Enantiopure Indolo[2,3-a]quinolizidines: Synthesis and Evaluation as NMDA Receptor Antagonists
Abstract
:1. Introduction
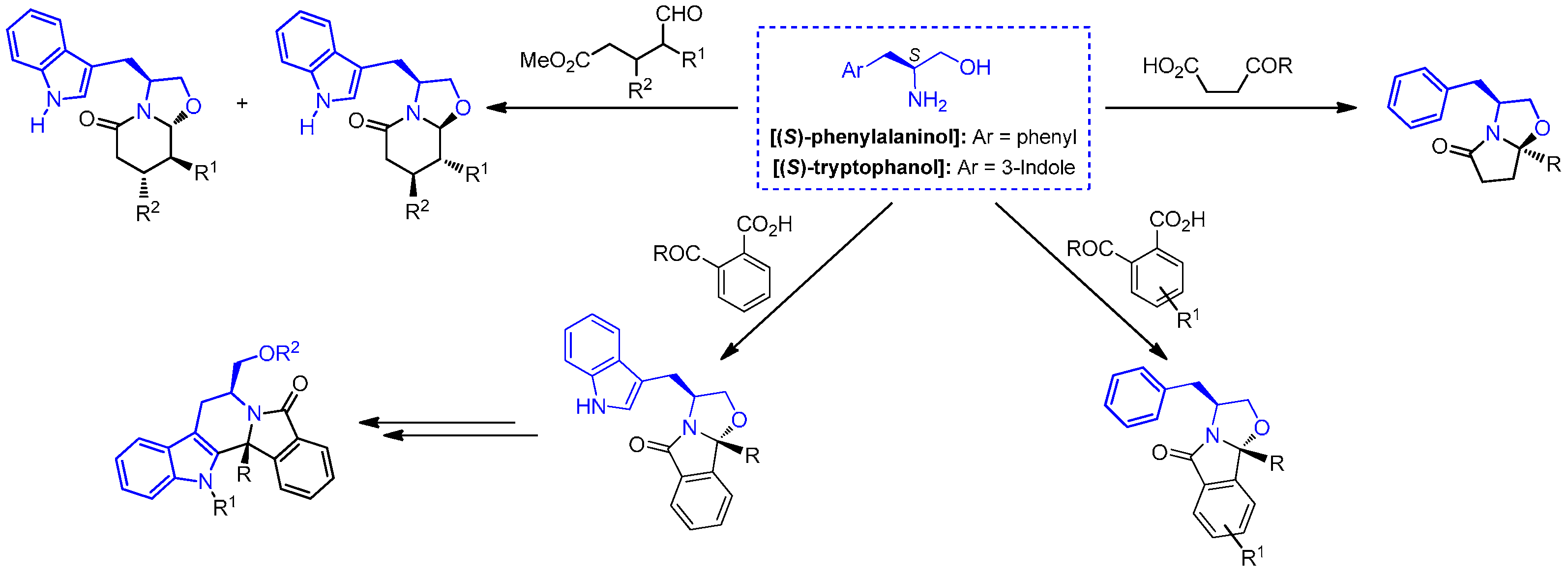
2. Results and Discussion
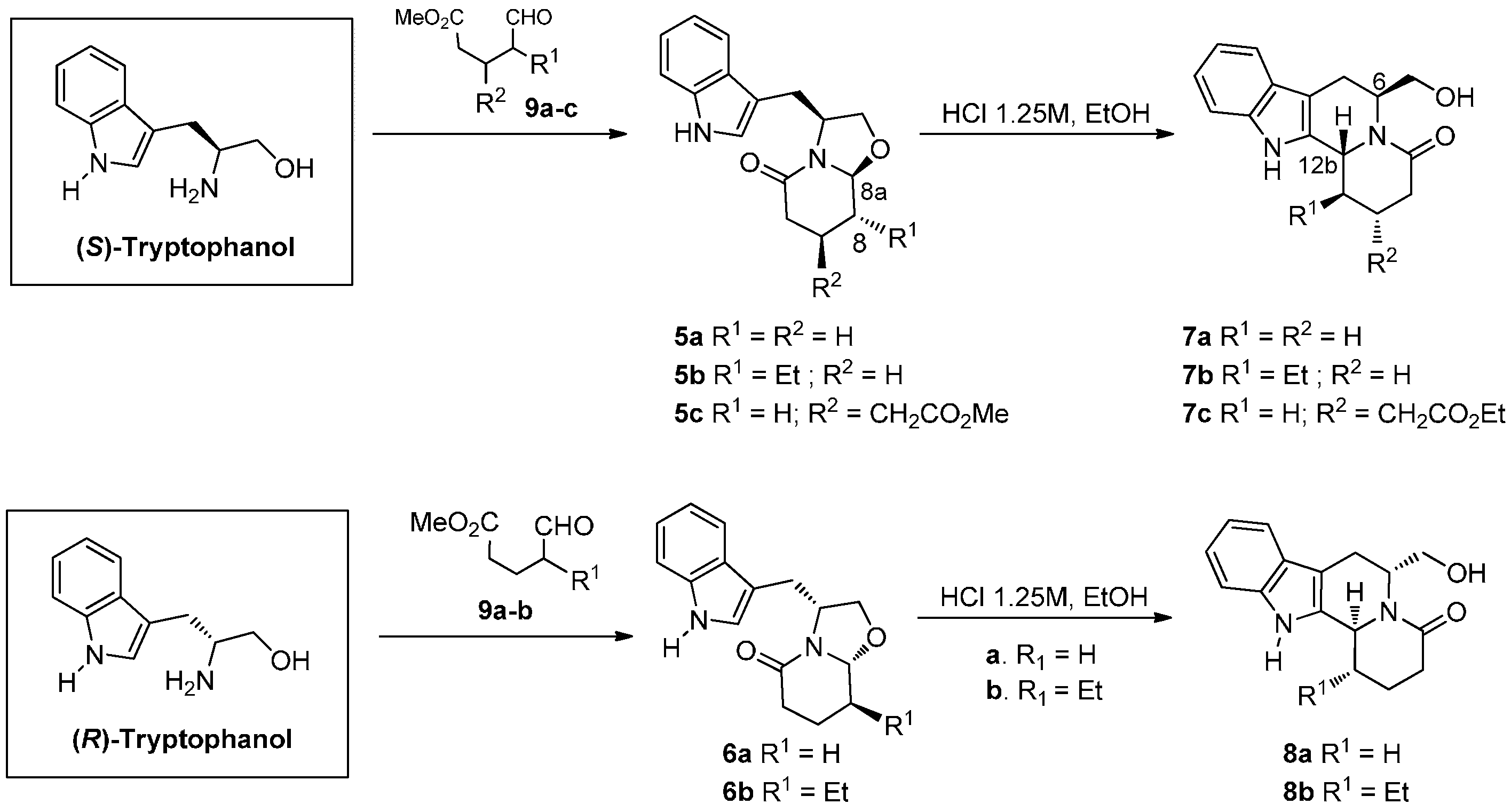
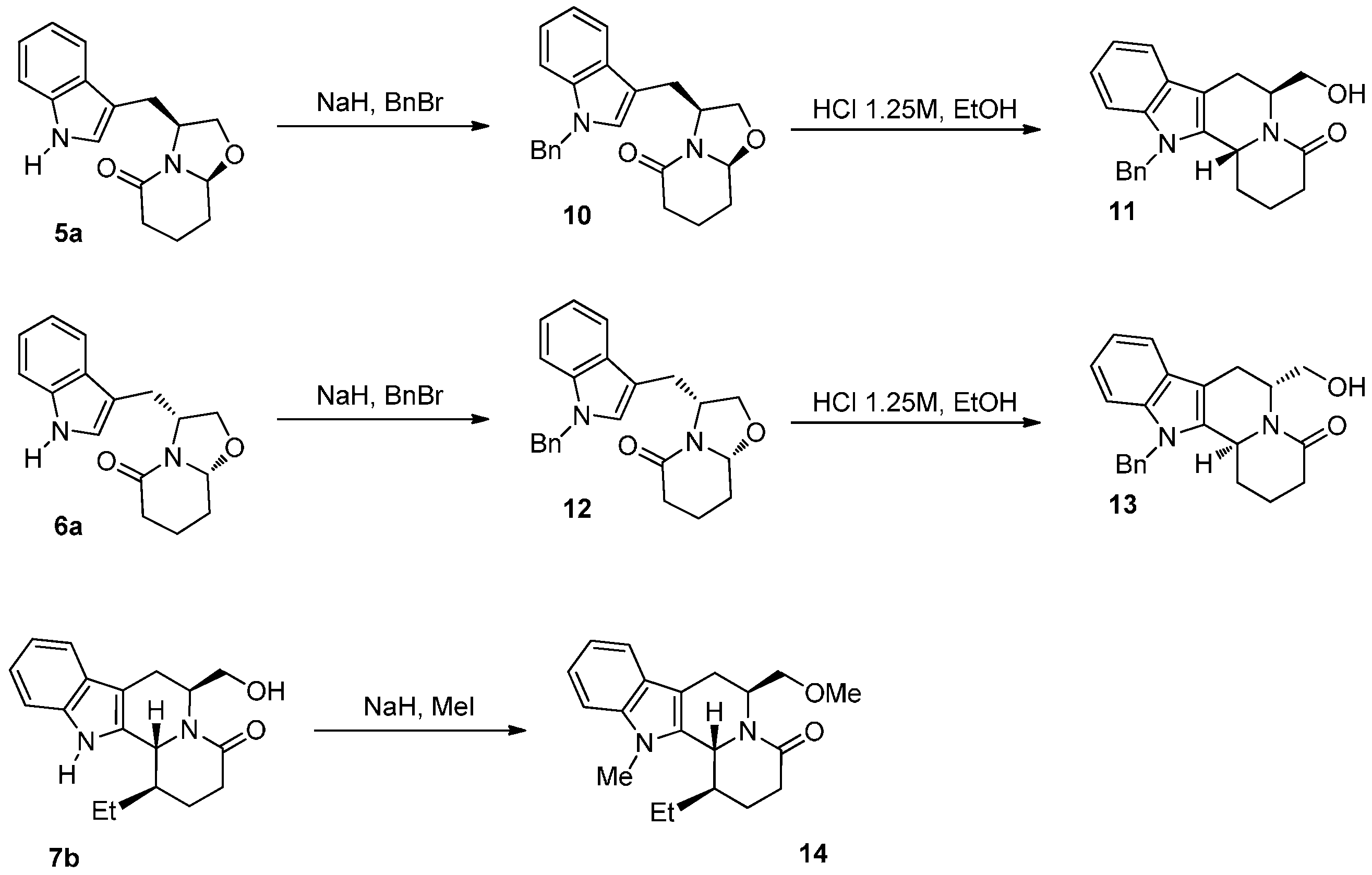
2.1. Chemistry
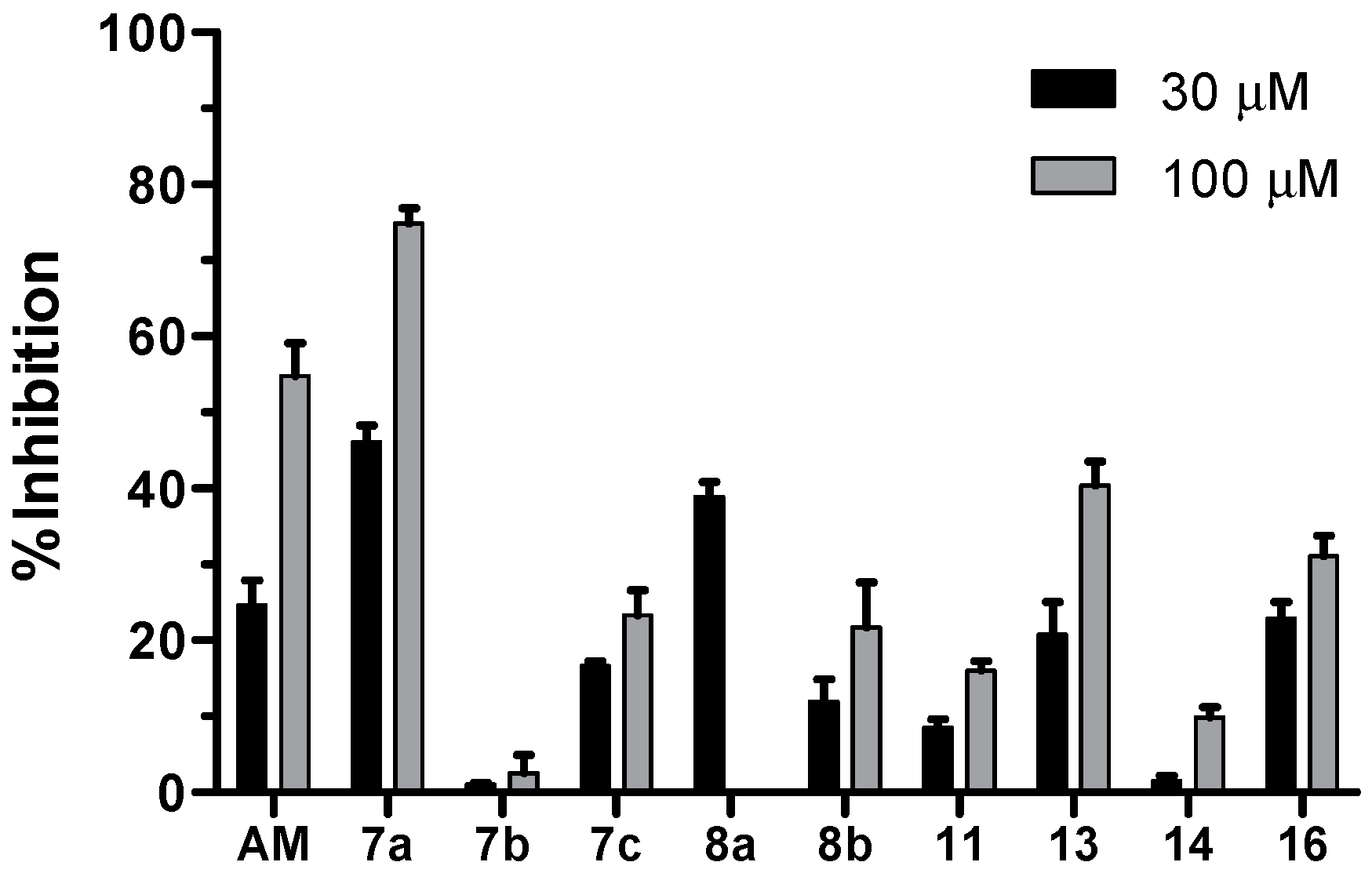
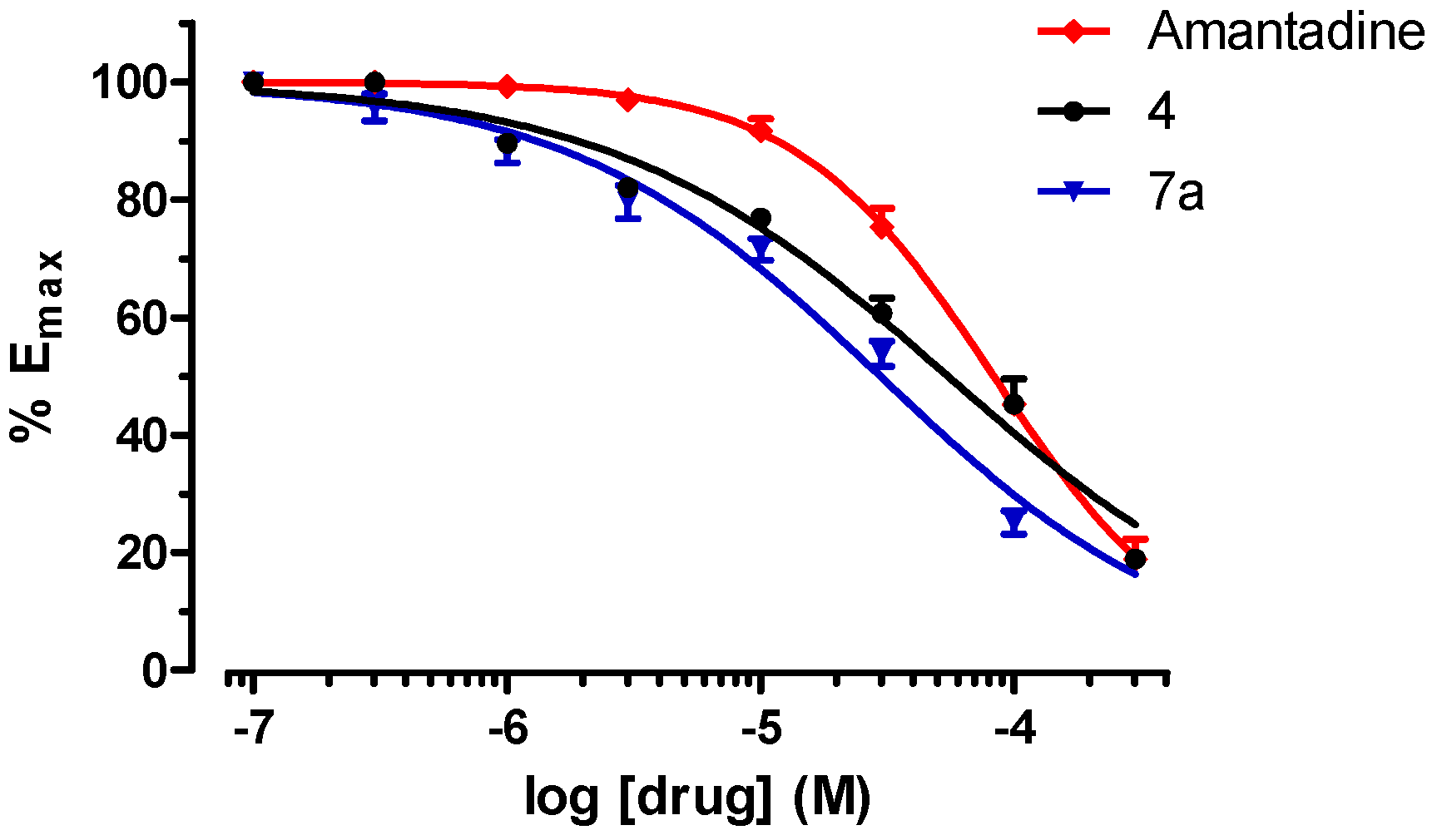
2.2. Biological Activity
3. Materials and Methods
3.1. General Information
3.2. Synthesis of (R)-Tryptophanol
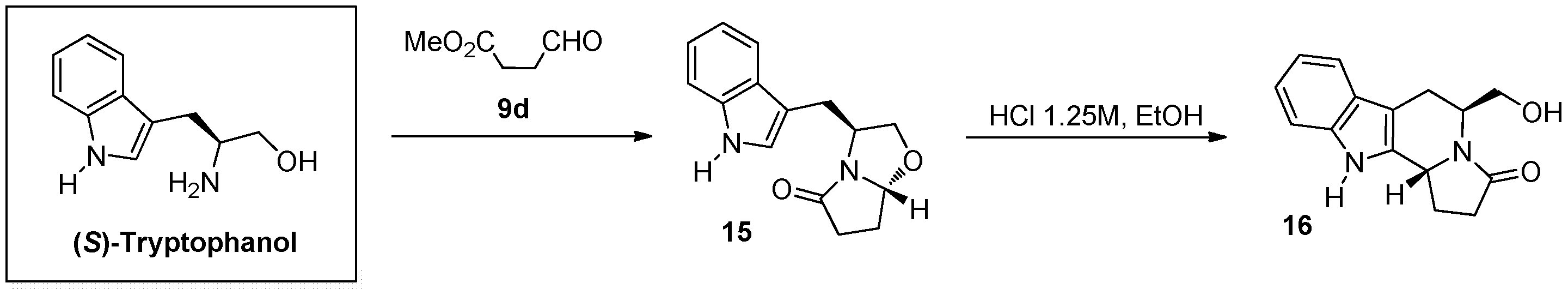
3.3. General Procedure for the Synthesis of Compounds 6a–b, and 15
3.4. General Procedure for the Synthesis of Compounds 10 and 12
3.5. General Procedure for the Synthesis of Compounds 7a–c, 8a–b, 11, 13 and 16
3.6. Synthesis of (1R,6S,12bR)-1-Ethyl-6-(methoxymethyl)-12-methyl-1,2,3,6,7,12b-hexahydroindolo [2,3-a]quinolizin-4(12H)-one (14)
3.7. NMDA Receptor Antagonist Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rouf, A.; Taneja, S.C. Synthesis of single-enantiomer bioactive molecules: A brief overview. Chirality 2014, 26, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Ager, D.J.; Prakash, I.; Schaad, D.R. 1,2-amino alcohols and their heterocyclic derivatives as chiral auxiliaries in asymmetric synthesis. Chem. Rev. 1996, 96, 835–875. