, P.C.H.; Vree, T.B.; Katan, M.B. Bioavailabilities of quercetin-3-glucoside and quercetin-4′-glucoside do not differ in humans. J. Nutr. 2000, 130, 1200–1203. [Google Scholar] [PubMed]
- Day, A.J.; Mellon, F.; Barron, D.; Sarrazin, G.; Morgan, M.R.; Williamson, G. Human metabolism of dietary flavonoids: Identification of plasma metabolites of quercetin. Free Radic. Res. 2001, 35, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Sawai, Y.; Kohsaka, K.; Nishiyama, Y.; Ando, K. Serum concentrations of rutoside metabolites after oral administration of a rutoside formulation to humans. Arzneim. Forsch. 1987, 37, 729–732. [Google Scholar]
- Noroozi, M.; Burns, J.; Crozier, A.; Kelly, I.E.; Lean, M.E. Prediction of dietary flavonol consumption from fasting plasma concentration or urinary excretion. Eur. J. Clin. Nutr. 2000, 54, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Erlund, I.; Silaste, M.L.; Alfthan, G.; Rantala, M.; Kesäniemi, Y.A.; Aro, A. Plasma concentrations of the flavonoids hesperetin, naringenin and quercetin in human subjects following their habitual diets, and diets high or low in fruit and vegetables. Eur. J. Clin. Nutr. 2002, 56, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans: I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [PubMed]
- Conquer, J.A.; Maiani, G.; Azzini, E.; Raguzzini, A.; Holub, B.J. Supplementation with quercetin markedly increases plasma quercetin concentration without effect on selected risk factors for heart disease in healthy subjects. J. Nutr. 1998, 128, 593–597. [Google Scholar] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kaur, M.; Silakari, O. Flavones: An important scaffold for medicinal chemistry. Eur. J. Med. Chem. 2014, 84, 206–239. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Yang, C.S.; Landau, J.M.; Huang, M.T.; Newmark, H.L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu. Rev. Nutr. 2001, 21, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.E.; Young, J.F.; Daneshvar, B.; Lauridsen, S.T.; Knuthsen, P.; Sandström, B.; Dragsted, L.O. Effect of parsley (Petroselinum crispum) intake on urinary apigenin excretion, blood antioxidant enzymes and biomarkers for oxidative stress in human subjects. Br. J. Nutr. 1999, 81, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Janssen, K.; Mensink, R.P.; Cox, F.J.J.; Harryvan, J.L.; Hovenier, R.; Hollman, P.C.H.; Katan, M.B. Effects of the fl avonoids quercetin and apigenin on hemostasis in healthy volunteers: Results from an in vitro and a dietary supplement study. Am. J. Clin. Nutr. 1998, 97, 255–262. [Google Scholar]
- Chen, J.; Lin, H.; Hu, M. Metabolism of flavonoids via enteric recycling: Role of intestinal disposition. Pharmacol. Exp. Ther. 2002, 304, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.K.; Kim, H.J.; Bae, S.M.; Park, M.Y.; Kim, Y.W.; Kim, T.E.; Ahn, W.S. Apigenin-induced apoptosis in cervical cancer cell lines. Korean J. Obstet. Gynec. 2008, 51, 874–881. [Google Scholar]
- Zheng, P.W.; Chiang, L.C.; Lin, C.C. Apigenin induced apoptosis through p53-dependent pathway in human cervical carcinoma cells. Life Sci. 2005, 76, 1367–1379. [Google Scholar] [CrossRef] [PubMed]
- Czyz, J.; Madeja, Z.; Irmer, U.; Korohoda, W.; Hulser, D.F. Flavonoid apigenin inhibits motility and invasiveness of carcinoma cells in vitro. Int. J. Cancer 2005, 114, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Brown, N.M.; Zimmer-Nechemias, L.; Brashear, W.T.; Wolfe, B.E.; Kirschner, A.S.; Heubi, J.E. Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. Am. J. Clin. Nutr. 2002, 76, 447–453. [Google Scholar] [PubMed]
- Izumi, T.; Piskula, M.K.; Osawa, S.; Obata, A.; Tobe, K.; Saito, M.; Kataoka, S.; Kikuchi, M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J. Nutr. 2000, 130, 1695–1699. [Google Scholar] [PubMed]
- Day, A.J.; DuPont, M.S.; Ridley, S.; Rhodes, M.; Rhodes, M.J.; Morgan, M.R. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver β-glucosidase activity. FEBS Lett. 1998, 436, 71–75. [Google Scholar] [CrossRef]
- Joannou, G.E.; Kelly, G.E.; Reeder, A.Y.; Waring, M.; Nelson, C. A urinary profile study of dietary phytoestrogens. The identification and mode of metabolism of new isoflavonoids. J. Steroid Biochem. Mol. Biol. 1995, 54, 167–184. [Google Scholar] [CrossRef]
- Adlercreutz, H.; Honjo, H.; Higashi, A.; Fotsis, T.; Hamalainen, E.; Hasegawa, T.; Okada, H. Urinary excretion of lignans and isoflavonoid phytoestrogens in Japanese men and women consuming a traditional Japanese diet. Am. J. Clin. Nutr. 1991, 54, 1093–1100. [Google Scholar] [PubMed]
- Watanabe, S.; Yamaguchi, M.; Sobue, T.; Takahashi, T.; Miura, T.; Arai, Y.; Mazur, W.; Wahala, K.; Adlercreutz, H. Pharmacokinetics of soybean isoflavones in plasma, urine, and feces of men after ingestion of 60 g baked soybean powder (Kinako). J. Nutr. 1998, 128, 1710–1715. [Google Scholar] [PubMed]
- Yashar, C.M.; Spanos, W.J.; Taylor, D.D.; Gercel-Taylor, C. Potentiation of the radiation effect with genistein in cervical cancer cells. Gynecol. Oncol. 2005, 99, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Hillman, G.G.; Forman, J.D.; Kucuk, O.; Yudelev, M.; Maughan, R.L.; Rubio, J.; Sarkar, F.H. Genistein potentiates the radiation effect on prostate carcinoma cells. Clin. Cancer Res. 2001, 7, 382–390. [Google Scholar] [PubMed]
