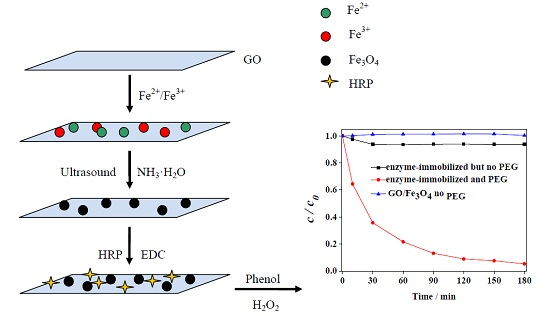
Catalytic Oxidation of Phenol and 2,4-Dichlorophenol by Using Horseradish Peroxidase Immobilized on Graphene Oxide/Fe3O4
Abstract
:1. Introduction
2. Results and Discussion
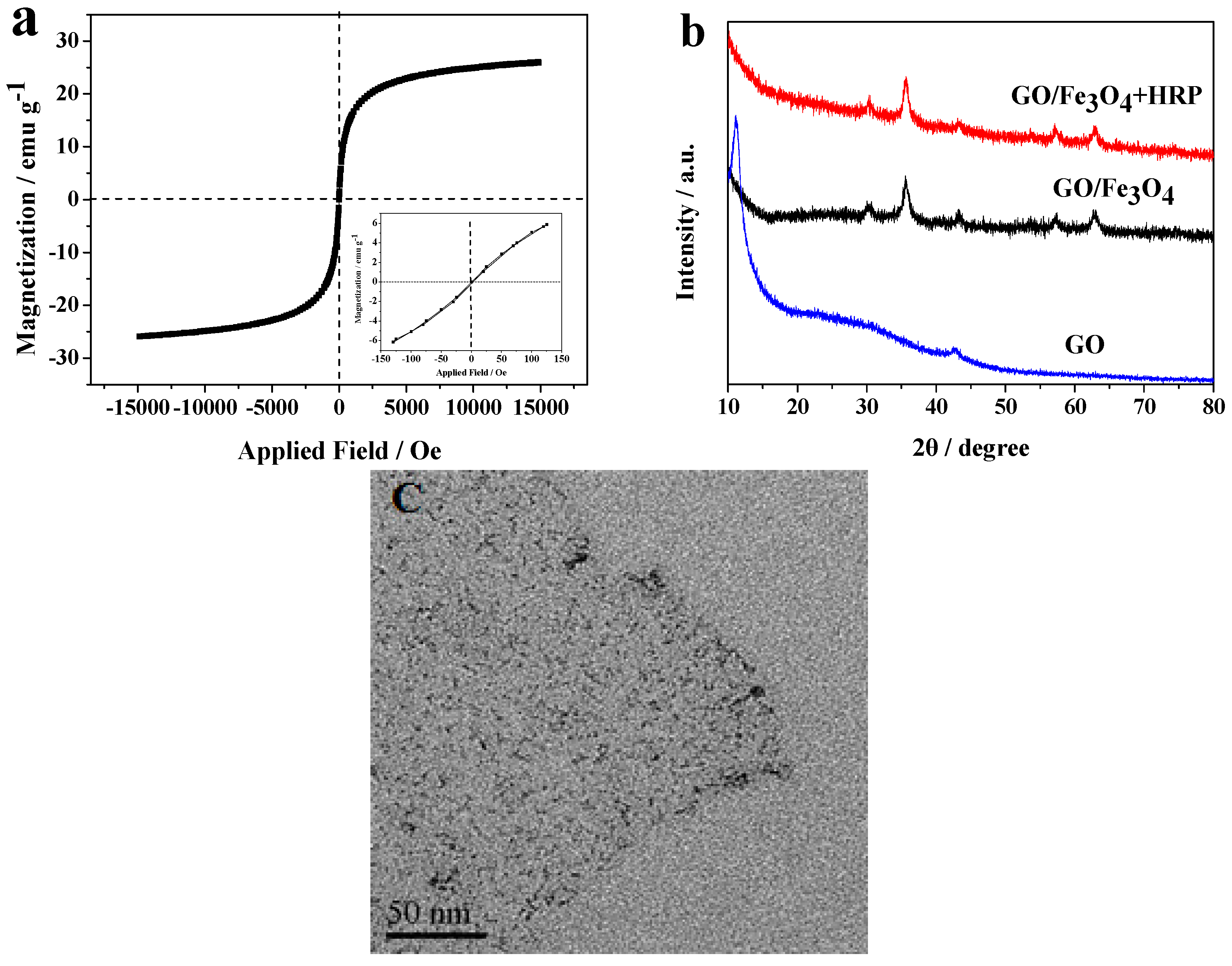
2.1. Characterization of Materials
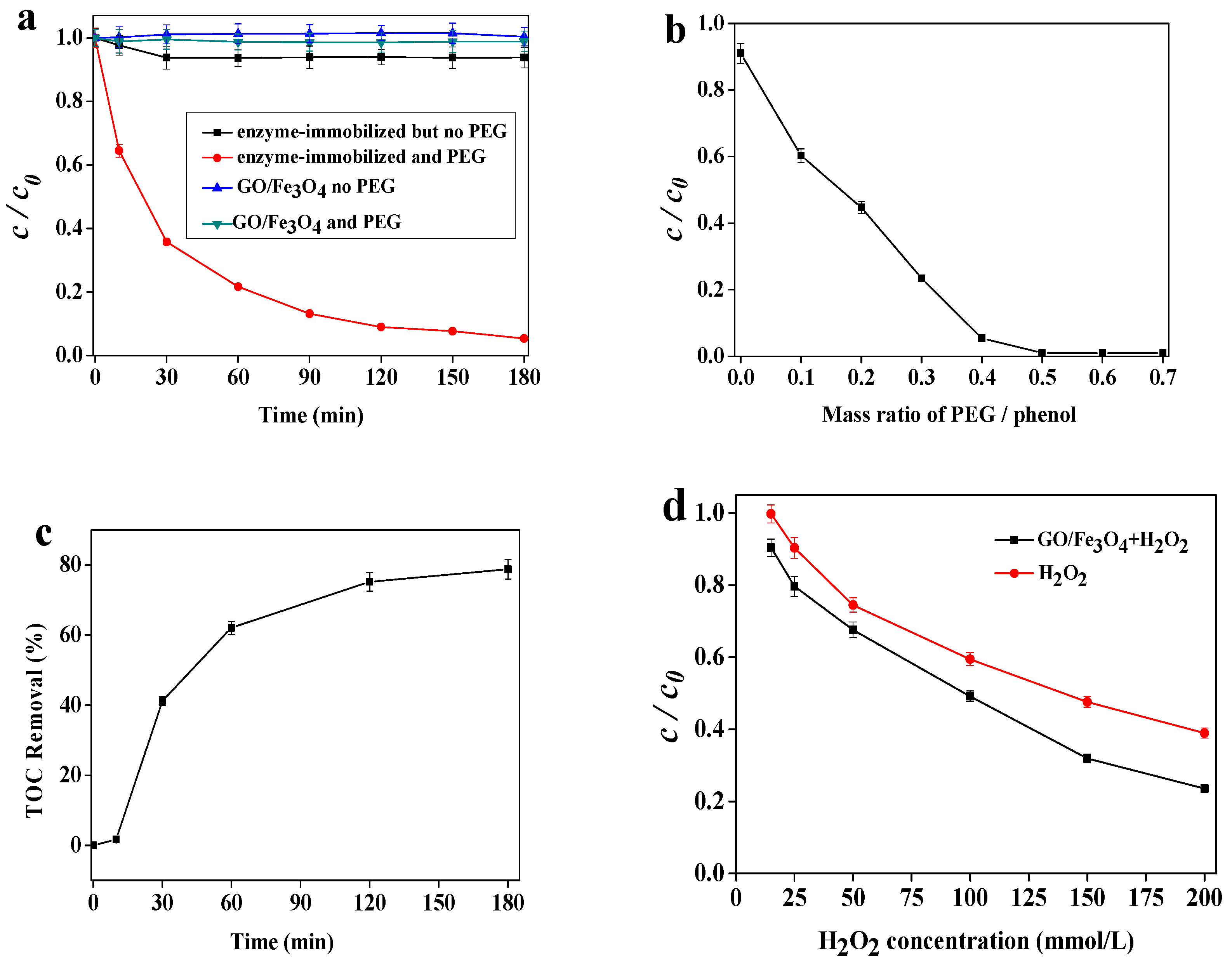
2.2. Reaction System and Effect of PEG
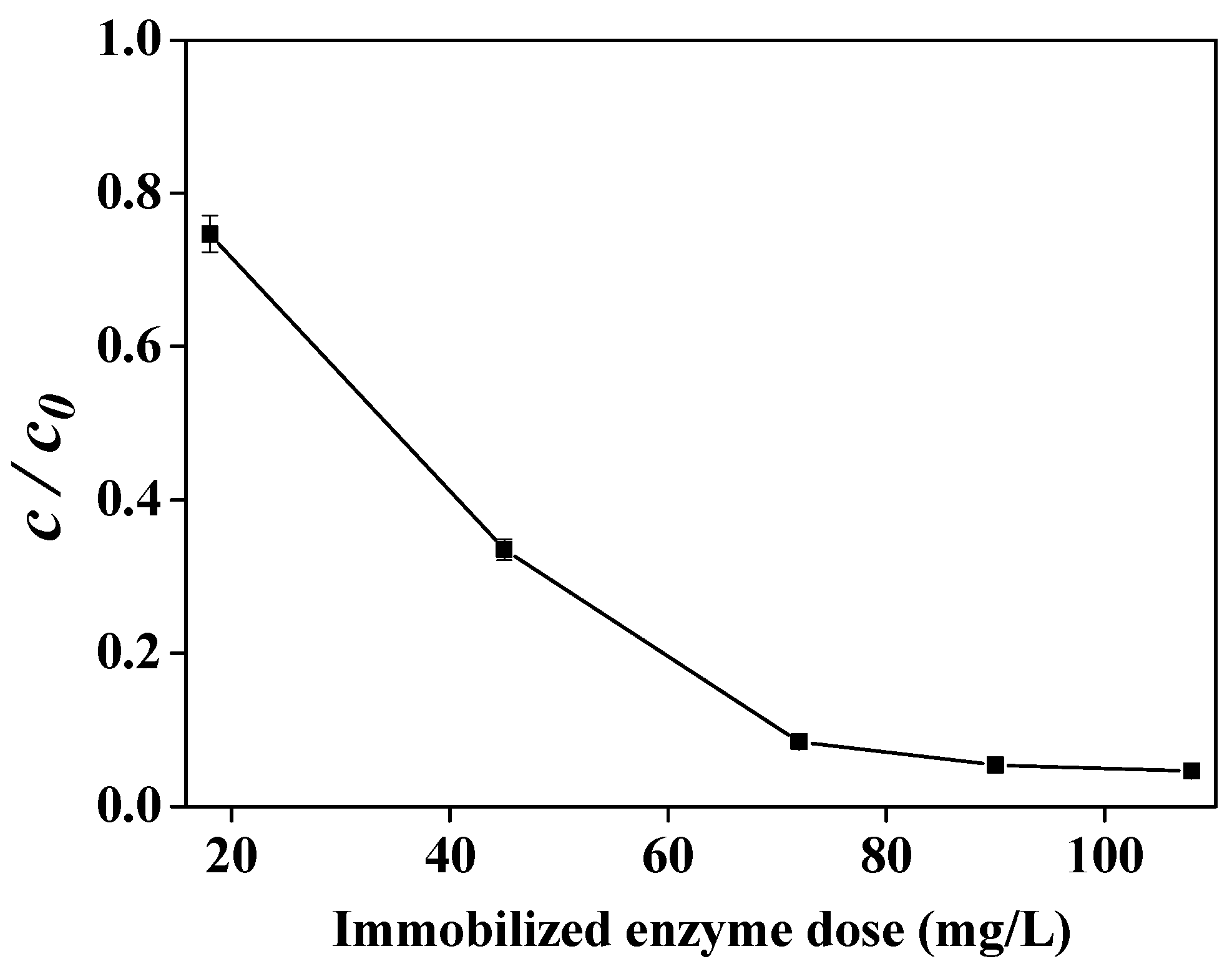
2.3. Effect of the Immobilized Enzyme Dose
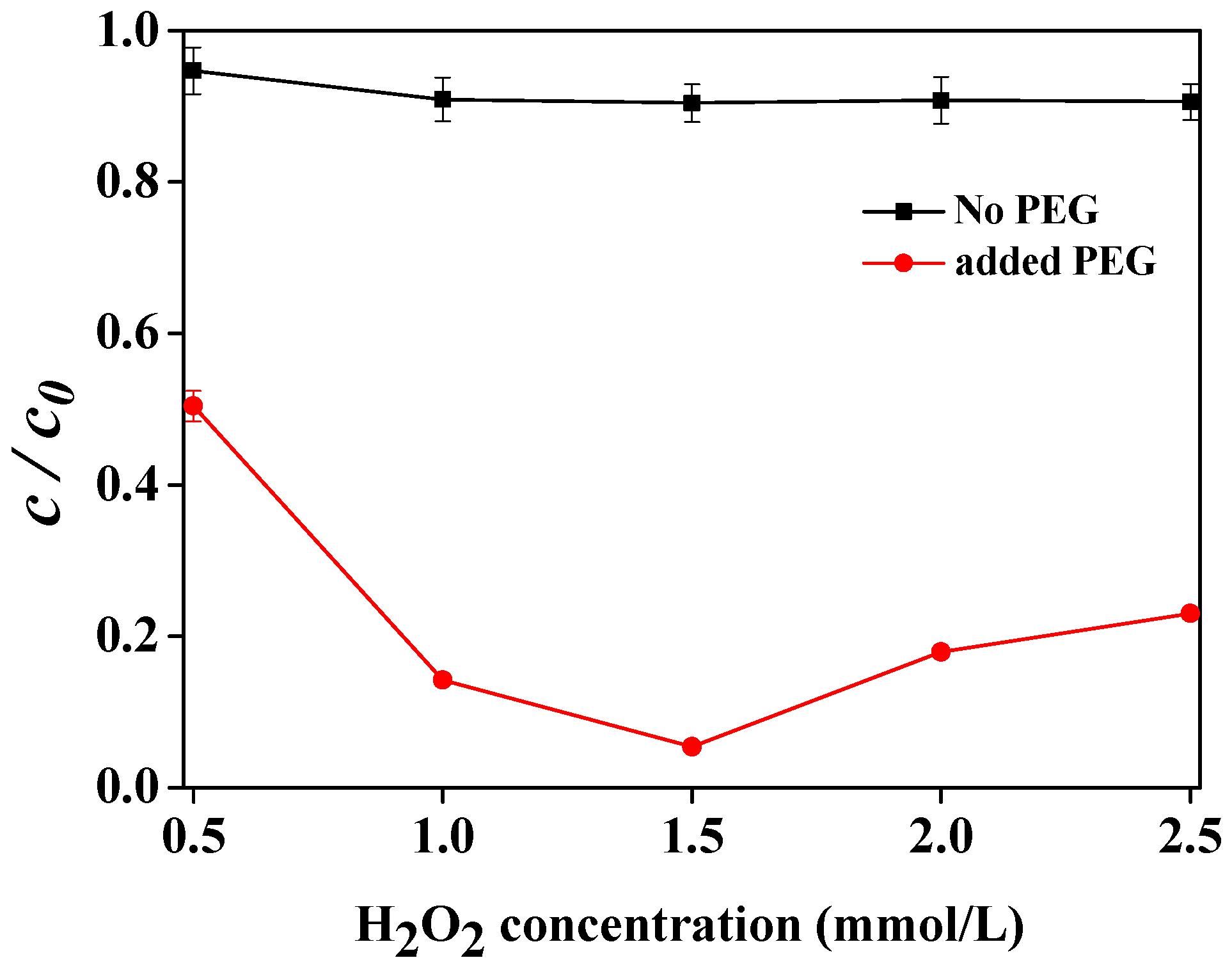
2.4. Effect of H2O2 Concentration
2.5. Degradation of Phenol and 2,4-Dichlorophenol in the Mixture
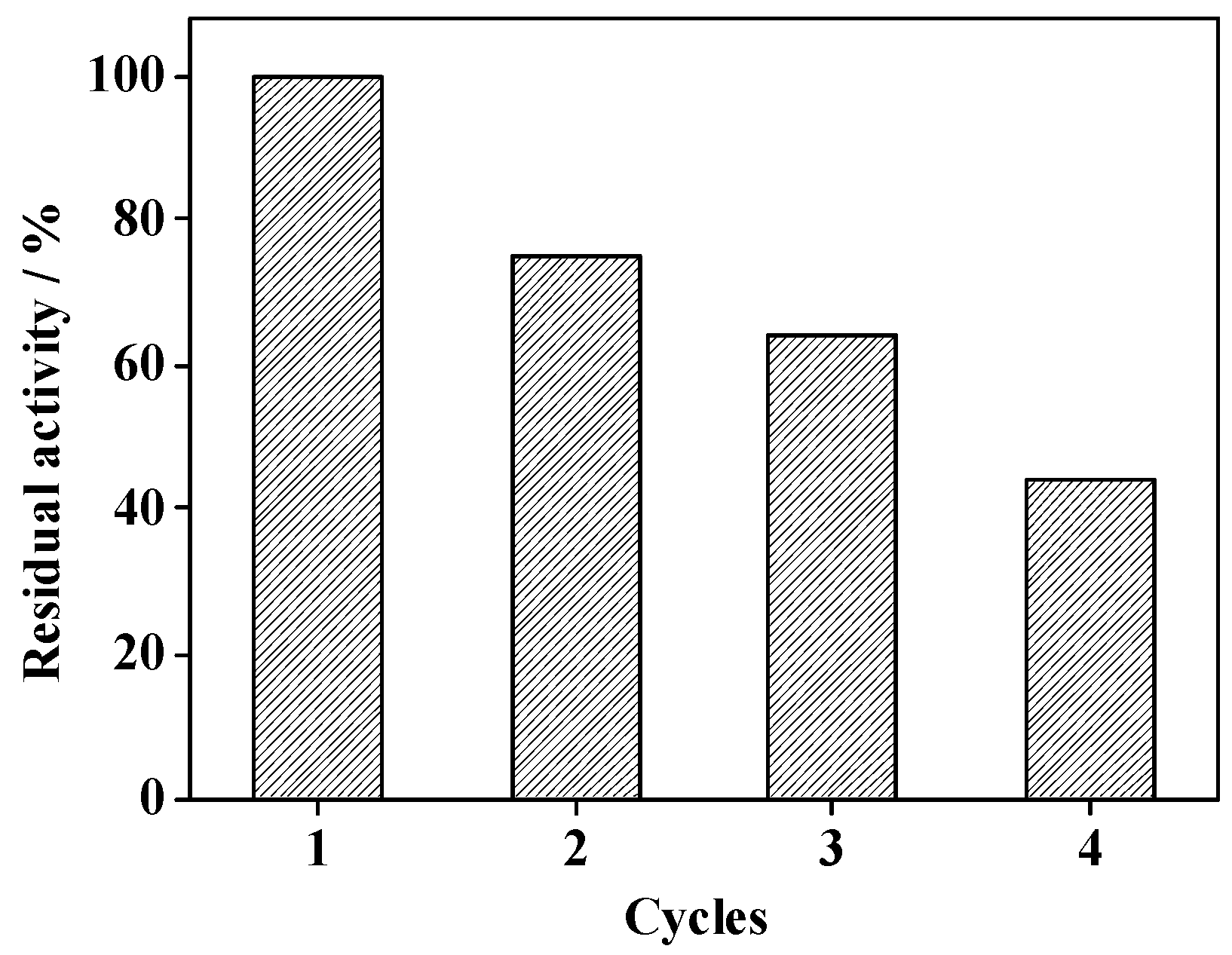
2.6. Reusability
3. Experimental Section
3.1. Reagents and Materials
3.2. Preparation of GO/Fe3O4 Nanoparticles
3.3. Immobilization of HRP on GO/Fe3O4
3.4. Characterization
3.5. Enzyme Activity Measurements
3.6. Experiments for Removing Phenol and the Mixture of Phenol and 2,4-Dichlorophenol
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, M.; Huang, Y.; Cao, H.J.; Lin, Y.H.; Li, X.; Li, H.M. Adsorption of phenol from aqueous solutions using a hydrophilic acrylic ester resin. Environ. Eng. Sci. 2015, 32, 881–890. [Google Scholar] [CrossRef]
