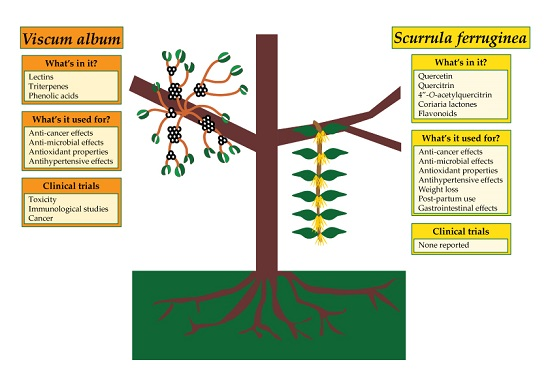
Parasitic Mistletoes of the Genera Scurrula and Viscum: From Bench to Bedside
Abstract
:1. Introduction
2. Parasitic Plant-Host Relationships
3. Phytochemistry
4. Ethnomedicinal Uses
4.1. Anti-Cancer Effects
4.2. Anti-Microbial Effects
4.3. Antioxidant Properties
4.4. Antihypertensive Effects
4.5. Weight Loss
4.6. Post-Partum Use
4.7. Gastrointestinal Effects
4.8. Toxicity Studies
5. Clinical Trials
5.1. Toxicity
5.2. Immunological Studies
5.3. Cancer
5.4. Quality of Life
6. Future Directions
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rubiales, D.; Heide-Jørgensen, H.S. Parasitic plants. Encicl. Life Sci. 2011, 1–10. [Google Scholar] [CrossRef]
- Kuijt, J. The Biology of Parasitic Flowering Plants; University of California Press: Berkeley, CA, USA, 1969. [Google Scholar]
- Heide-Jørgensen, H.S. Parasitic Flowering Plants; Brill: Leiden, The Netherlands, 2008. [Google Scholar]
- Tennakoon, K.; Weerasuriya, A. Nature’s scroungers. The fascinating world of plant parasites. Sri Lanka Nat. 1998, 46–58. [Google Scholar]
- Press, M.C.; Graves, J.D.; Stewart, G.R. Physiology of the interaction of angiosperm parasites and their higher plant hosts. Plant Cell Environ. 1990, 13, 91–104. [Google Scholar] [CrossRef]
- Musselman, L.J.; Press, M.C. Introduction to Parasitic Plants. In Parasitic Plants; Press, M.C., Graves, J.D., Eds.; Chapman & Hall: London, UK, 1995; pp. 1–13. [Google Scholar]
- Bell, T.L.; Adams, M.A.; Rennenberg, H. Attack on all fronts: Functional relationships between aerial and root parasitic plants and their woody hosts and consequences for ecosystems. Tree Physiol. 2011, 31, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Mathiasen, R.; Nickrent, D. Mistletoes: Pathology, systematics, ecology, and management. Plant Dis. 2008, 98, 988–1006. [Google Scholar] [CrossRef]
- Ameer, O.Z.; Salman, I.M.; Quek, K.J.; Asmawi, M.Z. Loranthus ferrugineus: A Mistletoe from Traditional Uses to Laboratory Bench. J. Pharmacopunct. 2015, 18, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Nickrent, D.L. Santalales (Including Mistletoes). In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd: Chichester, UK, 2011. [Google Scholar]
- Glatzel, G.; Geils, B. Mistletoe ecophysiology: Host-parasite interactions. Botany 2009, 87, 10–15. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Ullmann, I.; Lange, O.L.; Farquhar, G.D.; Cowan, I.R.; Schulze, E.D.; Ziegler, H. Mistletoes: A hypothesis concerning morphological and chemical avoidance of herbivory. Oecologia 1986, 70, 234–237. [Google Scholar] [CrossRef]
- Stewart, G.R.; Press, M.C. The physiology and biochemistry of parasitic protozoa. Annu. Rev. Plant Biol. 1990, 41, 127–151. [Google Scholar] [CrossRef]
- Shen, H.; Hong, L.; Ye, W.; Cao, H.; Wang, Z. The influence of the holoparasitic plant Cuscuta campestris on the growth and photosynthesis of its host Mikania micrantha. J. Exp. Bot. 2007, 58, 2929–2937. [Google Scholar] [CrossRef] [PubMed]
- Nickrent, D.L.; Musselman, L.J. Introduction to Parasitic Flowering Plants. Plant Heal. Instr. 2004, 13, 300–315. [Google Scholar] [CrossRef]
- Press, M.C.; Phoenix, G.K. Impacts of parasitic plants on natural communities. New Phytol. 2005, 166, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.M. Parasitic plants as facilitators: More Dryad than Dracula? J. Ecol. 2009, 97, 1151–1159. [Google Scholar] [CrossRef]
- Jeschke, W.; Baig, A.; Hilpert, A. Sink-stimulated photosynthesis, increased transpiration and increased demand-dependent stimulation of nitrate uptake: Nitrogen and carbon relations in the parasitic association Cuscuta reflexa-Coleus blumei. J. Exp. Bot. 1997, 48, 915–925. [Google Scholar] [CrossRef]
- Jeschke, W.; Hilpert, A. Sink-Stimulated photosynthesis and sink-dependent increase in nitrate uptake: Nitrogen and carbon relations of the parasitic association Cuscuta reflexa-Ricinus communis. Plant Cell Environ. 1997, 47–56. [Google Scholar] [CrossRef]
- Clark, J.; Bonga, J.M. Photosynthesis and respiration in black spruce (Picea mariana) parasitized by eastern dwarf mistletoe (Arceuthobium pusillum). Can. J. Bot. 1970, 48, 2029–2031. [Google Scholar] [CrossRef]
- Cechin, I.; Press, M.C. Nitrogen relations of the sorghm-Striga hermonthica host-parasite association: Germination, attachment and early growth. New Phytol. 1993, 124, 681–687. [Google Scholar] [CrossRef]
- Watling, J.R.; Press, M.C. Impacts of Infection by Parasitic Angiosperms on Host Photosynthesis. Plant Biol. 2001, 3, 244–250. [Google Scholar] [CrossRef]
- Bickford, C.P.; Kolb, T.E.; Geils, B.W. Host physiological condition regulates parasitic plant performance: Arceuthobium vaginatum subsp. cryptopodum on Pinus ponderosa. Oecologia 2005, 146, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Vicas, S.I.; Rugina, D.; Socaciu, C. Comparative study about antioxidant activities of Viscum album from different host trees, harvested in different seasons. J. Med. Plants Res. 2011, 5, 2237–2244. [Google Scholar]
- Le, Q.-V.; Tennakoon, U.K.; Metali, F.; Lim, L.B.L.; Bolin, J.F. Host specific variation in photosynthesis of an obligate xylem-tapping mistletoe Dendrophthoe curvata in a Bornean heath forest. Nord. J. Bot. 2016, 34, 235–243. [Google Scholar] [CrossRef]
- Le, Q.-V.; Tennakoon, K.U.; Metali, F.; Lim, L.B.; Bolin, J.F. Ecophysiological responses of mistletoe Dendrophthoe Curvata (Loranthaceae) to varying environmental parameters. J. Trop. For. Sci. 2016, 28, 59–67. [Google Scholar]