- Akimoto, T.; Nonaka, T.; Ishikawa, H.; Sakurai, H.; Saitoh, J.I.; Takahashi, T.; Mitsuhashi, N. Genistein, a tyrosine kinase inhibitor, enhanced radiosensitivity in human esophageal cancer cell lines in vitro: Possible involvement of inhibition of survival signal transduction pathways. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 195–201. [Google Scholar] [CrossRef]
- Akiyama, T.; Ogawara, H. Use and specificity of genistein as inhibitor of protein-tyrosine kinases. Methods Enzymol. 1991, 201, 362–370. [Google Scholar] [PubMed]
- Markovits, J.; Larsen, A.K.; Segal-Bendirdjian, E.; Fosse, P.; Sancier, T.M.; Gazit, A.; Levitzki, A.; Umezawa, K.; Jacquemin-Sablon, A. Inhibition of DNA topoisomerases I and II and induction of apoptosis by erbstatin and tyrphostin derivatives. Biochem. Pharmacol. 1994, 48, 549–560. [Google Scholar] [CrossRef]
- Cassidy, A.; Bingham, S.; Setchell, K.D. Biological effect of a diet of soy protein rich in isoflavones on the menstrual cycle of premenopausal women. Am. J. Clin. Nutr. 1994, 60, 333–340. [Google Scholar] [PubMed]
- Wei, H.; Wei, L.; Frenkel, K.; Bowen, R.; Barnes, S. Inhibition of tumorpromoter-induced hydrogen peroxide formation in vitro and in vivo by genistein. Nutr. Cancer 1993, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yanagihara, K.; Ito, A.; Toge, T.; Numoto, M. Antiproliferative effect of isoflavones on human cancer cell lines established from the gastrointestinal tract. Cancer Res. 1993, 53, 5815–5821. [Google Scholar] [PubMed]
- Busby, M.G.; Jeffcoat, A.R.; Bloedon, M.A.; Koch, M.A.; Black, T.; Dix, K.J.; Heizer, K.; Thomas, B.; Hill, J.; Crowell, J.; et al. Clinical characteristics and pharmacokinetics of purified soy isoflavones: Single-dose administration to healthy men. Am. J. Clin. Nutr. 2002, 75, 126–136. [Google Scholar] [PubMed]
- Zhang, B.; Liu, J.Y.; Pan, J.S.; Han, S.P.; Yin, X.X.; Wang, B.; Hu, G. Combined treatment of ionizing radiation with genistein on cervical cancer HeLa cells. J. Pharmacol. Sci. 2006, 102, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.M.; Kang, G.Z.; Xiao, B.X.; Liu, D.H.; Zhang, S. Effect of daidzein on cell growth, cell cycle, and telomerase activity of human cervical cancer in vitro. Int. J. Gynecol. Cancer 2004, 14, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Huber, G.M.; Rupasinghe, H.P.V. Phenolic profiles and antioxidant properties of apple skin extracts. J. Food Sci. 2009, 74, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Ratnasooriya, C.; Rupasinghe, H.P.V.; Jamieson, A. Juice quality and polyphenol concentration of fresh fruits and pomace of selected Nova Scotia-grown grape cultivars. Can. J. Plant Sci. 2010, 90, 193–205. [Google Scholar] [CrossRef]
- Otaki, N.; Kimira, M.; Katsumata, S.; Uehara, M.; Watanabe, S.; Suzuki, K. Distribution and major sources of flavonoid intakes in the middle-aged Japanese women. J. Clin. Biochem. Nutr. 2009, 44, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, U.; Haller, J.; Decourt, J.P.; Girault, N.; Girault, J.; Richard-Caudron, A.S.; Pineau, B.; Weber, P. A single ascending dose study of epigallocatechin gallate in healthy volunteers. J. Int. Med. Res. 2003, 3, 88–101. [Google Scholar] [CrossRef]
- Van Amelsvoort, J.M.; Van Hof, K.H.; Mathot, J.N.; Mulder, T.P.; Wiersma, A.; Tijburg, L.B. Plasma concentrations of individual tea catechins after a single oral dose in humans. Xenobiotica 2001, 31, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Natsume, M.; Osakabe, N.; Oyama, M.; Sasaki, M.; Baba, S.; Nakamura, Y.; Osawa, T.; Terao, J. Structures of (−)-epicatechin glucuronide identified from plasma and urine after oral ingestion of (−)-epicatechin: Differences between human and rat. Free Radic. Biol. Med. 2003, 34, 840–849. [Google Scholar] [CrossRef]
- Meng, X.; Sang, S.; Zhu, N.; Lu, H.; Sheng, S.; Lee, M.-J.; Ho, C.-T.; Yang, C.S. Identification and characterization of methylated and ring-fission metabolites of tea catechins formed in humans, mice, and rats. Chem. Res. Toxicol. 2002, 15, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032. [Google Scholar]
- Chow, H.H.S.; Cai, Y.; Alberts, D.S.; Hakim, I.; Dorr, R.; Shahi, F.; Crowell, J.A.; Yang, C.S.; Hara, Y. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol. Biomark. Prev. 2001, 10, 53–58. [Google Scholar]
- Meng, X.; Lee, M.J.; Li, C.; Sheng, S.; Zhu, N.; Sang, S.; Ho, C.-T.; Yang, C.S. Formation and identification of 4′-O-methyl-(−)-epigallocatechin in humans. Drug Metab. Dispos. 2001, 29, 789–793. [Google Scholar] [PubMed]
- Ahn, W.S.; Huh, S.W.; Bae, S.M.; Lee, I.P.; Lee, J.M.; Namkoong, S.E.; Kim, C.K.; Sin, J.-I. A major constituent of green tea, EGCG, inhibits the growth of a human cervical cancer cell line, CaSki cells, through apoptosis, G1 arrest, and regulation of gene expression. DNA Cell Biol. 2003, 22, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Hause, Z. Papilomaviruses and cancer: From basic studies to clinical application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar]