- Zhou, L.C.; Meng, X.G.; Fu, J.W.; Yang, Y.C.; Yang, P.; Mi, C. Highly efficient adsorption of chlorophenols onto chemically modified chitosan. Appl. Surf. Sci. 2014, 292, 735–741. [Google Scholar] [CrossRef]
- Chen, Z.; Li, H.; Gao, Y.Z.; Peng, A.P. Removal of phenanthrene and acenaphthene from aqueous solution by enzyme-catalyzed phenol coupling reaction. Chem. Eng. J. 2015, 265, 27–33. [Google Scholar] [CrossRef]
- Nanayakkara, S.; Zhao, Z.Y.; Patti, A.F.; He, L.Z.; Saito, K. Immobilized horseradish peroxidase (I-HRP) as biocatalyst for oxidative polymerization of 2,6-dimethylphenol. ACS Sustain. Chem. Eng. 2014, 2, 1947–1950. [Google Scholar] [CrossRef]
- Dong, S.P.; Mao, L.; Luo, S.Q.; Zhou, L.; Feng, Y.P.; Gao, S.X. Comparison of lignin peroxidase and horseradish peroxidase for catalyzing the removal of nonylphenol from water. Environ. Sci. Pollut. Res. 2014, 21, 2358–2366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Zhang, F.; Yang, H.J.; Huang, X.L.; Liu, H.; Zhang, J.Y.; Guo, S.W. Graphene oxide as a matrix for enzyme immobilization. Langmuir 2010, 26, 6083–6085. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Huang, Q.G.; Pinto, R.A.; Griebenow, K.; Stenner, R.S.; Weber, W.J. Inactivation of horseradish peroxidase by phenoxyl radical attack. J. Am. Chem. Soc. 2005, 127, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Nicell, J.A.; Saadi, K.W.; Buchanan, I.D. Phenol polymerization and precipitation by horseradish peroxidase enzyme and an additive. Bioresour. Technol. 1995, 54, 5–16. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fuentes, M.; Betancor, L.; Grazu, V.; López-Gallego, F.; Pessela, B.C.C.; Hidalgo, A.; Fernández-Lorente, G.; Fernández-Lafuente, R.; et al. Glyoxyl agarose: A fully inert and hydrophilic support for immobilization and high stabilization of proteins. Enzyme Microb. Technol. 2006, 39, 274–280. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Guzik, U.; Hupert-Kocurek, K.; Wojcieszyńska, D. Immobilization as a strategy for improving enzyme properties-application to oxidoreductases. Molecules 2014, 19, 8995–9018. [Google Scholar] [CrossRef] [PubMed]
- Gusan, J.M.; Alvaro, G.; Fernandez-Lafuente, R. Industrial design of enzymic processes catalysed by very active immobilized derivatives: Utilization of diffusional limitations (gradients of pH) as a profitable tool in enzyme engineering. Biotechnol. Appl. Biochem. 1994, 20, 357–369. [Google Scholar]
- Chang, Q.; Tang, H.Q. Immobilization of horseradish peroxidase on NH2-modified magnetic Fe3O4/SiO2 particles and its application in removal of 2,4-dichlorophenol. Molecules 2014, 19, 15768–15782. [Google Scholar] [CrossRef] [PubMed]
- Garlet, T.B.; Weber, C.T.; Klaic, R.; Foletto, E.L.; Jahn, S.L.; Mazutti, M.A.; Kuhn, R.C. Carbon nanotubes as supports for inulinase immobilization. Molecules 2014, 19, 14615–14624. [Google Scholar] [CrossRef] [PubMed]
- Zucca, P.; Sanjust, E. Inorganic materials as supports for covalent enzyme immobilization: methods and mechanisms. Molecules 2014, 19, 14139–14194. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Deng, K.J.; Zhu, L.H.; Jiang, G.D.; Yu, C.; Tang, H.Q. Determination of hydrogen peroxide with the aid of peroxidase-like Fe3O4 magnetic nanoparticles as the catalyst. Microchim. Acta 2009, 165, 299–305. [Google Scholar] [CrossRef]