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.A.L.; Sureda, F.X.; Turch, M.; Amat, M.; Bosch, J.; Santos, M.M.M. Synthesis of phenylalaninol-derived oxazolopyrrolidone lactams and evaluation as nmda receptor antagonists. Monatsh. Chem. 2013, 144, 473–477. [Google Scholar] [CrossRef]
- Pereira, N.A.L.; Sureda, F.X.; Esplugas, R.; Perez, M.; Amat, M.; Santos, M.M.M. Tryptophanol-derived oxazolopiperidone lactams: Identification of a hit compound as nmda receptor antagonist. Bioorg. Med. Chem. Lett. 2014, 24, 3333–3336. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.; Raimundo, L.; Pereira, N.A.L.; dos Santos, D.J.V.A.; Perez, M.; Queiroz, G.; Leao, M.; Santos, M.M.M.; Saraiva, L. A tryptophanol-derived oxazolopiperidone lactam is cytotoxic against tumors via inhibition of p53 interaction with murine double minute proteins. Pharmacol. Res. 2015, 95–96, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.; Pereira, N.A.L.; Monteiro, A.; Leao, M.; Bessa, C.; dos Santos, D.J.V.A.; Rairnundo, L.; Queiroz, G.; Bisio, A.; Inga, A.; et al. Oxazoloisoindolinones with in vitro antitumor activity selectively activate a p53-pathway through potential inhibition of the p53-mdm2 interaction. Eur. J. Pharm. Sci. 2015, 66, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.; Raimundo, L.; Pereira, N.A.L.; Monteiro, A.; Gomes, S.; Bessa, C.; Pereira, C.; Queiroz, G.; Bisio, A.; Fernandes, J.; et