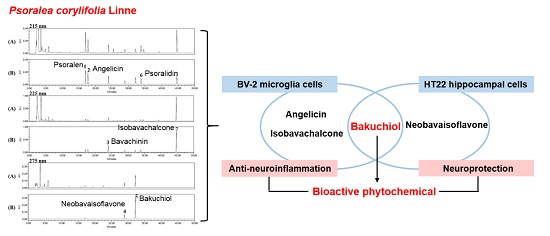
Quantitative Analysis of Psoralea corylifolia Linne and its Neuroprotective and Anti-Neuroinflammatory Effects in HT22 Hippocampal Cells and BV-2 Microglia
Abstract
:1. Introduction
2. Results
2.1. Optimization of High-Performance Liquid Chromatography (HPLC) Separation
2.2. Linearity, Limits of Detection (LOD), and Limits of Quantification (LOQ)
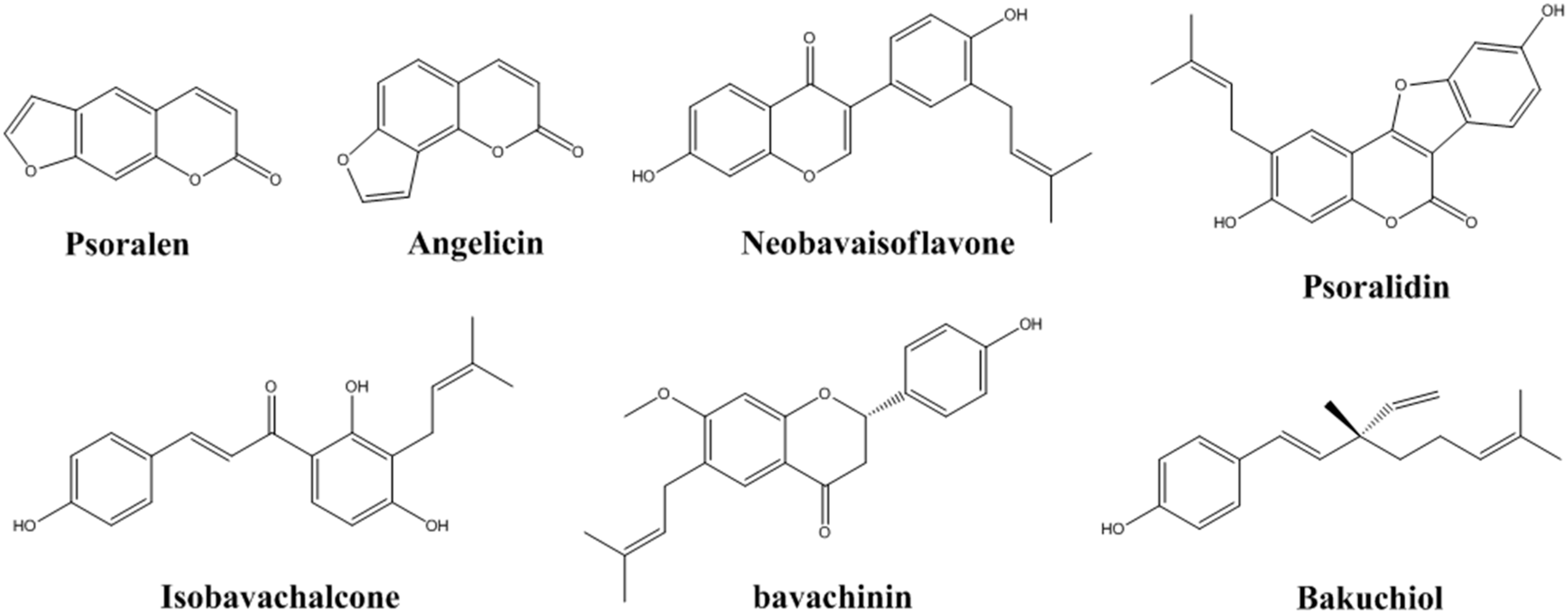
2.3. Determination of the Seven Standard Components in P. corylifolia
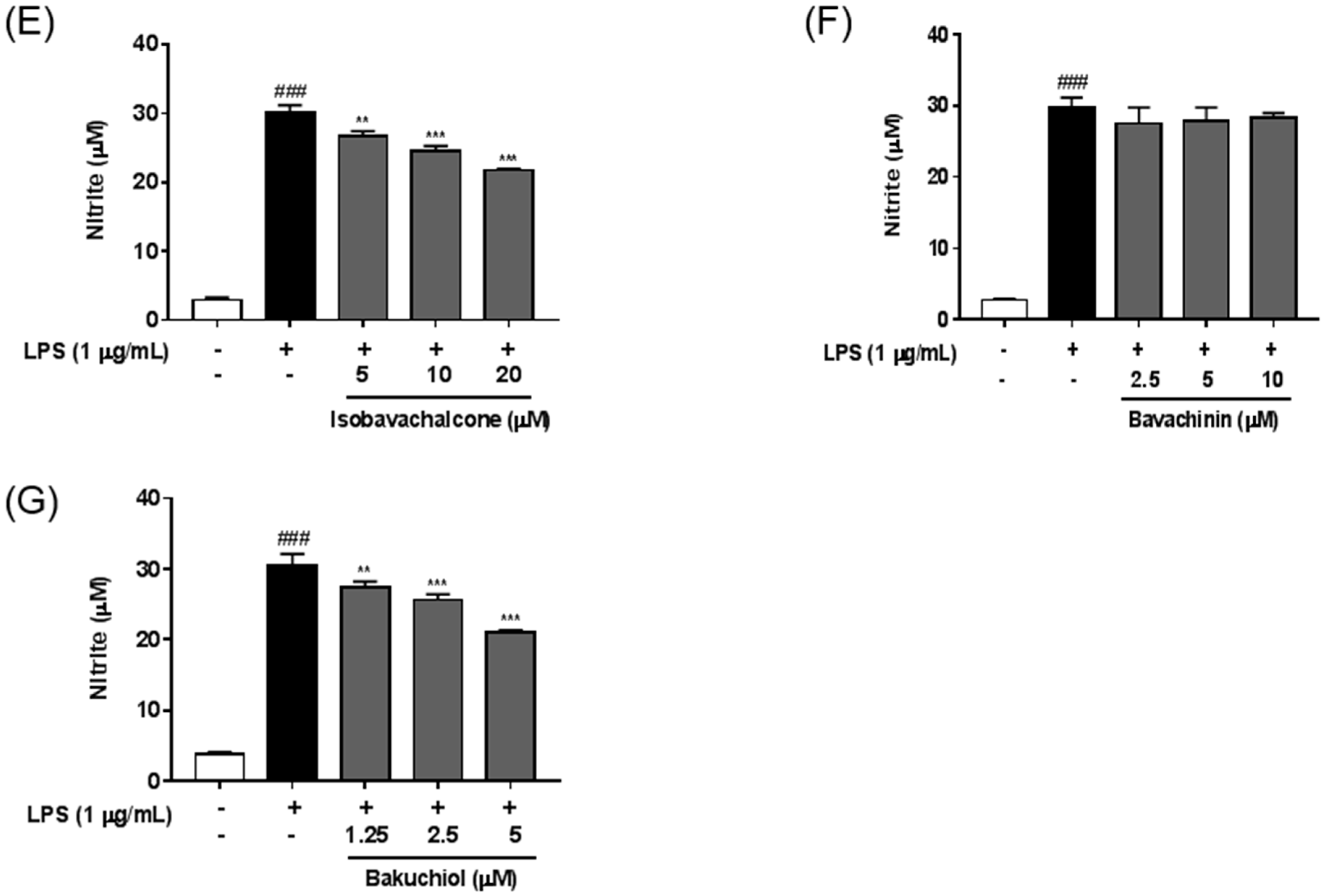
2.4. Anti-Neuroinflammatory Effects of Seven Standard Components in P. corylifolia
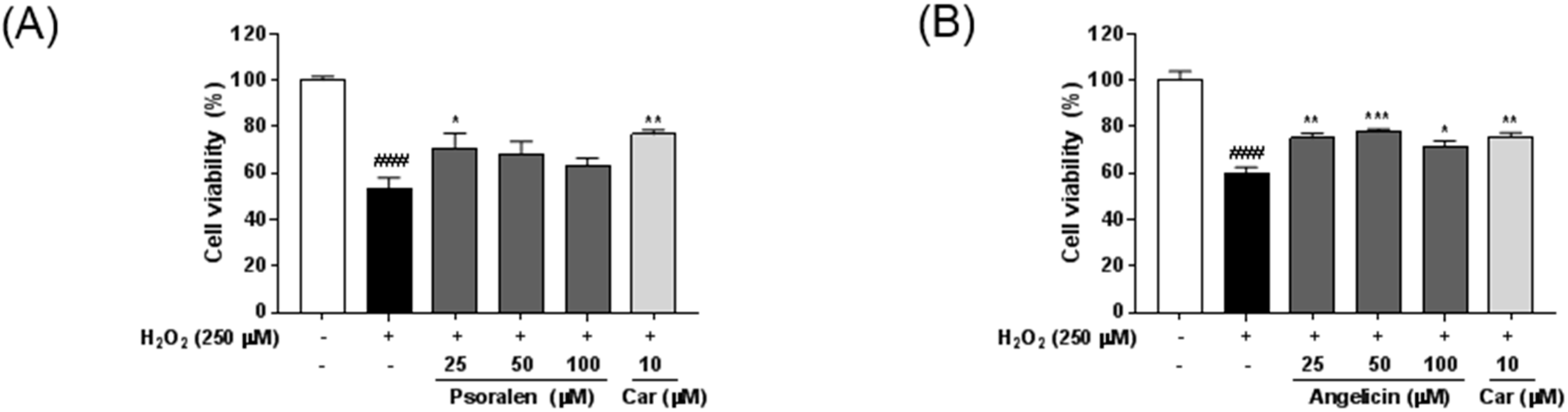
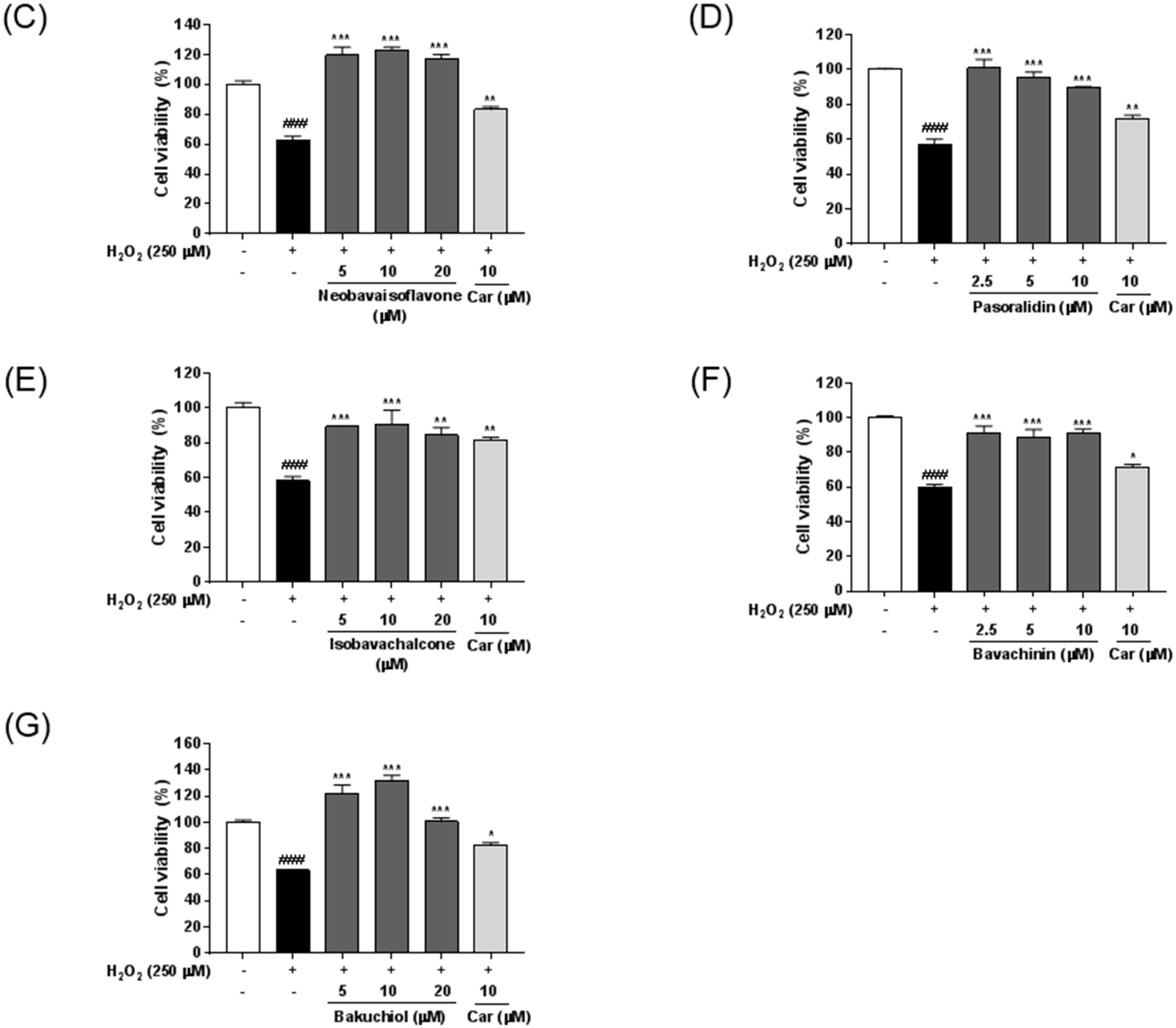
2.5. Neuroprotective Effects of Seven Standard Compounds in P. corylifolia
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals and Reagents
4.3. Apparatus and Chromatographic Conditions
4.4. Preparation of Standard Solutions
4.5. Preparation of Sample Solutions
4.6. Calibration Curve and Determination of the Limit of Detection (LOD) and Limit of Quantification (LOQ)
4.7. Cell Lines and Culture
4.8. Nitric Oxide (NO) Assay
4.9. Measurement of Neuroprotective Activity
4.10. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Uikey, S.K.; Yadav, A.; Sharma, A.K.; Rai, A.K.; Raghuwanshi, D.; Badkhane, Y. The botany, chemistry, pharmacological and therapeutic application of Psoralea corylifolia L.: A review. Int. J. Phytomed. 2011, 2, 100–107. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, W.; Wang, Y.; Lu, J.; Chen, X. The Chemical Constituents and Bioactivities of Psoralea corylifolia Linn.: A Review. Am. J. Chin. Med. 2016, 44, 35–60. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Li, H.; Noh, M.; Ryu, J.H. Bavachin from Psoralea corylifolia Improves Insulin-Dependent Glucose Uptake through Insulin Signaling and AMPK Activation in 3T3-L1 Adipocytes. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.; Lee, E.K.; Lee, C.S.; Chun, K.H.; Lee, M.Y.; Jun, H.S. Psoralea corylifolia L. seed extract ameliorates streptozotocin-induced diabetes in mice by inhibition of oxidative stress. Oxid. Med. Cell. Longev. 2014. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.; Oh, Y.S.; Jun, H.S. Psoralea corylifolia L. Seed Extract Attenuates Nonalcoholic Fatty Liver Disease in High-Fat Diet-Induced Obese Mice. Nutrients 2016, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.; Jang, E.H.; Hong, D.; Cho, I.H.; Park, M.J.; Kim, J.H. Aqueous extract of Psoralea corylifolia L. inhibits lipopolysaccharide-induced endothelial-mesenchymal transition via downregulation of the NF-kappaB-SNAIL signaling pathway. Oncol. Rep. 2015, 34, 2040–2046. [Google Scholar] [PubMed]
- Rajan, V.; Tripathi, J.; Variyar, P.; Pandey, B.N. Mechanism of cytotoxicity by Psoralea corylifolia extract in human breast carcinoma cells. J. Environ. Pathol. Toxicol. Oncol. 2014, 33, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Ling, S.; Duan, J.; Ma, J.; Ni, R.; Xu, J.W. Bavachalcone-induced manganese superoxide dismutase expression through the AMP-activated protein kinase pathway in human endothelial cells. Pharmacology 2015, 95, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Kim, J.M.; Baik, E.J.; Ryu, J.H.; Lee, S.H. Isobavachalcone attenuates lipopolysaccharide-induced ICAM-1 expression in brain endothelial cells through blockade of toll-like receptor 4 signaling pathways. Eur. J. Pharmacol. 