- Wang, M.Q.; Wang, N.; Tang, H.Q.; Cao, M.J.; She, Y.B.; Zhu, L.H. Surface modification of nano-Fe3O4 with EDTA and its use in H2O2 activation for removing organic pollutants. Catal. Sci. Technol. 2012, 2, 187–194. [Google Scholar] [CrossRef]
- Chang, Q.; Tang, H.Q. Optical determination of glucose and hydrogen peroxide using a nanocomposite prepared from glucose oxidase and magnetite nanoparticles immobilized on graphene oxide. Microchim. Acta 2014, 181, 527–534. [Google Scholar] [CrossRef]
- Si, Y.C.; Samulski, E.T. Exfoliated graphene separated by platinum nanoparticles. Chem. Mater. 2008, 20, 6792–6797. [Google Scholar] [CrossRef]
- Bai, L.Z.; Zhao, D.L.; Xu, Y.; Zhang, J.M.; Gao, Y.L.; Zhao, L.Y.; Tang, J.T. Inductive heating property of graphene oxide-Fe3O4 nanoparticles hybrid in an AC magnetic field for localized hyperthermia. Mater. Lett. 2012, 68, 399–401. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, J.; You, C.; Song, Z.; Yu, B.; Shen, Y. Influences of different synthesis conditions on properties of Fe3O4 nanoparticles. Mater. Chem. Phys. 2009, 113, 46–52. [Google Scholar] [CrossRef]