al. Reactivation of wild-type and mutant p53 by tryptophanol-derived oxazoloisoindolinone slmp53-1, a novel anticancer small-molecule. Oncotarget 2016, 7, 4326–4343. [Google Scholar] [PubMed]
- Pereira, N.A.L.; Monteiro, A.; Machado, M.; Gut, J.; Molins, E.; Perry, M.J.; Dourado, J.; Moreira, R.; Rosenthal, P.J.; Prudencio, M.; et al. Enantiopure indolizinoindolones with in vitro activity against blood- and liver-stage malaria parasites. Chem. Med. Chem. 2015, 10, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, P.; Neyton, J. Nmda receptor subunits: Function and pharmacology. Curr. Opin. Pharmacol. 2007, 7, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.G.M. Nmda receptors and memory encoding. Neuropharmacology 2013, 74, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, R.M.; Acker, T.M.; Zimmerman, S.S.; Katzman, B.M.; Strong, K.L.; Traynelis, S.F.; Liotta, D.C. Novel nmda receptor modulators: An update. Expert Opin. Ther. Pat. 2012, 22, 1337–1352. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, M.A. Safety overview of fda-approved medications for the treatment of the motor symptoms of parkinson’s disease. Expert Opin. Drug Saf. 2014, 13, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- Hellweg, R.; Wirth, Y.; Janetzky, W.; Hartmann, S. Efficacy of memantine in delaying clinical worsening in alzheimer’s disease (ad): Responder analyses of nine clinical trials with patients with moderate to severe ad. Int. J. Geriatr. Psychiatry 2012, 27, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Son, D.; Lee, P.; Kim, S.Y.; Kim, H.; Kim, C.J.; Lim, E. Alkaloid fraction of uncaria rhynchophylla protects against n-methyl-d-aspartate-induced apoptosis in rat hippocampal slices. Neurosci. Lett. 2003, 348, 51–55. [Google Scholar] [CrossRef]
- Shimada, Y.; Goto, H.; Itoh, T.; Sakakibara, I.; Kubo, M.; Sasaki, H.; Terasawa, K. Evaluation of the protective effects of alkaloids isolated from the hooks and stems of uncaria sinensis on glutamate-induced neuronal death in cultured cerebellar granule cells from rats. J. Pharm. Pharmacol. 1999, 51, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.Y.; Nam, K.N.; Woo, B.-C.; Kim, K.-P.; Kim, S.-O.; Lee, E.H. Hirsutine, an indole alkaloid of uncaria rhynchophylla, inhibits inflammation-mediated neurotoxicity and microglial activation. Mol. Med. Rep. 2013, 7, 154–158. [Google Scholar] [PubMed]