- Schulze, E.D.; Ehleringer, J.R. The effect of nitrogen supply on growth and water-use efficiency of xylem-tapping mistletoes. Planta 1984, 162, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.D.; Dawson, T.E.; Ehleringer, J.R. Integrated Nitrogen, Carbon, and Water Relations of a Xylem-Tapping Mistletoe following nitrogen fertilization of the host. Oecologia 1994, 100, 430–438. [Google Scholar]
- Rahmad, Z.B.; Addo-Fordjour, P.; Asyraf, M.; Nik Rosely, N.F. Mistletoe abundance, distribution and associations with trees along roadsides in Penang, Malaysia. Trop. Ecol. 2014, 55, 255–262. [Google Scholar]
- Richteri, B.Y.A.; Popp, M. The physiological importance of accumulation of cyclitols in Viscum album. New Phytol. 1992, 121, 431–434. [Google Scholar] [CrossRef]
- Murwani, R. Indonesian tea mistletoe (Scurrula oortiana) stem extract increases tumour cell sensitivity to tumour necrosis factor alpha (TNFalpha). Phytother. Res. 2003, 17, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Murtini, S.; Murwani, R.; Satrija, F.; Handaryani, E. Anti Marek’s Disease Virus Activity of Scurrula oortiana (Tea Mistletoe) Stem Extract in Embryonated Chicken Eggs*. Int. J. Poult. Sci. 2010, 9, 879–885. [Google Scholar]
- Johri, B.M.; Bhojwani, S. Embryo Morphogenesis in the Stem Parasite Scurrula pulverulenta. Ann. Bot. 1970, 34, 685–690. [Google Scholar]
- Ghosh, A.; Saha, S. Distribution of epiphytes, parasites and mistletoes on six species of Phorophytes in upper montane tropical forest of Darjeeling, West Bengal. Indian J. Appl. Pure Biol. 2013, 28, 45–54. [Google Scholar]
- Ameer, O.Z.; Salman, I.M.; Yam, M.F.; Allah, H.H.A.; Abdulla, M.H.; Shah, A.M.; Sadikun, A.; Asmawi, M.Z. Vasorelaxant Properties of Loranthus ferrugineus Roxb. Methanolic Extract. Int. J. Pharmacol. 2009, 5, 44–50. [Google Scholar] [CrossRef]
- Ameer, O.Z.; Salman, I.M.; Siddiqui, M.J.A.; Yam, M.F.; Sriramaneni, R.N.; Mohamed, A.J.; Sadikun, A.; Ismail, Z.; Shah, A.M.; Asmawi, M.Z. Pharmacological mechanisms underlying the vascular activities of Loranthus ferrugineus Roxb. in rat thoracic aorta. J. Ethnopharmacol. 2010, 127, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Priyanto, J.A.; Pujiyanto, S.; Rukmi, I. Flavonoids Production Capability Test of Tea Mistletoe (Scurrula atropurpurea BL. Dans) Endophytic Bacteria Isolates. Jurnal Sains dan Matematika. 2014, 22, 89–96. [Google Scholar]
- Shen, C.-C.; Chang, Y.-S.; Hott, L.-K. Nuclear magnetic resonance studies of 5,7-dihydroxyflavonoids. Phytochemistry 1993, 34, 843–845. [Google Scholar] [CrossRef]
- Shibuya, H.; Ohashi, K.; Kitagawa, I. Search for pharmacological leads from tropical rainforest plants. Pure Appl. Chem. 1999, 71, 1109–1113. [Google Scholar] [CrossRef]
- Ishizu, T.; Tsujino, E.; Winarno, H.; Ohashi, K.; Shibuya, H. A complex of perseitol and K+ ion from Scurrula fusca (Loranthaceae). Tetrahedron Lett. 2001, 42, 6887–6889. [Google Scholar] [CrossRef]
- Lohézic-Le Dévéhat, F.; Tomasi, S.; Fontanel, D.; Boustie, J. Flavonols from Scurrula ferruginea Danser (Loranthaceae). Z. Naturforsch. C. 2002, 57, 1092–1095. [Google Scholar] [PubMed]
- Zhi, K.; Li, M.; Bai, J.; Wu, Y.; Zhou, S.; Zhang, X.; Qu, L. Quercitrin treatment protects endothelial progenitor cells from oxidative damage via inducing autophagy through extracellular signal-regulated kinase. Angiogenesis 2016, 19, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, L.; Fang, L.; Xie, H.; Yao, W.; Zhou, X.; Xiong, Z.; Wang, L.; Li, Z.; Luo, F. Quercetin reduces cyclin D1 activity and induces G1 phase arrest in HepG2 cells. Oncol. Lett. 2016, 12, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Pratheeshkumar, P.; Son, Y.; Divya, S.P. Quercetin inhibits Cr(VI)-induced malignant cell transformation by targeting miR-21-PDCD4 signaling pathway. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Moghadamtousi, S.Z.; Kamarudin, M.N.A.; Chan, C.K.; Goh, B.H.; Kadir, H.A. Phytochemistry and Biology of Loranthus parasiticus Merr, a Commonly Used Herbal Medicine. Am. J. Chin. Med. 2014, 42, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Winarno, H.; Mukai, M.; Shibuya, H. Preparation and Cancer Cell Invasion Inhibitory Effects of C16-Alkynic Fatty Acids. Chem. Pharm. Bull. (Tokyo) 2003, 51, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Winarno, H.; Mukai, M.; Inoue, M.; Prana, M.S.; Simanjuntak, P.; Shibuya, H. Indonesian medicinal plants. XXV. Cancer cell invasion inhibitory effects of chemical constituents in the parasitic plant Scurrula atropurpurea (Loranthaceae). Chem. Pharm. Bull. (Tokyo) 2003, 51, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.-Y.; Wang, F.; Zhang, L.; Xie, J.-M.; Li, P.; Zhang, Y.-H. A Hydroxylated Lupeol-Based Triterpenoid Ester Isolated from the Scurrula parasitica Parasitic on Nerium indicum. Helv. Chim. Acta 2015, 98, 627–632. [Google Scholar] [CrossRef]
- Ravishankar, D.; Rajora, A.K.; Greco, F.; Osborn, H.M.I. Flavonoids as prospective compounds for anti-cancer therapy. Int. J. Biochem. Cell Biol. 2013, 45, 2821–2831. [Google Scholar] [CrossRef] [PubMed]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Sak, K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn. Rev. 2014, 8, 122–146. [Google Scholar] [CrossRef] [PubMed]