- Moody, C.A.; Laiminis, L.A. Human papillomavirus oncoproteins: Pathways to transformation. Nat. Rev. Cancer 2010, 10, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Von Knebel Doeberitz, M. New markers for cervical dysplasia to visualize the genomic chaos created by aberrant oncogenic papillomavirus infection. Eur. J. Cancer 2002, 38, 2229–2242. [Google Scholar] [CrossRef]
- Qiao, Y.; Cao, J.; Xie, L.; Shi, X. Cell growth inhibition and gene expression regulation by (−)-epigallocatechin-3-gallate in human cervical cancer cells. Arch. Pharm. Res. 2009, 32, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Nusri, Q.E.A.; Begum, S.; Javed, E.; Rizvi, T.A.; Hussain, A. Epigallocatechin-3-gallate induces apoptosis and inhibits invasion and migration of human cervical cancer cells. Asian Pac. J. Cancer Prev. 2012, 13, 4815–4822. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Liu, H.; Feugang, J.M.; Hao, Z.; Chow, H.S.; Garcia, F. Green tea compound in chemoprevention of cervical cancer. Int. J. Gynecol. Cancer 2010, 20, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Tripoli, E.; Guardia, M.L.; Giammanco, S.; Danila, D.; Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Manthey, J.A.; Grohmann, K.; Guthrie, N. Biological properties of citrus flavonoids pertaining to cancer and inflammation. Curr. Med. Chem. 2001, 8, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Amaro, M.I.; Rocha, J.; Vila-Real, H.; Eduardo-Figueira, M.; Mota-Filipe, H.; Sepodes, B.; Ribeiro, M.H. Anti-inflammatory activity of naringin and the biosynthesised naringenin by naringinase immobilized in microstructured materials in a model of DSS-induced colitis in mice. Food Res. Int. 2009, 42, 1010–1017. [Google Scholar] [CrossRef]
- Choi, J.S.; Park, K.Y.; Moon, S.H.; Rhee, S.H.; Young, H.S. Antimutagenic effect of plant flavonoids in the salmonella assay system. Arch. Pharm. Res. 1994, 17, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Kanno, S.; Tomizawa, A.; Ohtake, T.; Koiwai, K.; Ujibe, M.; Ishikawa, M. Naringenininduced apoptosis via activation of NF-κB and necrosis involving the loss of ATP in human promyeloleukemia HL-60 cells. Toxicol. Lett. 2006, 166, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.D.; Yang, Z.Y.; Wang, Q.; Cai, T.K.; Crewdson, P. Synthesis, characterization, cytotoxic activities and DNA-binding properties of the La(III) complex with naringenin schiff-base. Bioorg. Med. Chem. 2006, 14, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Ratnam, D.V.; Ankola, D.D.; Baradwaj, V.; Sahana, D.K.; Ravikumar, M.N.V. Role of antioxidants in prophylaxis and therapy: A pharmaceutical perspective. J. Control. Release 2006, 113, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, N.; Sulfikkarali, N.; RajendraPrasad, N.; Karthikeyan, S. Enhanced anticancer activity of naringenin-loaded nanoparticles in human cervical (HeLa) cancer cells. Biomed. Prev. Nutr. 2011, 1, 223–231. [Google Scholar] [CrossRef]
- Yen, F.L.; Wu, T.H.; Lin, L.T.; Cham, T.M.; Lin, C.C. Naringenin-loaded nanoparticles improve the physicochemical properties and the hepatoprotective effect of naringenin in orally administrated rats with CCl4-induced acute liver failure. Pharm. Res. 2008, 26, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Flori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Park, J.W.; Lee, J.W.; Paik, H.D.; Cho, S.G.; Nah, S.Y.; Park, Y.S.; Han, Y.S. Cytotoxic effect of 7-Obutyl naringenin on human breast cancer MCF-7 cells. Food Sci. Biotechnol. 2010, 19, 717–724. [Google Scholar] [CrossRef]
- Chang, H.; Mi, M.; Ling, W.; Zhu, J.; Zhang, Q.; Wei, N.; Zhou, Y.; Tang, Y.; Yuan, J. Structurally related cytotoxic effect of flavonoids on human cancer cells in vitro. Arch. Pharm. Res. 2008, 31, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, E.; Alshatwi, A.A. Naringin induces death receptor and mitochondria-mediated apoptosis in human cervical cancer (SiHa) cells. Food Chem. Toxicol. 2013, 51, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zhen, Y.; Chen, Y.; Zou, L.; Zhang, Y.; Hu, F.; Feng, J.; Shen, J.; Wei, B. Naringin inhibits growth and induces apoptosis by a mechanism dependent on reduced activation of NF-κB/COX-2-caspase-1 pathway in HeLa cervical cancer cells. Int. J. Oncol. 2014, 45, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Vanamala, J.; Leonardi, T.; Patil, B.S.; Taddeo, S.S.; Murphy, M.E.; Pike, L.M.; Chapkin, R.S.; Lupton, J.R.; Turner, N.D. Suppression of colon carcinogenesis by bioactive compounds in grapefruit. Carcinogenesis 2006, 27, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-I.; Lee, S.J.; Lee, S.B.; Park, K.; Kim, W.J.; Moon, S.K. Requirement for Ras/Raf/ERK pathway in naringin-induced G1-cell-cycle arrest via p21WAF1 expression. Carcinogenesis 2008, 29, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Kahkonen, M.P.; Heinonen, M. Antioxidant activity of anthocyanins and their aglycons. J. Agric. Food Chem. 2003, 51, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Kuhnau, J. The flavonoids. A class of semi-essential food components: Their role in human nutrition. World Rev. Nutr. Diet. 1976, 24, 117–191. [Google Scholar] [PubMed]