- Imamura, S.; Tabuchi, M.; Kushida, H.; Nishi, A.; Kanno, H.; Yamaguchi, T.; Sekiguchi, K.; Ikarashi, Y.; Kase, Y. The blood-brain barrier permeability of geissoschizine methyl ether in uncaria hook, a galenical constituent of the traditional japanese medicine yokukansan. Cell. Mol. Neurobiol. 2011, 31, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Ndagijimana, A.; Wang, X.; Pan, G.; Zhang, F.; Feng, H.; Olaleye, O. A review on indole alkaloids isolated from uncaria rhynchophylla and their pharmacological studies. Fitoterapia 2013, 86, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Allin, S.M.; Thomas, C.I.; Doyle, K.; Elsegood, M.R.J. An asymmetric synthesis of both enantiomers of the indole alkaloid deplancheine. J. Org. Chem. 2005, 70, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Allin, S.M.; Thomas, C.I.; Allard, J.E.; Doyle, K.; Elsegood, M.R.J. A highly stereoselective synthesis of the indolo 2,3-a quinolizine ring system and application to natural product synthesis. Eur. J. Org. Chem. 2005, 4179–4186. [Google Scholar] [CrossRef]
- Bassas, O.; Llor, N.; Santos, M.M.M.; Griera, R.; Molins, E.; Amat, M.; Bosch, J. Biogenetically inspired enantioselective approach to indolo [2,3-a]- and benzo a quinolizidine alkaloids from a synthetic equivalent of secologanin. Org. Lett. 2005, 7, 2817–2820. [Google Scholar] [CrossRef] [PubMed]