2015, 754, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Youn, H.; Seong, K.M.; Yun, Y.J.; Kim, W.; Kim, Y.H.; Lee, J.Y.; Kim, C.S.; Jin, Y.W.; Youn, B. Psoralidin, a dual inhibitor of COX-2 and 5-LOX, regulates ionizing radiation (IR)-induced pulmonary inflammation. Biochem. Pharmacol. 2011, 82, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Nam, J.W.; Song, Y.S.; Viswanath, A.N.; Pae, A.N.; Kil, Y.S.; Kim, H.D.; Park, J.H.; Seo, E.K.; Chang, M. Psoralidin, a coumestan analogue, as a novel potent estrogen receptor signaling molecule isolated from Psoralea corylifolia. Bioorg. Med. Chem. Lett. 2014, 24, 1403–1406. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; do Kim, H.; Ahn, H.N.; Song, Y.S.; Lee, Y.J.; Ryu, J.H. Activation of Estrogen Receptor by Bavachin from Psoralea corylifolia. Biomol. Ther. 2012, 20, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, H.D.; Xia, X.; Kung, H.F.; Pan, Y.; Kong, L.D. Behavioral and biochemical studies of total furocoumarins from seeds of Psoralea corylifolia in the chronic mild stress model of depression in mice. Phytomedicine 2007, 14, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.T.; Li, Y.C.; Pan, Y.; Li, J.M.; Xu, Q.; Mo, S.F.; Qiao, C.F.; Jiang, F.X.; Xu, H.; Lu, X.B.; et al. Antidepressant-like effects of psoralidin isolated from the seeds of Psoralea corylifolia in the forced swimming test in mice. Prog Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Im, A.R.; Chae, S.W.; Zhang, G.J.; Lee, M. Neuroprotective effects of Psoralea corylifolia Linn seed extracts on mitochondrial dysfunction induced by 3-nitropropionic acid. BMC Complement. Altern. Med. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Chen, Z.; Tan, M.; Liu, A.; Chen, M.; Liu, J.; Pi, R.; Fang, J. Carvedilol, a third-generation beta-blocker prevents oxidative stress-induced neuronal death and activates Nrf2/ARE pathway in HT22 cells. Biochem. Biophys. Res. Commun. 2013, 441, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Kielian, T. Neuroinflammation: Good, bad, or indifferent? J. Neurochem. 2014, 130, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kraft, A.D.; Harry, G.J. Features of microglia and neuroinflammation relevant to environmental exposure and neurotoxicity. Int. J. Environ. Res. Public Health 2011, 8, 2980–3018. [Google Scholar] [CrossRef] [PubMed]
- Streit, W.J.; Mrak, R.E.; Griffin, W.S. Microglia and neuroinflammation: A pathological perspective. J. Neuroinflamm. 2004, 1, 14. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J.L.; Uemura, E.; Cunnick, J.E. Microglial release of nitric oxide by the synergistic action of beta-amyloid and IFN-gamma. Brain Res. 1995, 692, 207–214. [Google Scholar] [CrossRef]
- Vincent, V.A.; Tilders, F.J.; van Dam, A.M. Production, regulation and role of nitric oxide in glial cells. Mediators Inflamm. 1998, 7, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Stefano, G.B.; Kim, E.; Liu, Y.; Zhu, W.; Casares, F.; Mantione, K.; Jones, D.A.; Cadet, P. Nitric oxide modulates microglial activation. Med. Sci. Monit. 2004, 10, 11594. [Google Scholar]