- Cevallos-Casals, B.A.; Byrne, D.; Okie, W.R.; Cisneros-Zevallos, L. Selecting new peach and plum genotypes rich in phenolic compounds and enhanced functional properties. Food Chem. 2006, 96, 273–328. [Google Scholar] [CrossRef]
- Chen, P.N.; Kuo, W.H.; Chiang, C.L.; Chiou, H.L.; Hsieh, Y.S.; Chu, S.C. Black rice anthocyanins inhibit cancer cells invasion via repressions of MMPs and u-PA expression. Chem. Biol. Interact. 2006, 163, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.; Farnsworth, R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Hsu, J.D.; Wang, S.F.; Chiang, H.C.; Yang, M.Y.; Kao, E.S.; Ho, Y.-C.; Wang, C.J. Hibiscus sabdariffa extract inhibits the development of atherosclerosis in cholesterol-fed rabbits. J. Agric. Food Chem. 2003, 51, 5472–5477. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Zhang, Y.; Nair, M.G. Inhibition of proliferation of human cancer cells and cyclooxygenase enzymes by anthocyanidins and catechins. Nutr. Cancer 2003, 46, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Fotsis, T.; Pepper, M.S.; Aktas, E.; Breit, S.; Rasku, S.; Adlercreutz, H.; Wahala, K.; Montesano, R.; Schweigerer, L. Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res. 1997, 57, 2916–2921. [Google Scholar] [PubMed]
- Song, Q.I.A.N.; Li-qin, J.I.N. The studies of cyanidin 3-glucoside-induced apoptosis in human cervical cancer Hela cells and its mechanism. Chin. J. Biochem. Pharm. 2008, 6. [Google Scholar]
- Shahidi, F.; Naczk, M. Food Phenolics, Sources, Chemistry, Effects, Applications; Technomic Publishing Co., Inc.: Lancaster, PA, USA, 1995. [Google Scholar]
- Faried, A.; Kurnia, D.; Faried, L.S.; Usman, N.; Miyazaki, T.; Kato, H.; Kuwano, H. Anticancer effects of gallic acid isolated from Indonesian herbal medicine. Phaleria macrocarpa (Scheff.) Boerl, on human cancer cell lines. Int. J. Oncol. 2007, 30, 605–613. [Google Scholar] [PubMed]
- You, B.R.; Moon, H.J.; Han, Y.H.; Park, W.H. Gallic acid inhibits the growth of HeLa cervical cancer cells via apoptosis and/or necrosis. Food Chem. Toxicol. 2010, 48, 1334–1340. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Scalbert, A. Ellagitannins—Occurrence in food, bioavailability and cancer prevention. J. Food Sci. Agric. 2000, 80, 1118–1125. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.H.; Katan, M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [PubMed]
- Sosulski, F.; Krygier, K.; Hogge, L. Free, esterified, and insoluble-bound phenolic acids. 3. Composition of phenolic acids in cereal and potato flours. J. Agric. Food Chem. 1982, 30, 337–340. [Google Scholar] [CrossRef]
- Van de Putte, B.; Hollman, P.C.H. Catechin contents of foods commonly consumed in The Netherlands. 1. Fruits, vegetables, staple foods, and processed foods. J. Agric. Food Chem. 2000, 48, 1746–1751. [Google Scholar]
- Kern, S.M.; Bennett, R.N.; Mellon, F.A.; Kroon, P.A.; Garcia-Conesa, M.T. Absorption of hydroxycinnamates in humans after high-bran cereal consumption. J. Agric. Food Chem. 2003, 51, 6050–6055. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The story so far. Eur. J. Cancer 2005, 41, 1955–1968. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Bar-Sela, G.; Epelbaum, R.; Schaffer, M. Curcumin as an anti-cancer agent: Review of the gap between basic and clinical applications. Curr. Med. Chem. 2010, 17, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Saengkrit, N.; Saesoo, S.; Srinuanchai, W.; Phunpee, S.; Ruktanonchai, U.R. Influence of curcumin-loaded cationic liposome on anticancer activity for cervical cancer therapy. Colloids Surf. B Biointerfaces 2014, 114, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, B.; Blennow, G. A study on the fate of curcumin in the rat. Acta Pharmacol. Toxicol. 1978, 43, 86–92. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Ryu, E.K.; Choe, Y.S.; Lee, K.H.; Choi, Y.; Kim, B.T. Curcumin and dehydrozingerone derivatives: Synthesis, radiolabeling, and evaluation for β-amyloid plaque imaging. J. Med. Chem. 2006, 49, 6111–6119. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Bhatt, I.D.; Ichikawa, H.; Ahn, K.S.; Sethi, G.; Sandur, S.K.; Natarajan, C.; Seeram, N.; Shishodia, S. Curcumin—Biological and medicinal properties. In Turmeric the Genus Curcuma; Ravindran, P.N., Babu, K.N., Sivaraman, K., Eds.; CRC Press: New York, NY, USA, 2007; pp. 297–368. [Google Scholar]
- Limtrakul, P.; Chearwae, W.; Shukla, S.; Phisalphong, C.; Ambudkar, S.V. Modulation of function of three ABC drug transporters, P-glycoprotein (ABCB1), mitoxantrone resistance protein (ABCG2) and multidrug resistance protein 1 (ABCC1) by tetrahydrocurcumin, a major metabolite of curcumin. Mol. Cell. Biochem. 2007, 296, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as Curecumin: From kitchen to clinic. Biochem. Pharmacol. 2007, 75, 787–809. [Google Scholar] [CrossRef] [PubMed]
- Shahrzad, S.; Aoyagi, K.; Winter, A.; Koyama, A.; Bitsch, I. Pharmacokinetics of gallic acid and its relative bioavailability from tea in healthy humans. J. Nutr. 2001, 131, 1207–1210. [Google Scholar] [PubMed]