- Amat, M.; Santos, M.M.M.; Bassas, O.; Llor, N.; Escolano, C.; Gomez-Esque, A.; Molins, E.; Allin, S.M.; McKee, V.; Bosch, J. Straightforward methodology for the enantioselective synthesis of benzo a - and indolo 2,3-a quinolizidines. J. Org. Chem. 2007, 72, 5193–5201. [Google Scholar] [CrossRef] [PubMed]
- Amat, M.; Santos, M.M.M.; Gomez, A.M.; Jokic, D.; Molins, E.; Bosch, J. Enantioselective spirocyclizations from tryptophanol-derived oxazolopiperidone lactams. Org. Lett. 2007, 9, 2907–2910. [Google Scholar] [CrossRef] [PubMed]
- Amat, M.; Gomez Esque, A.; Escolano, C.; Santos, M.M.M.; Molins, E.; Bosch, J. Enantioselective formal synthesis of (+)-dihydrocorynantheine and (−)-dihydrocorynantheol. J. Org. Chem. 2009, 74, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Arioli, F.; Rigacci, G.; Santos, M.M.M.; Gomez-Esque, A.; Florindo, P.; Ramos, C.; Bosch, J.; Amat, M. Stereocontrolled generation of benzo[α]- and indolo[2,3-α]quinolizidines from (s)-tryptophanol and (s)-(3,4-dimethoxyphenyl)alaninol-derived lactams. Eur. J. Org. Chem. 2011, 3858–3863. [Google Scholar] [CrossRef]
- Amat, M.; Ramos, C.; Perez, M.; Molins, E.; Florindo, P.; Santos, M.M.M.; Bosch, J. Enantioselective formal synthesis of ent-rhynchophylline and ent-isorhynchophylline. Chem. Commun. 2013, 49, 1954–1956. [Google Scholar] [CrossRef] [PubMed]