- Lohézic-Le Dévéhat, F.; Bakhtiar, A.; Bézivin, C.; Amoros, M.; Boustie, J. Antiviral and cytotoxic activities of some Indonesian plants. Fitoterapia 2002, 73, 400–405. [Google Scholar] [CrossRef]
- Parwati, N.W.M.; Lindayani, I.K.; Ratnawati, R.; Winarsih, S.; Nurseta, T. Possible effect of tea plant parasite, Scurrula atropurpurea (Blume) Danser, on growth inhibition of culture HeLa cells in vitro through DNA repair and apoptosis intrinsic pathways mechanism. Asian Pacific J. Trop. Dis. 2015, 5, 743–746. [Google Scholar] [CrossRef]
- Iwakuma, T.; Lozano, G. MDM2, An Introduction. Mol. Cancer Res. 2003, 1, 993–1000. [Google Scholar] [PubMed]
- Wang, X.; Lin, Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol. Sin. 2008, 29, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Van Horssen, R.; Hagen, T.L.M.; Eggermont, A.M.M. TNF-α in cancer treatment: Molecular insights, antitumor effects, and clinical utility. Oncologist 2006, 11, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Cengizeroglu, A.; Farkasova, K.; Viola, J.R.; Anton, M.; Ellwart, J.W.; Haase, R.; Wagner, E.; Ogris, M. Systemic TNFα gene therapy synergizes with liposomal doxorubicine in the treatment of metastatic cancer. Mol. Ther. 2013, 21, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Gaskill, H.V. Continuous infusion of tumor necrosis factor: Mechanisms of toxicity in the rat. J. Surg. Res. 1988, 44, 664–671. [Google Scholar] [CrossRef]
- Lehmann, V.; Freudenberg, M.A.; Galanos, C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and d-galactosamine-treated mice. J. Exp. Med. 1987, 165, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Dondossola, E.; Dobroff, A.S.; Marchiò, S.; Cardó-Vila, M.; Hosoya, H.; Libutti, S.K.; Corti, A.; Sidman, R.L.; Arap, W.; Pasqualini, R. Self-targeting of TNF-releasing cancer cells in preclinical models of primary and metastatic tumors. Proc. Natl. Acad. Sci. USA 2016, 113, 2223–2228. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.J.; Yoo, Y.C.; Choi, O.B.; Do, M.S.; Kang, T.B.; Lee, S.W.; Azuma, I.; Kim, J.B. Inhibitory effect of Korean mistletoe (Viscum album coloratum) extract on tumour angiogenesis and metastasis of haematogenous and non-haematogenous tumour cells in mice. Cancer Lett. 1995, 97, 83–91. [Google Scholar] [CrossRef]
- Hostanska, K.; Hajto, T.; Spagnoli, G.C.; Fischer, J.; Lentzen, H.; Herrmann, R. A plant lectin derived from Viscum album induces cytokine gene expression and protein production in cultures of human peripheral blood mononuclear cells. Nat. Immun. 1995, 14, 295–304. [Google Scholar] [PubMed]
- Estko, M.; Baumgartner, S.; Urech, K.; Kunz, M.; Regueiro, U.; Heusser, P.; Weissenstein, U. Tumour cell derived effects on monocyte/macrophage polarization and function and modulatory potential of Viscum album lipophilic extract in vitro. BMC Complement. Altern. Med. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Elluru, S.R.; Duong van Huyen, J.-P.; Delignat, S.; Kazatchkine, M.D.; Friboulet, A.; Kaveri, S.V.; Bayry, J. Induction of maturation and activation of human dendritic cells: A mechanism underlying the beneficial effect of Viscum album as complimentary therapy in cancer. BMC Cancer 2008, 8. [Google Scholar] [CrossRef] [PubMed]
- Hegde, P.; Maddur, M.S.; Friboulet, A.; Bayry, J.; Kaveri, S.V. Viscum album exerts anti-inflammatory effect by selectively inhibiting cytokine-induced expression of cyclooxygenase-2. PLoS ONE 2011, 6, e26312. [Google Scholar] [CrossRef] [PubMed]
- Saha, C.; Hegde, P.; Friboulet, A.; Bayry, J.; Kaveri, S.V. Viscum album-mediated COX-2 inhibition implicates destabilization of COX-2 mRNA. PLoS ONE 2015, 10, e0114965. [Google Scholar] [CrossRef] [PubMed]
- Stein, G.M.; Edlund, U.; Pfüller, U.; Büssing, A.; Schietzel, M. Influence of polysaccharides from Viscum album L. on human lymphocytes, monocytes and granulocytes in vitro. Anticancer Res. 1999, 19, 3907–3914. [Google Scholar] [PubMed]
- Delebinski, C.I.; Jaeger, S.; Kemnitz-Hassanin, K.; Henze, G.; Lode, H.N.; Seifert, G.J. A new development of triterpene acid-containing extracts from Viscum album L. displays synergistic induction of apoptosis in acute lymphoblastic leukaemia. Cell Prolif. 2012, 45, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Delebinski, C.I.; Twardziok, M.; Kleinsimon, S.; Hoff, F.; Mulsow, K.; Rolff, J.; Jäger, S.; Eggert, A.; Seifert, G. A natural combination extract of Viscum album L. containing both triterpene acids and lectins is highly effective against AML in vivo. PLoS ONE 2015, 10, e0133892. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.B.; Yeh, E.S.; Soloff, A.C. Tumor-associated macrophages: Unwitting accomplices in breast cancer malignancy. NPJ Breast Cancer 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Grossarth-Maticek, R.; Kiene, H.; Baumgartner, S.M.; Ziegler, R. Use of Iscador, an extract of European mistletoe (Viscum album), in cancer treatment: Prospective nonrandomized and randomized matched-pair studies nested within a cohort study. Altern. Ther. Health Med. 2001, 7, 57–66, 68–72, 74–76. [Google Scholar] [PubMed]
- Bock, P.R.; Hanisch, J.; Matthes, H.; Zänker, K.S. Targeting inflammation in cancer-related-fatigue: A rationale for mistletoe therapy as supportive care in colorectal cancer patients. Inflamm. Allergy Drug Targets. 2014, 13, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Sunjic, S.B.; Gasparovic, A.C.; Vukovic, T.; Weiss, T.; Weiss, E.S. Adjuvant cancer biotherapy by Viscum album Extract Isorel: Overview of evidence based medicine findings. Coll. Antropol. 2015, 39, 701–708. [Google Scholar] [PubMed]