- Seeram, N.P.; Lee, R.; Heber, D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin. Chim. Acta 2004, 348, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Strimpakos, A.S.; Sharma, R.A. Curcumin: Preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid. Redox Signal. 2008, 10, 511–545. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Jhurani, S.; Aggarwal, B.B. Multi-targeted therapy by curcumin: How spicy is it? Mol. Nutr. Food Res. 2008, 52, 1010–1030. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, R.; Singh, A.K.; Gaddipati, J.; Srimal, R.C. Multiple biological activities of curcumin: A short review. Life Sci. 2006, 78, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Prusty, B.K.; Das, B.C. Constitutive activation of transcription factor AP-1 in cervical cancer and suppression of human papillomavirus (HPV) transcription and AP-1 activity in HeLa cells by curcumin. Int. J. Cancer 2005, 113, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Berezutskaya, E.; Bagchi, S. The human papillomavirus E7 oncoprotein functionally interacts with the S4 subunit of the 26 S proteasome. J. Biol. Chem. 1997, 272, 135–140. [Google Scholar] [CrossRef]
- Smitha, B.; Sreekanth, C.N.; Thulasidasan, A.K.T.; Anto, N.P.; Cheriyan, V.T.; Puliyappadamba, V.T.; Menon, S.G.; Ravichandran, S.D.; Anto, R.J. Akt is upstream and MAPKs are downstream of NF-κB in paclitaxel-induced survival signaling events, which are down-regulated by curcumin contributing to their synergism. Int. J. Biochem. Cell Biol. 2011, 43, 331–341. [Google Scholar]
- Ganta, S.; Mansoor, A. Coadministration of paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol. Pharm. 2009, 6, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.-T.; Lin, B.-R.; Wu, M.S.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar] [PubMed]
- Kunwar, A.; Sandur, S.K.; Krishna, M.; Priyadarsini, K.I. Curcumin mediates time and concentration dependent regulation of redox homeostasis leading to cytotoxicity in macrophage cells. Eur. J. Pharmacol. 2009, 611, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.R.; Pandit, B.; Bhasin, D.; Etter, J.P.; Regan, N.; Abdelhamid, D.; Li, C.; Lin, H.; Li, P.K. Structure-activity relationship studies of curcumin analogues. Bioorg. Med. Chem. Lett. 2009, 19, 2065–2069. [Google Scholar] [CrossRef] [PubMed]
- Maher, D.M.; Bell, M.C.; O’Donnell, E.A.; Gupta, B.K.; Jaggi, M.; Chauhan, S.C. Curcumin suppresses human papillomavirus oncoproteins, restores p53, rb, and ptpn13 proteins and inhibits benzo[a]pyrene-induced upregulation of HPV E7. Mol. Carcinog. 2011, 50, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004, 24, 2783–2840. [Google Scholar] [PubMed]
- Goldberg, D.M.; Yan, J.; Soleas, G.J. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin. Biochem. 2003, 36, 79–87. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhang, Y.; Liu, H.; Yuan, J.; Zheng, Z.; Zou, G. Transport of a cancer chemopreventive polyphenol, resveratrol: Interaction with serum albumin and hemoglobin. J. Fluoresc. 2007, 17, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Sale, S.; Verschoyle, R.D.; Boocock, D.; Jones, D.J.L.; Wilsher, N.; Ruparelia, K.C.; Potter, P.A.; Farmer, P.B.; Steward, W.P.; Gescher, A.J. Pharmacokinetics in mice and growth-inhibitory properties of the putative cancer chemopreventive agent resveratrol and the synthetic analogue trans 3,4,5,4′-tetramethoxystilbene. Br. J. Cancer 2004, 90, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.-F.; Wu, C.L.; Huang, S.C.; Wu, C.M.; Hsiao, J.R.; Yo, Y.T.; Chen, Y.H.; Shiau, A.-L.; Chou, C.Y. Cathepsin L mediates resveratrol-induced autophagy and apoptotic cell death in cervical cancer cells. Autophagy 2009, 5, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Sull, J.W.; Sung, H.J. Suppressing effect of resveratrol on the migration and invasion of human metastatic lung and cervical cancer cells. Mol. Boil. Rep. 2012, 39, 8709–8716. [Google Scholar] [CrossRef] [PubMed]
- Zoberi, I.; Bradbury, C.M.; Curry, H.A.; Bisht, K.S.; Goswami, P.C.; Roti, J.L.R.; Gius, D. Radiosensitizing and anti-proliferative effects of resveratrol in two human cervical tumor cell lines. Cancer Lett. 2002, 175, 165–173. [Google Scholar] [CrossRef]
- García-Zepeda, S.P.; García-Villa, E.; Díaz-Chávez, J.; Hernández-Pando, R.; Gariglio, P. Resveratrol induces cell death in cervical cancer cells through apoptosis and autophagy. Eur. J. Cancer Care 2013, 22, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.P.; Węsierska-Gądek, J. Monitoring of Long-Term Effects of Resveratrol on Cell Cycle Progression of Human HeLa Cells after Administration of a Single Dose. Ann. N. Y. Acad. Sci. 2009, 1171, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Talwar, G.P. A Clinical Study on the Clearance of Human Papilloma Virus (HPV) Infection in Uterine Cervix by Basant (a Polyherbal Cream) and Curcumin Soft Gelatin Capsule in Females Infected with HPV Clinical Trial Registry—India National Institute of Medical Statistics; CTRI/2008/091/000095; ICMR: New Delhi, India, 2008. [Google Scholar]
- Basu, P.; Dutta, S.; Begum, R.; Mittal, S.; Dutta, P.D.; Bharti, A.C.; Panda, K.C.; Biswas, J.; Dey, B.; Talwar, G.P.; et al. Clearance of cervical human papillomavirus infection by topical application of curcumin and curcumin containing polyherbal cream: A phase II randomized controlled study. Asian Pac. J. Cancer Prev. 2013, 14, 5753–5759. [Google Scholar] [CrossRef] [PubMed]