- Cheng, J.; Yu, S.M.; Zuo, P. Horseradish peroxidase immobilized on aluminum-pillared interlayered clay for the catalytic oxidation of phenolic wastewater. Water Res. 2006, 40, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Nicell, J.A.; Bewtra, J.K.; Taylor, K.E.; Biswas, N.; St Pierre, C. Enzyme catalyzed polymerization and precipitation of aromatic compounds from wastewater. Water Sci. Technol. 1992, 25, 157–164. [Google Scholar]
- Liu, J.Z.; Song, H.Y.; Weng, L.P.; Ji, L.N. Increased thermostability and phenol removal efficiency by chemical modified horseradish peroxidase. J. Mol. Catal. B Enzym. 2002, 18, 225–232. [Google Scholar] [CrossRef]
- Entezaria, M.H.; Petrier, C. A combination of ultrasound and oxidative enzyme: Sono-enzyme degradation of phenols in a mixture. Ultrason. Sonochem. 2005, 12, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Yang, Q.H.; Yang, Y.G.; Lv, W.; Wen, Y.F.; Hou, P.X.; Wang, M.Z.; Cheng, H.M. Self-assembled free-standing graphite oxide membrane. Adv. Mater. 2009, 21, 3007–3011. [Google Scholar] [CrossRef]