- Von Schoen-Angerer, T.; Goyert, A.; Vagedes, J.; Kiene, H.; Merckens, H.; Kienle, G.S. Disappearance of an advanced adenomatous colon polyp after intratumoural injection with Viscum album (european mistletoe) extract: A case report. J. Gastrointest. Liver Dis. 2014, 23, 449–452. [Google Scholar]
- Von Schoen-Angerer, T.; Wilkens, J.; Kienle, G.S.; Kiene, H.; Vagedes, J. High-dose Viscum album extract treatment in the prevention of recurrent bladder cancer: A retrospective case series. Perm. J. 2015, 19, 76–83. [Google Scholar] [PubMed]
- Podlech, O.; Harter, P.N.; Mittelbronn, M.; Pöschel, S.; Naumann, U. Fermented mistletoe extract as a multimodal antitumoral agent in gliomas. Evid. Based Complement. Altern. Med. 2012. [Google Scholar] [CrossRef] [PubMed]
- Kuonen, R.; Weissenstein, U.; Urech, K.; Kunz, M.; Hostanska, K.; Estko, M.; Heusser, P.; Baumgartner, S. Effects of lipophilic extract of Viscum album L. and oleanolic acid on migratory activity of NIH/3T3 fibroblasts and on HaCat keratinocytes. In Evid. Based Complement. Altern. Med.; 2013. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, M.; Zhang, S.-Y.; Zhou, Z.-K.; Tian, W.-X. Parasitic loranthus from Loranthaceae rather than Viscaceae potently inhibits fatty acid synthase and reduces body weight in mice. J. Ethnopharmacol. 2008, 118, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, V.; Botti, C.; di Tondo, U.; Visca, P.; Pizzuti, L.; Santeusanio, G.; Alo, P.L. Tissue microarray analysis of FAS, Bcl-2, Bcl-x, ER, PgR, Hsp60, p53 and Her2-neu in breast carcinoma. Anticancer Res. 2006, 26, 2983–2987. [Google Scholar] [PubMed]
- Flavin, R.; Peluso, S.; Nguyen, P.L.; Loda, M. Fatty acid synthase as a potential therapeutic target in cancer. Future Oncol. 2010, 6, 551–562. [Google Scholar] [CrossRef]
- Kurnia, I.; Lestari, A.; Nazip, K.; Estuningsih, S. Test of effectiveness of antibacterial of ethanol extract of Loranthus of tea (Scurulla atropurpurea BL Danser) on the growth of enterobacter sakazakii. In Proceedings of the International Conference on Food, Biological and Medical Sciences, Bangkok, Thailand, 28–29 January 2014; pp. 10–15.
- Marvibaigi, M.; Amini, N.; Supriyanto, E.; Jamil, S.; Abdul Majid, F.A.; Khangholi, S. Total phenolic content, antioxidant and antibacterial properties of Scurrula ferruginea extracts. J. Teknol. 2014, 5, 65–72. [Google Scholar]
- Tripathi, S.; Ray, S.; Das, P.K.; Mondal, A.K.; Verma, N.K. Antimicrobial activities of some rare aerial hemi parasitic taxa of south West Bengal, India. Int. J. Phytopharm. 2013, 4, 106–112. [Google Scholar]
- Ukwueze, S.E.; Osadebe, P.O.; Ezenobi, N.O. Bioassay-guided evaluation of the antibacterial activity of Loranthus species of the African mistletoe. Int. J. Pharm. Biomed. Res. 2013, 4, 79–82. [Google Scholar]
- Iwalokun, B.A.; Oyenuga, A.O.; Saibu, G.M.; Ayorinde, J.; Lagos, Y.; Polytechnic, L.S. Analyses of cytotoxic and genotoxic potentials of Loranthus micranthus using the Allium cepa test. J. Biol. Sci. 2011, 3, 459–467. [Google Scholar]
- Ameer, O.Z.; Salman, I.M.; Siddiqui, M.J.A.; Yam, M.F.; Sriramaneni, R.N.; Sadikun, A.; Ismail, Z.; Shah, A.M.; Asmawi, M.Z. Characterization of the possible mechanisms underlying the hypotensive and spasmogenic effects of Loranthus ferrugineus methanolic extract. Am. J. Chin. Med. 2009, 37, 991–1008. [Google Scholar] [CrossRef] [PubMed]
- Ameer, O.Z.; Salman, I.M.; Siddiqui, M.J.A.; Yam, M.F.; Sriramaneni, R.N.; Sadikun, A.; Ismail, Z.; Shah, A.M.; Asmawi, M.Z. Cardiovascular activity of the n-butanol fraction of the methanol extract of Loranthus ferrugineus Roxb. Braz. J. Med. Biol. Res. 2010, 43, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Ameer, O.Z.; Salman, I.M.; Najim, H.S.; Abdullah, G.Z.; Abdulkarim, M.F.; Yam, M.F.; Sadikun, A.; Asmawi, M.Z. In vitro pharmacodynamic profile of Loranthus ferrugineus: Evidence for noncompetitive antagonism of norepinephrine-induced vascular contraction. J. Acupunct. Meridian Stud. 2010, 3, 272–282. [Google Scholar] [CrossRef]
- Mothana, R.A.A.; Al-Said, M.S.; Al-Rehaily, A.J.; Thabet, T.M.; Awad, N.A.; Lalk, M.; Lindequist, U. Anti-inflammatory, antinociceptive, antipyretic and antioxidant activities and phenolic constituents from Loranthus regularis Steud. ex Sprague. Food Chem. 2012, 130, 344–349. [Google Scholar] [CrossRef]
- Ali, M.A.; Chanu, K.H.V.; Devi, L.I. Scurrula parasitica L.: A medicinal plant with high antioxidant activity. Int. J. Pharm. Pharm. Sci. 2013, 5, 5–8. [Google Scholar]
- Wong, D.Z.H.; Kadir, H.A.; Lee, C.L.; Goh, B.H. Neuroprotective properties of Loranthus parasiticus aqueous fraction against oxidative stress-induced damage in NG108-15 cells. J. Nat. Med. 2012, 66, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.Z.H.; Kadir, H.A.; Ling, S.K. Bioassay-guided isolation of neuroprotective compounds from Loranthus parasiticus against H2O2-induced oxidative damage in NG108-15 cells. J. Ethnopharmacol. 2012, 139, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-N. Ethanol Extracts from Mistletoe (Viscum album L.) Act as natural antioxidants and antimicrobial agents in uncooked pork patties during refrigerated storage. Asian-Aust. J. Anim. Sci. 2016, 29, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Onay-Uçar, E.; Erol, O.; Kandemir, B.; Mertoğlu, E.; Karagöz, A.; Arda, N. Viscum album L. Extracts protects HeLa cells against nuclear and mitochondrial DNA damage. Evid. Based. Complement. Alternat. Med. 2012, 2012. [Google Scholar] [CrossRef]