- Arioli, F.; Perez, M.; Are, C.; Estarellas, C.; Luque, F.J.; Bosch, J.; Amat, M. Stereocontrolled annulations of indolo 2,3-a quinolizidine-derived lactams with a silylated nazarov reagent: Access to allo and epiallo yohimbine-type derivatives. Chem. Eur. J. 2015, 21, 13382–13389. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.M.M. Tryptophanol-derived oxazolopiperidone lactams: Valuable building blocks for the enantioselective synthesis of piperidine-containing alkaloids. In Heterocyclic Targets in Advanced Organic Synthesis; Carreiras, M.C., Marco-Contelles, J., Eds.; Research Signpost: Kerala, India, 2011; pp. 69–82. [Google Scholar]
- Perez, M.; Espadinha, M.; Santos, M.M.M. Indolo 2,3-a quinolizidines and derivatives: Bioactivity and asymmetric synthesis. Curr. Pharm. Des. 2015, 21, 5518–5546. [Google Scholar] [CrossRef] [PubMed]
- Horrocks, P.; Fallon, S.; Denman, L.; Devine, O.; Duffy, L.J.; Harper, A.; Meredith, E.-L.; Hasenkamp, S.; Sidaway, A.; Monnery, D.; et al. Synthesis and evaluation of a novel series of indoloisoquinolines as small molecule anti-malarial leads. Bioorg. Med. Chem. Lett. 2012, 22, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Amat, M.; Bassas, O.; Cantó, M.; Llor, N.; Santos, M.M.M.; Bosch, J. Synthesis of 3-acetonyl- and 3-(2-oxoethyl)glutarates. Tetrahedron 2005, 61, 7693–7702. [Google Scholar] [CrossRef]