- Wang, N.; Zhu, L.; Wang, D.; Wang, M.; Lin, Z.; Tang, H. Sono-assisted preparation of highly-efficient peroxidase-like Fe3O4 magnetic nanoparticles for catalytic removal of organic pollutants with H2O2. Ultrason. Sonochem. 2010, 17, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds Fe3O4 are available from the authors.


© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Q.; Huang, J.; Ding, Y.; Tang, H. Catalytic Oxidation of Phenol and 2,4-Dichlorophenol by Using Horseradish Peroxidase Immobilized on Graphene Oxide/Fe3O4. Molecules 2016, 21, 1044. https://doi.org/10.3390/molecules21081044
Chang Q, Huang J, Ding Y, Tang H. Catalytic Oxidation of Phenol and 2,4-Dichlorophenol by Using Horseradish Peroxidase Immobilized on Graphene Oxide/Fe3O4. Molecules. 2016; 21(8):1044. https://doi.org/10.3390/molecules21081044
Chicago/Turabian StyleChang, Qing, Jia Huang, Yaobin Ding, and Heqing Tang. 2016. "Catalytic Oxidation of Phenol and 2,4-Dichlorophenol by Using Horseradish Peroxidase Immobilized on Graphene Oxide/Fe3O4" Molecules 21, no. 8: 1044. https://doi.org/10.3390/molecules21081044