- Nugrahaa, A.S.; Keller, P.A. Revealing indigenous Indonesian traditional medicine: Anti-infective agents. Nat. Prod. Commun. 2011, 6, 1953–1966. [Google Scholar]
- Ameer, O.Z.; Salman, I.M.; Siddiqui, M.J.; Yam, M.F.; Sriramaneni, R.N.; Sadikun, A.; Ismail, Z.; Shah, A.M.; Asmawi, M.Z. In vitro cholinomimetic effect of Loranthus ferrugineus in isolated guinea pig ileum. J. Acupunct. Meridian Stud. 2009, 2, 288–293. [Google Scholar] [CrossRef]
- Ventura, R.; Mordec, K.; Waszczuk, J.; Wang, Z.; Lai, J.; Fridlib, M.; Buckley, D.; Kemble, G.; Heuer, T.S.; Abulrob, A.; et al. Inhibition of de novo Palmitate Synthesis by Fatty Acid Synthase Induces Apoptosis in Tumor Cells by Remodeling Cell Membranes, Inhibiting Signaling Pathways, and Reprogramming Gene Expression. EBioMedicine 2015, 2, 808–824. [Google Scholar] [CrossRef] [PubMed]
- Iversen, C.; Lane, M.; Forsythe, S.J. The growth profile, thermotolerance and biofilm formation of Enterobacter sakazakii grown in infant formula milk. Lett. Appl. Microbiol. 2004, 38, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Sulistiarini, R.; Soemardji, A.A.; Fidrianny, I. Study activity of Scurrula atropurpurea (Blume) danser on wound healing phase. Int. J. Trop. Nat. Sci. 2014, 1, 11–14. [Google Scholar]
- Limananti, A.I.; Triratnawati, A. Ramuan Jamu Cekok Sebagai Penyembuhan Kurang Nafsu Makan Pada Anak: Suatu Kajian Etnomedisin. Makara Kesehat. 2003, 7, 11–20. [Google Scholar]
- Deliorman, D.; Caliş, I.; Ergun, F. A new acyclic monoterpene glucoside from Viscum album ssp. album. Fitoterapia 2001, 72, 101–105. [Google Scholar] [CrossRef]
- Ertürk, Ö.; Katı, H.; Yaylı, N.; Demirbağ, Z. Antimicrobial activity of Viscum album L. subsp. abietis (Wiesb). Turk J. Biol. 2003, 27, 255–258. [Google Scholar]
- Dulger, B.; Gonuz, A. Antimicrobial activity of some Turkish Medicinal Plants. Pak. J. Biol. Sci. 2004, 7, 1559–1562. [Google Scholar]
- Hussain, M.A.; Khan, M.Q.; Hussain, N.; Habib, T. Antibacterial and antifungal potential of leaves and twigs of Viscum album L. J. Med. Plants Res. 2011, 5, 5545–5549. [Google Scholar]
- Sadananda, T.; Govindappa, M.; Ramachandra, Y. Antibacterial activity of Viscum album endophytic fungal lectin. Int. J. Biol. Pharm. Res. 2013, 4, 878–884. [Google Scholar]
- Karagöz, A.; Önay, E.; Arda, N.; Kuru, A. Antiviral potency of mistletoe (Viscum album ssp. album) extracts against human parainfluenza virus type 2 in Vero cells. Phyther. Res. 2003, 17, 560–562. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence? Lancet 1994, 344, 721–724. [Google Scholar] [CrossRef]
- Kothari, S.; Thompson, A.; Agarwal, A.; du Plessis, S.S. Free radicals: Their beneficial and detrimental effects on sperm function. Indian J. Exp. Biol. 2010, 48, 425–435. [Google Scholar] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [PubMed]
- Popa-Wagner, A.; Mitran, S.; Sivanesan, S.; Chang, E.; Buga, A.-M. ROS and brain diseases: The Good, the Bad, and the Ugly. Oxid. Med. Cell. Longev. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Antioxidants in human health and disease. Annu. Rev. Nutr. 1996, 16, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Osadebe, P.O.; Omeje, E.O.; Uzor, P.F.; David, E.K.; Obiorah, D.C. Seasonal variation for the antidiabetic activity of Loranthus micranthus methanol extract. Asian Pac. J. Trop. Med. 2010, 3, 196–199. [Google Scholar] [CrossRef]
- Agbo, M.O.; Lai, D.; Okoye, F.B.C.; Osadebe, P.O.; Proksch, P. Antioxidative polyphenols from Nigerian mistletoe Loranthus micranthus (Linn.) parasitizing on Hevea brasiliensis. Fitoterapia 2013, 86, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Ogechukwu, O.E.; Ogoamaka, O.P.; Kawamura, A.; Hassan, A.; Debbab, A.; Charles, E. Three - (−) Catechin-O-Rhamnosides from the Eastern Nigeria Mistletoe with Potent Immunostimulatory and Antioxidant Activities. Biomolecules 2012, 1. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Kim, Y.S.; Choi, S.U.; Ryu, S.Y. Isolation of flavonol rhamnosides from Loranthus tanakae and cytotoxic effect of them on human tumor cell lines. Arch. Pharm. Res. 2004, 27, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Behl, C.; Moosmann, B. Antioxidant neuroprotection in Alzheimer’s disease as preventive and therapeutic approach. Free Radic. Biol. Med. 2002, 33, 182–191. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Zhang, M.; An, C.; Gao, Y.; Leak, R.K.; Chen, J.; Zhang, F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog. Neurobiol. 2013, 100, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xu, B.T.; Gan, R.Y.; Zhang, Y.; Xu, X.R.; Xia, E.Q.; Li, H.B. Total phenolic contents and antioxidant capacities of herbal and tea infusions. Int. J. Mol. Sci. 2011, 12, 2112–2124. [Google Scholar] [CrossRef] [PubMed]