- Ahn, W.S.; Yoo, J.; Huh, S.W.; Kim, C.K.; Lee, J.M.; Namkoong, S.E.; Bae, S.-M.; Lee, I.P. Protective effects of green tea extracts (polyphenon E and EGCG) on human cervical lesions. Eur. J. Cancer Prev. 2003, 12, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-J.; Zheng, C.J.; Peng, C.; Zhang, H.; Jiang, Y.P.; Han, T.; Qin, L.P. Plants and cervical cancer: An overview. Expert Opin. Investig. Drugs 2013, 22, 1133–1156. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, R.V.; Murugan, R.S.; Maitreyi, S.; Ramalingam, K.; Karunagaran, D.; Nagini, S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer HeLa cells through p53 induction and NF-κB inhibition. Eur. J. Pharmacol. 2010, 649, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liu, J.; Li, C.; Wu, H.Z.; Liu, Y.W. Kaempferol-7-O-β-d-glucoside (KG) isolated from Smilax china L. rhizome induces G 2/M phase arrest and apoptosis on HeLa cells in a p53-independent manner. Cancer Lett. 2008, 264, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Ying, T.H.; Yang, S.F.; Tsai, S.J.; Hsieh, S.C.; Huang, Y.C.; Bau, D.T.; Hsieh, Y.H. Fisetin induces apoptosis in human cervical cancer HeLa cells through ERK1/2-mediated activation of caspase-8-/caspase-3-dependent pathway. Arch. Toxicol. 2012, 86, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, Q.; Zheng, X.; Sun, H.; Zhou, Y.; Li, D.; Lin, Y. Luteolin enhances TNF-related apoptosis-inducing ligand’s anticancer activity in a lung cancer xenograft mouse model. Biochem. Biophys. Res. Commun. 2012, 417, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Alshatwi, A.A.; Ramesh, E.; Periasamy, V.S.; Subash-Babu, P. The apoptotic effect of hesperetin on human cervical cancer cells is mediated through cell cycle arrest, death receptor, and mitochondrial pathways. Fundam. Clin. Pharmacol. 2013, 27, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Roohbakhsh, A.; Parhiz, H.; Soltani, F.; Rezaee, R.; Iranshahi, M. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sci. 2015, 124, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Noguchi, M.; Nakao, Y.; Ysunaga, M.; Yamasaki, F.; Iwasaka, T. Antiproliferative effects of the major tea polyphenol, (−)-epigallocatechin gallate and retinoic acid in cervical adenocarcinoma. Gynecol. Oncol. 2008, 108, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Shulan, L.; Zhang, J.; Li, S.; Gao, J.; Pan, C. Effects of Res on proliferation and apoptosis of human cervical carcinoma cell lines C33A, SiHa and HeLa. J. Med. Coll. PLA 2009, 24, 148–154. [Google Scholar]
- Srinivas, G.; Anto, R.J.; Srinivas, P.; Vidhyalakshmi, S.; Senan, V.P.; Karunagaran, D. Emodin induces apoptosis of human cervical cancer cells through poly (ADP-ribose) polymerase cleavage and activation of caspase-9. Eur. J. Pharmacol. 2003, 473, 117–125. [Google Scholar] [CrossRef]
- Singh, M.; Singh, N. Molecular mechanism of curcumin induced cytotoxicity in human cervical carcinoma cells. Mol. Cell. Biochem. 2009, 325, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Giridharan, P.; Somasundaram, S.T.; Perumal, K.; Vishwakarma, R.A.; Karthikeyan, N.P.; Velmurugan, R.; Balakrishnan, A. Novel substituted methylenedioxy lignan suppresses proliferation of cancer cells by inhibiting telomerase and activation of c-myc and caspases leading to apoptosis. Br. J. Cancer 2002, 87, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Bhui, K.; Singh, R.; Shukla, Y. Tea polyphenols enhance cisplatin chemosensitivity in cervical cancer cells via induction of apoptosis. Life Sci. 2013, 93, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, F.; Foppoli, C.; Coccia, R.; Perluigi, M. Antioxidants in cervical cancer: Chemopreventive and chemotherapeutic effects of polyphenols. Biochim. Biophys. Acta 2012, 1822, 737–747. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Wang, Q.; Zheng, X.L.; Yan, J.Q.; Yang, L.; Sun, H.; Hu, N.; Lin, Y.; Wang, X. Wogonin potentiates cisplatin-induced cancer cell apoptosis through accumulation of intracellular reactive oxygen species. Oncol. Rep. 2012, 28, 601–605. [Google Scholar] [PubMed]
- Jakubowicz-Gil, J.; Paduch, R.; Piersiak, T.; Głowniak, K.; Gawron, A.; Kandefer-Szerszeń, M. The effect of quercetin of pro-apoptotic activity of cisplatin in HeLa cells. Biochem. Pharmacol. 2005, 69, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xin, Y.; Diao, Y.; Lu, C.; Fu, J.; Luo, L.; Yin, Z. Synergistic effects of apigenin and paclitaxel on apoptosis of cancer cells. PLoS ONE 2011, 6, 29169. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.L.; Wang, W. Formononetin potentiates epirubicininduced apoptosis via ROS production in HeLa cells in vitro. Chem. Biol. Interact. 2013, 205, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.L.; Wang, W.; Ho, C.T. 7,3′,4′-Trihydroxyisoflavone modulates multidrug resistance transporters and induces apoptosis via production of reactive oxygen species. Toxicology 2012, 302, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Yu, Y.; Zhao, H.G.; Yang, A.; Yan, H.; Cui, Y. Combination of quercetin with radiotherapy enhances tumor radiosensitivity in vitro and in vivo. Radiother. Oncol. 2012, 104, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.I.; Shim, J.H.; Kim, K.H.; Choi, H.S.; Kim, J.W.; Lee, H.G.; Kim, B.Y.; Park, S.N.; Park, O.J.; Yoon, D.Y. Sensitization of the apoptotic effect of gamma-irradiation in genistein-pretreated CaSki cervical cancer cells. J. Microbiol. Biotechnol. 2008, 18, 523–531. [Google Scholar] [PubMed]