- Yuste, J.E.; Tarragon, E.; Campuzano, C.M.; Ros-Bernal, F. Implications of glial nitric oxide in neurodegenerative diseases. Front. Cell. Neurosci. 2015, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Y.; Luan, P.; Huang, S.X.; Xiao, S.H.; Zhao, J.; Zhang, B.; Gu, B.B.; Pi, R.B.; Liu, J. Edaravone protects HT22 neurons from H2O2-induced apoptosis by inhibiting the MAPK signaling pathway. CNS Neurosci. Ther. 2013, 19, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Vedder, H.; Teepker, M.; Fischer, S.; Krieg, J.C. Characterization of the neuroprotective effects of estrogens on hydrogen peroxide-induced cell death in hippocampal HT22 cells: Time and dose-dependency. Exp. Clin. Endocrinol. Diabetes 2008, 108, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Teepker, M.; Anthes, N.; Krieg, J.C.; Vedder, H. 2-OH-estradiol, an endogenous hormone with neuroprotective functions. J. Psychiatr. Res. 2003, 37, 517–523. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, J.H.; Lee, Y.; Yang, H.; Heo, Y.S.; Bode, A.M.; Lee, K.W.; Dong, Z. Bakuchiol suppresses proliferation of skin cancer cells by directly targeting Hck, Blk, and p38 MAP kinase. Oncotarget 2016, 7, 14616–14627. [Google Scholar] [PubMed]
- Li, L.; Chen, X.; Liu, C.C.; Lee, L.S.; Man, C.; Cheng, S.H. Phytoestrogen Bakuchiol Exhibits in Vitro and in Vivo Anti-breast Cancer Effects by Inducing S Phase Arrest and Apoptosis. Front. Pharmacol. 2016, 7, 128. [Google Scholar] [CrossRef] [PubMed]
- Nordin, M.A.; Abdul Razak, F.; Himratul-Aznita, W.H. Assessment of Antifungal Activity of Bakuchiol on Oral-Associated Candida spp. Evid. Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Shoji, M.; Arakaki, Y.; Esumi, T.; Kohnomi, S.; Yamamoto, C.; Suzuki, Y.; Takahashi, E.; Konishi, S.; Kido, H.; Kuzuhara, T. Bakuchiol Is a Phenolic Isoprenoid with Novel Enantiomer-selective Anti-influenza A Virus Activity Involving Nrf2 Activation. J. Biol. Chem. 2015, 290, 28001–28017. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Ha, T.Y.; Kim, S.R.; Ahn, J.; Park, H.J.; Kim, S. Ethanol extract of Psoralea corylifolia L. and its main constituent, bakuchiol, reduce bone loss in ovariectomised Sprague-Dawley rats. Br. J. Nutr. 2009, 101, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Zhao, Y.Z.; Kim, Y.C.; Sohn, D.H. Protective effect of (S)-bakuchiol from Psoralea corylifolia on rat liver injury in vitro and in vivo. Planta Med. 2005, 71, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Wang, H.; Ma, S.; Xu, Y.; Zhang, H.; Wang, Y.; Niu, Z.; Fan, G.; Zhu, Y.; Gao, X.M. Bidirectional regulation of bakuchiol, an estrogenic-like compound, on catecholamine secretion. Toxicol. Appl. Pharmacol. 2014, 274, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, R.K.; Bojanowski, K. Bakuchiol: A retinol-like functional compound revealed by gene expression profiling and clinically proven to have anti-aging effects. Int. J. Cosmet. Sci. 2014, 36, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Blasi, E.; Barluzzi, R.; Bocchini, V.; Mazzolla, R.; Bistoni, F. Immortalization of microflial cells by a v-raf/v-myc carrying retrovirus. J. Neuroimmunol. 1990, 27, 229–237. [Google Scholar] [CrossRef]
- Morimoto, B.H.; Koshland, D.E., Jr. Induction and expressio of long- and short-term neurosecretory potentiation in a neural cell line. Neuron 1990, 5, 875–880. [Google Scholar] [CrossRef]
- Sample Availability: Not available.