- Manning, J.; Roberts, J.C. Analysis of catechin content of commercial green tea products. J. Herb. Pharmacother. 2003, 3, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, H.; Halliwell, B. Damage to DNA by reactive oxygen and nitrogen species: Role in inflammatory disease and progression to cancer. Biochem. J. 1996, 313, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Vicas, S.I.; Rugina, D.; Socaciu, C. Antioxidant Activity of European Mistletoe (Viscum album). In Phytochemicals as Nutraceuticals–Global Approaches to Their Role in Nutrition and Health; InTech: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Önay-Uçar, E.; Karagöz, A.; Arda, N. Antioxidant activity of Viscum album ssp. album. Fitoterapia 2006, 77, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Simona, V.; Rugină, D.; Socaciu, C. Antioxdant Activities Of Viscum Album’s Leaves From Various Host Trees. Bull. UASVM Agric. 2008, 65, 322–327. [Google Scholar]
- Bowman, I. The everlasting mistletoe and the cardiovascular system. Texas Hear. Inst. J. 1990, 17, 310–314. [Google Scholar]
- Ofem, O.; Eno, A.; Imoru, J.; Nkanu, E.; Unoh, F.; Ibu, J.; Ofem, O. Effect of crude aqueous leaf extract of Viscum album (mistletoe) in hypertensive rats. Indian J. Pharmacol. 2007, 39, 15–19. [Google Scholar] [CrossRef]
- Poruthukaren, K.J.; Palatty, P.L.; Baliga, M.S.; Suresh, S. Clinical evaluation of Viscum album mother tincture as an antihypertensive: A pilot study. J. Evid. Based Complement. Altern. Med. 2013, 19. [Google Scholar] [CrossRef] [PubMed]
- Nazaruk, J.; Orlikowski, P. Phytochemical profile and therapeutic potential of Viscum album L. Nat. Prod. Res. 2016, 30, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Bachhav, S.S.; Patil, S.D.; Bhutada, M.S.; Surana, S.J. Oleanolic acid prevents glucocorticoid-induced hypertension in rats. Phyther. Res. 2011, 25, 1435–1439. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, M.; Hrupka, B.; Langhans, W. New approaches in the pharmacological treatment of obesity. Eur. J. Nutr. 1999, 38. [Google Scholar] [CrossRef]
- Guo, C.; Wei, D.; Chen, H.; Zeng, X. A review of the study on anti-obesity therapies with traditional Chinese medicine. J. Chin. Med. Mater. 2002, 25, 534–537. [Google Scholar]
- Mobbs, C.V.; Makimura, H. Block the FAS, lose the fat. Nat. Med. 2002, 8, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Artanti, N.; Firmansyah, T.; Darmawan, A.; Selatan, T. Bioactivities Evaluation of Indonesian Mistletoes (Dendrophthoe pentandra (L.) Miq.) Leaves Extracts. J. Appl. Pharm. Sci. 2012, 2, 24–27. [Google Scholar]
- Edem, D.O.; Usoh, I.F. Biochemical Changes in Wistar Rats on Oral Doses of Mistletoe (Loranthus micranthus). Am. J. Pharmacol. Toxicol. 2009, 4, 94–97. [Google Scholar] [CrossRef]
- Kienle, G.S.; Grugel, R.; Kiene, H. Safety of higher dosages of Viscum album L. in animals and humans—Systematic review of immune changes and safety parameters. BMC Complement. Altern. Med. 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Mansky, P.J.; Grem, J.; Wallerstedt, D.B.; Monahan, B.P.; Blackman, M.R. Mistletoe and gemcitabine in patients with advanced cancer: A model for the Phase I study of botanicals and botanical-drug interactions in cancer therapy. Integr. Cancer Ther. 2003, 2, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Kienle, G.S.; Kiene, H. Review article: Influence of Viscum album L (European Mistletoe) extracts on quality of life in cancer patients: A systematic review of controlled clinical studies. Integr. Cancer Ther. 2010, 9, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Büssing, A.; Stumpf, C.; Tröger, W.; Schietzel, M. Course of mitogen-stimulated T lymphocytes in cancer patients treated with Viscum album extracts. Anticancer Res. 2007, 27, 2903–2910. [Google Scholar] [PubMed]
- Horneber, M.A.; Bueschel, G.; Huber, R.; Linde, K.; Rostock, M. Mistletoe therapy in oncology. Cochrane Database Syst. Rev. 2008. [Google Scholar] [CrossRef]
- Huber, R.; Lüdtke, H.; Wieber, J.; Beckmann, C. Safety and effects of two mistletoe preparations on production of Interleukin-6 and other immune parameters—a placebo controlled clinical trial in healthy subjects. BMC Complement. Altern. Med. 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Kienle, G.S.; Glockmann, A.; Schink, M.; Kiene, H. Viscum album L. extracts in breast and gynaecological cancers: A systematic review of clinical and preclinical research. J. Exp. Clin. Cancer Res. 2009, 28, 79–112. [Google Scholar] [CrossRef] [PubMed]
- Mansky, P.J.; Wallerstedt, D.B. Complementary medicine in palliative care and cancer symptom management. Cancer J. 2006, 12, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Melzer, J.; Iten, F.; Hostanska, K.; Saller, R. Efficacy and Safety of Mistletoe Preparations (Viscum album) for Patients with Cancer Diseases A Systematic Review. Forschende Komplementärmedizin / Res. Complement. Med. 2009, 16, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Marvibaigi, M.; Supriyanto, E.; Amini, N.; Abdul Majid, F.A.; Jaganathan, S.K. Preclinical and clinical effects of mistletoe against breast cancer. Biomed Res. Int. 2014, 2014, 785479. [Google Scholar] [CrossRef] [PubMed]
- Elsasserbeile, U.; Leiber, C.; Wolf, P.; Lucht, M.; Mengs, U.; Wetterauer, U. Adjuvant intravesical treatment of superficial bladder cancer with a standardized mistletoe extract. J. Urol. 2005, 174, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-C.; Yook, J.-H.; Eisenbraun, J.; Kim, B.-S.; Huber, R. Quality of life, immunomodulation and safety of adjuvant mistletoe treatment in patients with gastric carcinoma—A randomized, controlled pilot study. BMC Complement. Altern. Med. 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, S.; Bayraktar, U.D.; Rocha-Lima, C.M. Recent developments in palliative chemotherapy for locally advanced and metastatic pancreas cancer. World J. Gastroenterol. 2010, 16, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Tröger, W.; Galun, D.; Reif, M.; Schumann, A.; Stanković, N.; Milićević, M. Quality of life of patients with advanced pancreatic cancer during treatment with mistletoe: A randomized controlled trial. Dtsch. Ärzteblatt Int. 2014, 111, 493–502. [Google Scholar]