| Compound | Chemical Structure | Dietary Sources | Biological Effects |
|---|---|---|---|
| Flavonols | |||
| Epicatechin |  | Apple, berries, grapes, red wine, green and black tea, chocolate | Antioxidative, anti-proliferative, pro-apoptotic, antiangiogenic, suppression of growth and invasion, anti-inflammatory, antimetastatic, antimutagenic, inhibition of telomerase activity and lipid peroxidation, modulation of estrogen activity, modulation and reversal of epigenetic changes |
| Catechin |  | Red wine, broad beans, black grapes, apricots, tea, strawberries | Antioxidative, anti-proliferative, pro-apoptotic, antiangiogenic, inhibition of tumor growth, anti-inflammatory, suppression of growth and invasion, pro-oxidative |
| Flavones | |||
| Apigenin |  | Parsley, celery, celeriac, and chamomile tea | Antioxidative, anti-mutagenic, anti-inflammatory, anti-viral, inhibition of tumor growth, pro-apoptotic, suppression of tumor progression, anti-invasive, antiangiogenic, antimetastatic, anti-proliferative, modulation of epigenetic changes |
| Luteolin |  | Celery, broccoli, green pepper, parsley, thyme, dandelion, chamomile tea, carrots, olive oil, peppermint, rosemary, navel oranges, and oregano | Anti-inflammatory, anti-mutagenic, anti-carcinogenic |
| Chrysin |  | Passion flowers, chamomile, honeycomb | Anti-proliferative, anti-anxiety, anticonvulsant, antioxidant, anti-inflammatory |
| Flavonols | |||
| Quercetin |  | Onions, broccoli, apples, apricots, berries, nuts, seeds, tea, wine, cocoa | Antioxidative; pro-oxidative, antiviral, inhibition of tumor formation and migration, pro-apoptotic, anti-proliferative, antimetastatic, anti-angiogenic, inhibition of lipid peroxidation, reduction of tumor incidence and multiplicity, prevention of GJIC inhibition, modulation of epigenetic changes |
| Kaempferol |  | Apples, grapes, tomatoes, green tea, potatoes, onions, broccoli, Brussels sprouts, squash, cucumbers, lettuce, green beans, peaches, blackberries, raspberries, and spinach | Antioxidant, anti-viral, antibacterial, antiproliferative, anti-inflammatory |
| Flavanones | |||
| Naringenin |  | Grapefruit, oranges, and tomatoes | Anti-oxidative, anti-inflammatory, anti-metastatic, delayed tumor development, reduction of tumor incidence, anticarcinogenic, lipid-lowering, superoxide scavenging, anti-apoptotic, metal chelating |
| Anthocyanins | |||
 | Blueberry, cranberry, bilberry; black raspberry, red raspberry, blackberry; blackcurrant, cherry, eggplant (aubergine) peel, black rice, Concord grape, red cabbage, and violet petals. Red-fleshed peaches and apples contain anthocyanins | Anti-inflammatory, anti-edema, antiproliferative, antioxidant, antiangiogenic, antimetastatic | |
| Proanthocyanidins | |||
 | Cinnamon, aronia fruit, cocoa beans, grape seed, grape skin, red wines, bilberry, cranberry, black currant, green tea, black tea | Antioxidant, antiproliferative, antibacterial, anti-inflammatory | |
| Isoflavones | |||
| Daidzein |  | Kwao Krua, Kudzu, Maackia amurensis cell cultures, tofu | Antioxidant, estrogenic and anti-estrogenic effects |
| Genistein |  | Lupin, fava beans, soybeans, kudzu, psoralea, coffee | Antioxidative, anti-invasive, anti-inflammatory, anti-metastatic, delay/repression of tumor development/growth, reduction of tumor multiplicity and volume, pro-apoptotic, antiproliferative, estrogenic activity, prevention of GJIC inhibition, modulation of epigenetic changes |
| Stilbenes | |||
| Resveratrol |  | Skin of grapes, blueberries, raspberries, mulberries | Antioxidative, anti-inflammatory, anti-cyclooxygenase, antiproliferative, proapoptotic, antiestrogenic, modulation of lipid metabolism, inhibition of platelet aggregation |
| Lignans | |||
| Secoisolari-ciresinol |  | Flax, sunflower, sesame, pumpkin seeds | Antioxidant, anti-inflammatory, antiproliferative, anticarcinogenic |
| Phenolic Acids | |||
| Benzoic acids (Gallic acid) |  | Gallnuts, sumac, witch hazel, tea leaves, oak bark | Antioxidative, pro-oxidative, anti-inflammatory, antibacterial, antiviral, anti-melanogenic, antimutagenic, suppression of tumor growth, anti-invasive, antiproliferative, inhibition of tumorigenesis, anti-angiogenic, modulation of androgen receptor |
| Cinnamic acids |  | Oil of cinnamon, balsams such as storax, shea butter | Antioxidative, antimicrobial, anti-inflammatory, antiproliferative |
| Tannins | |||
 | Grape skins, seeds and stems, cranberries, strawberries, blueberries, hazelnuts, walnuts, pecans, Cloves, tarragon, cumin, thyme, vanilla, and cinnamon | Antimicrobial activities, Antitumor activities, Inhibition of the mutagenicity of carcinogens, Inhibition of tumor promotion | |
| Other Polyphenols | |||