| Time (min) | Flow Rate (mL/min) | Mobile Phase | |
|---|---|---|---|
| Water (%) | Acetonitrile (%) | ||
| 0 | 1.0 | 75 | 25 |
| 40 | 1.0 | 20 | 80 |
| 46 | 1.0 | 0 | 100 |
| 52 | 1.0 | 0 | 100 |
| Compound | Linear Range (μg/mL) | Regression Equation (y = ax + b) a | Correlation Coefficient (r2) | LOD b (μg/mL) | LOQ c (μg/mL) | |
|---|---|---|---|---|---|---|
| Slope (a) | Intercept (b) | |||||
| Psoralen | 3.125–100 | 81466 | 73697 | 0.9999 | 0.102 | 0.309 |
| Angelicin | 3.125–100 | 68433 | 61942 | 0.9999 | 0.103 | 0.313 |
| Neobavaisoflavone | 3.125–100 | 46488 | 25575 | 1.0000 | 0.239 | 0.725 |
| Psoralidin | 3.125–100 | 24213 | 1833.9 | 1.0000 | 0.134 | 0.407 |
| Isobavachalcone | 3.125–100 | 113946 | 60594 | 1.0000 | 0.175 | 0.529 |
| Bavachinin | 3.125–100 | 39030 | 33550 | 0.9999 | 0.190 | 0.576 |
| Bakuchiol | 12.5–400 | 38481 | −29888 | 1.0000 | 0.988 | 2.995 |
| Compound | Content (mg/g) |
|---|---|
| Psoralen | 1.902 ± 0.003 |
| Angelicin | 1.506 ± 0.003 |
| Neobavaisoflavone | 1.321 ± 0.011 |
| Psoralidin | 1.310 ± 0.010 |
| Isobavachalcone | 0.736 ± 0.006 |
| Bavachinin | 1.623 ± 0.011 |
| Bakuchiol | 11.713 ± 0.088 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.J.; Lim, H.-S.; Lee, J.; Jeong, S.-J. Quantitative Analysis of Psoralea corylifolia Linne and its Neuroprotective and Anti-Neuroinflammatory Effects in HT22 Hippocampal Cells and BV-2 Microglia. Molecules 2016, 21, 1076. https://doi.org/10.3390/molecules21081076
Kim YJ, Lim H-S, Lee J, Jeong S-J. Quantitative Analysis of Psoralea corylifolia Linne and its Neuroprotective and Anti-Neuroinflammatory Effects in HT22 Hippocampal Cells and BV-2 Microglia. Molecules. 2016; 21(8):1076. https://doi.org/10.3390/molecules21081076
Chicago/Turabian StyleKim, Yu Jin, Hye-Sun Lim, Jun Lee, and Soo-Jin Jeong. 2016. "Quantitative Analysis of Psoralea corylifolia Linne and its Neuroprotective and Anti-Neuroinflammatory Effects in HT22 Hippocampal Cells and BV-2 Microglia" Molecules 21, no. 8: 1076. https://doi.org/10.3390/molecules21081076