- Tröger, W.; Galun, D.; Reif, M.; Schumann, A.; Stanković, N.; Milićević, M. Viscum album [L.] extract therapy in patients with locally advanced or metastatic pancreatic cancer: A randomised clinical trial on overall survival. Eur. J. Cancer. 2013, 49, 3788–3797. [Google Scholar] [CrossRef] [PubMed]
- Bar-Sela, G.; Goldberg, H.; Beck, D.; Amit, A.; Kuten, A. Reducing malignant ascites accumulation by repeated intraperitoneal administrations of a Viscum album extract. Anticancer Res. 2006, 26, 709–713. [Google Scholar] [PubMed]
- Langmead, L.; Rampton, D. Herbal treatment in gastrointestinal and liver disease—Benenefits and dangers. Aliment. Pharmacol. Ther. 2001, 15, 1239–1252. [Google Scholar]
- Ostermann, T.; Raak, C.; Büssing, A. Survival of cancer patients treated with mistletoe extract (Iscador): A systematic literature review. BMC Cancer 2009, 9, 451. [Google Scholar] [CrossRef] [PubMed]

| Parasite | Example(s) of Host Plant | Reference(s) |
|---|---|---|
| Arceuthobium vaginatum subsp. cryptopodum | Pinus ponderosa | [23] |
| Dendrophthoe curvata Blume | Acacia auriculiformis A. Cunn. Ex Benth Andira inermis (W. Wright) DC. Mangifera indica L. Vitex pinnata L. | [25,26] |
| Phoradendron californicum | Acacia greggii | [27] |
| Phoradendron juniperinum | Juniperus osteospermum | [27,28] |
| Scurrula ferruginea | Tabebuia Rosea Tabebuia pallida | [29] |
| Viscum album | Acer platanoides L. Betula pendula Roth Salix alba Malus domestica | [24,30] |
| Botanical Name | Synonym(s) | Common Name | Other Vernacular Name(s) | Reference(s) |
|---|---|---|---|---|
| Scurrula oortiana | Dendrophthoe oortiana(Korth.) Miq. Loranthus oortianus Korth. | Indonesian tea mistletoe | ‘Benalu teh’ | [31,32] |
| Scurrula pulverulenta | - | Powdery mistletoe | Leafy mistletoe | [33] |
| Scurrula elata | Loranthus elatus Edgew. | Butterfly-Bush mistletoe | Tall mistletoe | [34] |
| Scurrula ferruginea | Loranthus ferrugineus Jack | Rusty mistletoe |
| [9,35,36] |
| Scurrula atropurpurea |
| Indonesian tea mistletoe | ‘Benalu teh’ | [37] |
| Name | Structure | Reference(s) |
|---|---|---|
| Scurrula ferruginea | ||
| Quercetin |  | [41] |
| Quercitrin |  | [41] |
| 4-O-acetylquercitrin |  | [41] |
| Loranthus parasiticus | ||
| Coriamyrtin |  | [45] |
| Tutin |  | [45] |
| Corianin |  | [45] |
| Coriatin |  | [45] |
| Proanthocyanidins of (+)-catechin |  | [45,46] |
| Proanthocyanidins of AC trimer |  | [45] |
| Scurrula atropurpurea | ||
| Z-octadec-12-ene-8,10-diynoic acid |  | [46] |
| octadeca-12-ene-8,10-triynoic acid |  | [47] |
| Flavanes-Epigallocatechin-3-O-gallate |  | [46] |
| Scurrula parasitica | ||
| Quercetin 3-O-β-l-galactopyranoside |  | [48] |
| Quercetin 3-O-β-l-arabinopyranoside |  | [48] |
| Quercetin 3-O-α-l-rhamnopyranoside |  | [48] |
| Plant | Parts of Plant Used | Type of Extract(s) | Observation(s) | Bioactive Compounds | Reference(s) |
|---|---|---|---|---|---|
| Antibacterial Activity | |||||
| Scurrula atropurpurea |
| Ethanol extract | Antibacterial in inhibiting the growth of Enterobacter sakazakii |
| [81] |
| Scurrula ferruginea |
| Aqueous extract | Antibacterial activity against (MIC)
| Phenolic compounds | [82] |
| Macrosolon cochichinensis |
| Methanolic extract and aqueous extract | Antibacterial activity (with inhibition zones from 4 mm to 8 mm) against
| - | [83] |
| Scurrula atropurpurea |
| Methanolic extract | Antibacterial activity against
| - | [83] |
| Viscum album |
| Methanolic extract | Antibacterial activity against Klebsiella pneumonia with inhibition one of 3–3.5 mm | - | [83] |
| Aqueous extract | Antibacterial activity against Bacillus substilis with inhibition one of 2–3 mm. | - | |||
| Loranthus micranthus |
| Crude methanol extract followed by fractionation with ethyl acetate and acetone | Antibacterial activity against
|
| [84] |
| Anticancer or cytotoxic | |||||
| Scurrula ferruginea |
| Extracted with petroleum Ether followed by isolation from ethyl acetate fraction | Quercetin was found to be the most active in the following four human cancer lines:
|
| [41] |
| Loranthus micranthus |
| Aqueous extract | Genotoxic effects against Allium cepa root cells |
| [85] |
| Loranthus parasiticus | - | The methanol extract, ethyl acetate and aqueous fractions | Cytotoxic against the ovarian cancer cell lines, namely SKOV3, CAOV3 and OVCAR-3 |
| [45] |
| Scurrula oortiana |
| Aqueous and methanolic extracts | WEHI-164 cells sensitive to TNFα when treated with extract | - | [31] |
| Scurrula atropurpurea |
| Extracted with 70% acetone followed by fractionation with ethyl acetate | Octadeca-8,10,12-triynoic acid was most potent against mesothelial cells isolated from Donryu rats |
| [47] |
| Scurrula atropurpurea | - | Preparation of C16-Alkynic fatty acid | inhibitory effects on cancer cell invasion assay mesothelium monolayer by using MM1 cell line isolated from rat ascites hepatoma AH130 cells |
| [46] |
| Anti-hypertensive | |||||
| Scurrula ferruginea Scurrula ferruginea |