| Curcumin |  | Turmeric | Antioxidative, anti-angiogenic, anti-adhesive, tumor growth suppressive, antiproliferative, proapoptotic, antimetastatic, anti-inflammatory, modulation and reversal of epigenetic changes |
| Rosmarinic acid |  | Basil, lemon balm, rosemary, marjoram, sage, thyme, peppermint | Antioxidative, reduction of HCA formation, modulation of epigenetic changes |
| 6-Gingerol |  | Fresh ginger | Antioxidative, anti-inflammatory |
| Class | Chemical Constituent | Study Type | Cell Type | Activity | Mechanism of Action | References |
|---|---|---|---|---|---|---|
| Flavanols | Quercetin | In vitro | HeLa | Antiproliferation Induction of apoptosis | Induction of G2/M phase cell cycle arrest and mitochondrial apoptosis; inhibition of anti-apoptotic AKT and Bcl-2 expression | [192] |
| Kaempferol | In vitro | HeLa | Antiproliferation | Induction of G2/M phase growth arrest, decrease of cyclin B1 and CDK1, inhibition of NF-κB nuclear translocation, upregulation of Bax and downregulation of Bcl-2 | [193] | |
| Fisetin | In vitro/in vivo | HeLa | Antiproliferation Induction of apoptosis | Significantly reduced tumor growth; Activation of the phosphorylation ERK1/2, inhibition of ERK1/2 by PD98059, activation of caspase-8/-3 pathway | [194] | |
| Flavones | Apigenin | In vitro | HeLa | cell cycle arrest and apoptosis | Decreased in the protein expression of Bcl-2 protein; induced p53 expression; down regulation of Bcl-2 expression | [82] |
| Luteolin | In vivo | HeLa | Induction of apoptosis and tumor growth | Luteolin sensitized HeLa cells to TRAIL-induced apoptosis by both extrinsic and intrinsic apoptotic pathways | [195] | |
| Isoflavones | Daidzein | In vitro | HeLa | Inhibition of tumor growth | Expression of human telomerase catalytic subunit mRNA decreased. Affected cell growth, cell cycle and telomerase activity in vitro | [100] |
| Genistein | In vitro | CaSki | inhibits growth of cervical cancer cells | Inhibition of Mcl-1 correlated with increase in radiosensitivity in Me180 cells. Activated pAKT (Thr 308) was inhibited enhancement of the radiation effect that may be partially mediated by G(2)M arrest, Mcl-1 and activation of the AKT gene. migration-inhibition in a time-dependent manner by modulating the expression of MMP-9 and TIMP-1 | [91,92,93] | |
| Flavanones | Naringenin | In vitro | SiHa | Antiproliferation Induction of apoptosis | Induction of apoptosis through both death-receptor and mitochondrial pathways | [130,133] |
| Hesperetin | In vitro | SiHa | reduction in cell viability and the induction of apoptosis | Attenuation of mitochondrial membrane potential with increased expression of caspase-3, caspase-8, caspase-9, p53, Bax, and Fas death receptor and its adaptor protein Fas-associated death domain-containing protein (FADD) induced apoptosis was confirmed by TUNEL and Annexin V-Cy3 | [196,197] | |
| Anthocyanidins | Cyanidin | In vitro | HeLa | Antiproliferative | Induced the accumulation of peroxides. inhibited HeLa human cervical tumor cell proliferation and increased generation of reactive oxygen species | [143] |
| Flavan-3-ols | EGCG | In vitro | HeLa | Antiproliferation | Combination of EGCG with RA induced apoptosis and inhibited telomerase activity | [198] |
| Phenolic acids | Gallic acid | In vitro | HeLa | Induction of apoptosis | Induction of cell death via apoptosis and/or necrosis was accompanied by ROS increase and GSH depletion | [146] |
| Stilbens | Resveratrol | In vitro | SiHa, HeLa, C-33A | Antiproliferation | Suppression of C-33A, SiHa and HeLa cells growth through induction of cell apoptosis | [199] |
| Tannins | Emodin | In vitro | Bu 25TK | Antiproliferative Induction of apoptosis | Inhibited DNA synthesis and induced apoptosis by increased nuclear condensation, annexin binding and DNA fragmentation apoptotic pathway is caspase-dependent | [200] |
| Curcuminoids | Curcumin | In vitro | HeLa SiHa CaSki | Antiproliferation Induction of apoptosis | Upregulation of Bax, AIF, release of cytochrome c and downregulation of Bcl-2, Bcl-XL, COX-2, iNOS and cyclin D1 | [201] |
| Lignans | methylenedioxy lignan | In vitro | HeLa | Antiproliferative Induction of apoptosis | Inhibiting telomerase and activation of c-mycand caspases leading to apoptosis induces apoptosis by bcl-2 suppression and activation of caspases | [202] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moga, M.A.; Dimienescu, O.G.; Arvatescu, C.A.; Mironescu, A.; Dracea, L.; Ples, L. The Role of Natural Polyphenols in the Prevention and Treatment of Cervical Cancer—An Overview. Molecules 2016, 21, 1055. https://doi.org/10.3390/molecules21081055
Moga MA, Dimienescu OG, Arvatescu CA, Mironescu A, Dracea L, Ples L. The Role of Natural Polyphenols in the Prevention and Treatment of Cervical Cancer—An Overview. Molecules. 2016; 21(8):1055. https://doi.org/10.3390/molecules21081055
Chicago/Turabian StyleMoga, Marius Alexandru, Oana Gabriela Dimienescu, Cristian Andrei Arvatescu, Aurel Mironescu, Laura Dracea, and Liana Ples. 2016. "The Role of Natural Polyphenols in the Prevention and Treatment of Cervical Cancer—An Overview" Molecules 21, no. 8: 1055. https://doi.org/10.3390/molecules21081055