- Allin, S.M.; Gaskell, S.N.; Elsegood, M.R.J.; Martin, W.P. A new asymmetric synthesis of the natural enantiomer of the indolizidino 8,7-b indole alkaloid (+)-harmicine. Tetrahedron Lett. 2007, 48, 5669–5671. [Google Scholar] [CrossRef]
- Sample Availability: Samples are not available from the authors.


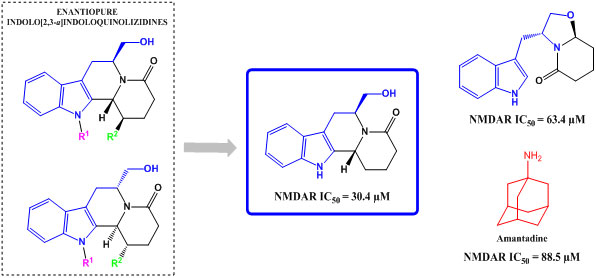
| Compound | NMDA (100 μM) |
|---|---|
| IC50 μM a | |
| 4 | 63.4 ± 9.0 |
| 7a | 30.4 ± 2.5 |
| Amantadine | 88.5 ± 11.8 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, N.A.L.; Sureda, F.X.; Pérez, M.; Amat, M.; Santos, M.M.M. Enantiopure Indolo[2,3-a]quinolizidines: Synthesis and Evaluation as NMDA Receptor Antagonists. Molecules 2016, 21, 1027. https://doi.org/10.3390/molecules21081027
Pereira NAL, Sureda FX, Pérez M, Amat M, Santos MMM. Enantiopure Indolo[2,3-a]quinolizidines: Synthesis and Evaluation as NMDA Receptor Antagonists. Molecules. 2016; 21(8):1027. https://doi.org/10.3390/molecules21081027
Chicago/Turabian StylePereira, Nuno A. L., Francesc X. Sureda, Maria Pérez, Mercedes Amat, and Maria M. M. Santos. 2016. "Enantiopure Indolo[2,3-a]quinolizidines: Synthesis and Evaluation as NMDA Receptor Antagonists" Molecules 21, no. 8: 1027. https://doi.org/10.3390/molecules21081027