| Crude methanol extract, chloroform extract, ethyl acetate extract | In vivo experiment: Vasorelaxant by using Rat thoracic aorta | Polyphenolic and Flavonoids compounds | [35] |
| Methanolic Extract | Guinea Pig Ileum: Hypotensive and Spasmogenic effects | Polyphenolic and Flavonoids compounds | [86] | ||
| Methanolic extract and by n-butanol fraction | Rat thoracic aorta rings: vascular smooth muscle relaxation in vitro and a dose-dependent hypotensive action in vivo. | Terpenoids | [87] | ||
| Methanolic extract | The vascular effects of three different concentrations of this extract by reversible noncompetitive antagonism of norepinephrine-induced vasoconstriction | Terpenoids | [88] | ||
| Methanolic extract and then successively fractionated using chloroform, ethyl acetate and n-butanol | The n-butanol fraction of LFME (NBF-LFME) was studied using isolated rat thoracic aorta: relaxation by stimulating muscarinic receptors, activating the endothelium-derived nitric oxide-cGMP-relaxant pathway | Terpenoids | [36] | ||
| Antioxidant | |||||
| Loranthus parasiticus |
| Water/aqueous extract followed by methanolic extract, then ethyl acetate extract. | Antioxidant activity by 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity | Sesquiterpene lactones:
| [45] |
| Scurrula ferruginea |
| Acetone extract (mostly stem) | Antioxidant capacity of extracts were evaluated using DPPH free radical scavenging assay | Phenolic compounds | [82] |
| Loranthus regularis Steud. ex Sprague |
| Ethyl acetate fraction of a methanol extract | The antioxidant power of the extract, its fractions and isolated compounds was studied using DPPH scavenging and b-carotene/linoleic acid tests |
| [89] |
| Scurrula parasitica | Leaves (mature and tender) | Methanolic extract of the matured leaves | DPPH free radical scavenging assay | Phenolic compounds | [90] |
| Loranthus parasiticus |
| Ethanolic extract and further partitioned into ethyl acetate fraction and followed by aqueous fraction | Measurement of intracellular reactive oxygen species (ROS); H2O2-induced oxidative damage in NG108-15 cells | Proanthocyanidins AC trimer and (+)-catechin | [91,92] |
| Viscum album |
| Aqueous extract Ethanolic extract Acetone extract Methanolic extract (leaves rich in phenolics and carotenoids) | DPPH free radical scavenging assay ORAC method TEAC method Folin-Ciocalteu FRAP method DCFH-DA assay (to measure intracellular ROS levels) | Phenolic acids | [24,93,94] |
| Antiviral | |||||
| Scurrula ferruginea |
| Methanolic extracts | Antiviral activity against poliovirusactive on Poliovirus and activity on the U251 glioblastoma cells |
| [52] |
| Loranthus parasiticus |
| Methanolic extracts | Anti HIV-1 effect | - | [95] |
| Scurrula oortiana |
| Aqueous extracts | Anti Marek’s Disease Virus (MDV) | - | [32] |
| Neuroprotection | |||||
| Loranthus parasiticus |
| Aqueous fraction | Neuroprotective role in NG108-15 cells | Proanthocyanidins of AC trimer | [91] |
| Aqueous fraction | Increased cell viability and decreased intracellular ROS level in a dose-dependent manner against H2O2-induced oxidative stress in NG108-15 cells | Proanthocyanidins of (+)-catechin | |||
| Anti-schizophrenic Activity | |||||
| Loranthus parasiticus |
| Ethanol extract | Coriamyrtin with strong catatonic action in mice were effective components of L. parasiticus for shock therapy in catatonia treatment. | Coriamyrtin | [45] |
| Non-toxic corianin with comparable activity to electric shock or insulin has been used for catatonia treatment by muscle injection in hospitals of various areas of China. | Corianin (non-toxic) | ||||
| Tutin with strong catatonic action in mice were effective components of L. parasiticus for shock therapy in catatonia treatment. | Tutin | ||||
| Miscellaneous studies | |||||
| Scurrula ferruginea |
| Methanolic Extract | Spasmogenic effect in isolated guinea pig ileum |
| [86] |
| S. ferruginea methanol extract and followed by ethyl acetate and n-butanol fraction | In vitro Cholinomimetic Effect | [96] | |||
| Taxillus chinensis |
| Extracted with 50% ethanol in the ratio 1:20 (w/v) | Potent inhibition on fatty acid synthase (FAS) that is proposed to be a potential therapeutic target for treatment of obesity. | - | [78] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, Y.C.; Rajabalaya, R.; Lee, S.H.F.; Tennakoon, K.U.; Le, Q.-V.; Idris, A.; Zulkipli, I.N.; Keasberry, N.; David, S.R. Parasitic Mistletoes of the Genera Scurrula and Viscum: From Bench to Bedside. Molecules 2016, 21, 1048. https://doi.org/10.3390/molecules21081048
Lim YC, Rajabalaya R, Lee SHF, Tennakoon KU, Le Q-V, Idris A, Zulkipli IN, Keasberry N, David SR. Parasitic Mistletoes of the Genera Scurrula and Viscum: From Bench to Bedside. Molecules. 2016; 21(8):1048. https://doi.org/10.3390/molecules21081048
Chicago/Turabian StyleLim, Ya Chee, Rajan Rajabalaya, Shirley Huan Fang Lee, Kushan U. Tennakoon, Quang-Vuong Le, Adi Idris, Ihsan N. Zulkipli, Natasha Keasberry, and Sheba R. David. 2016. "Parasitic Mistletoes of the Genera Scurrula and Viscum: From Bench to Bedside" Molecules 21, no. 8: 1048. https://doi.org/10.3390/molecules21081048
APA StyleLim, Y. C., Rajabalaya, R., Lee, S. H. F., Tennakoon, K. U., Le, Q.-V., Idris, A., Zulkipli, I. N., Keasberry, N., & David, S. R. (2016). Parasitic Mistletoes of the Genera Scurrula and Viscum: From Bench to Bedside. Molecules, 21(8), 1048. https://doi.org/10.3390/molecules21081048