Stimuli-Responsive Polymeric Systems for Controlled Protein and Peptide Delivery: Future Implications for Ocular Delivery
Abstract
:1. Introduction
2. Responsive Protein and Peptide Delivery
2.1. pH-Responsive Systems
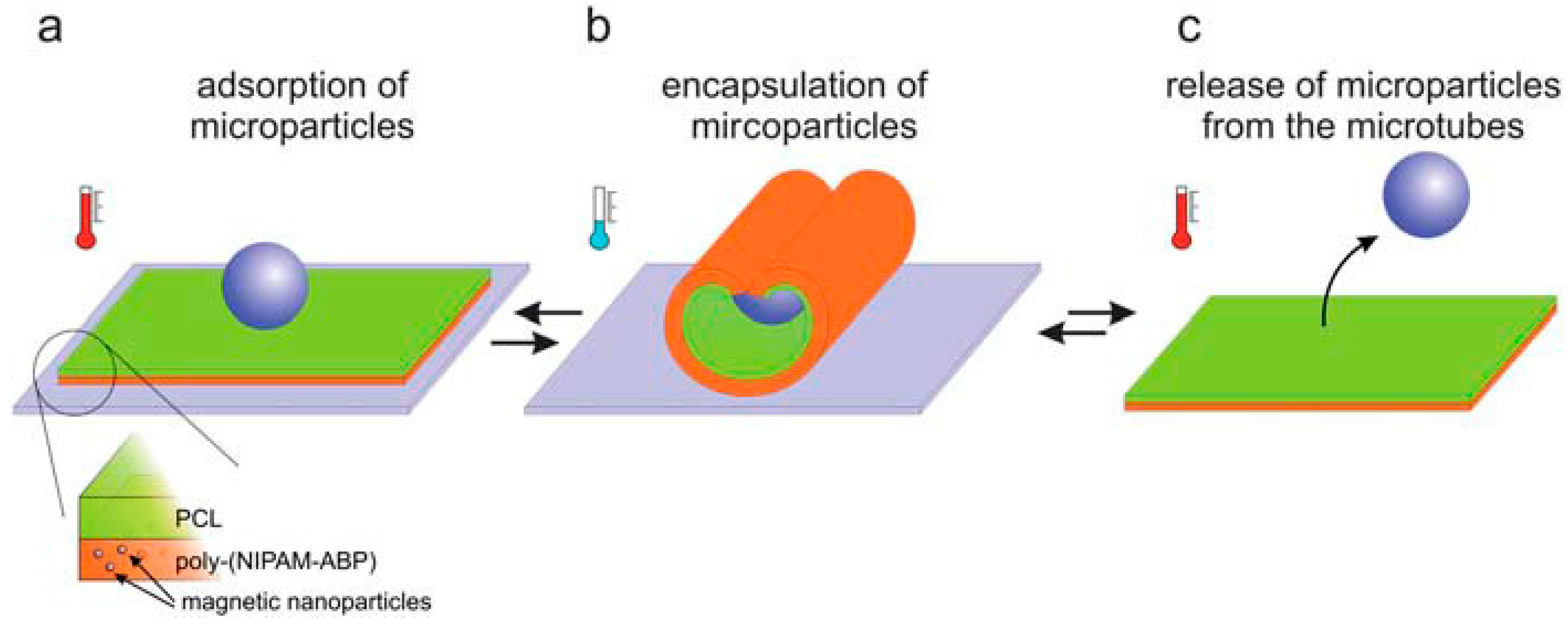
2.2. Thermo-Responsive Systems
2.3. Enzyme-Responsive Systems
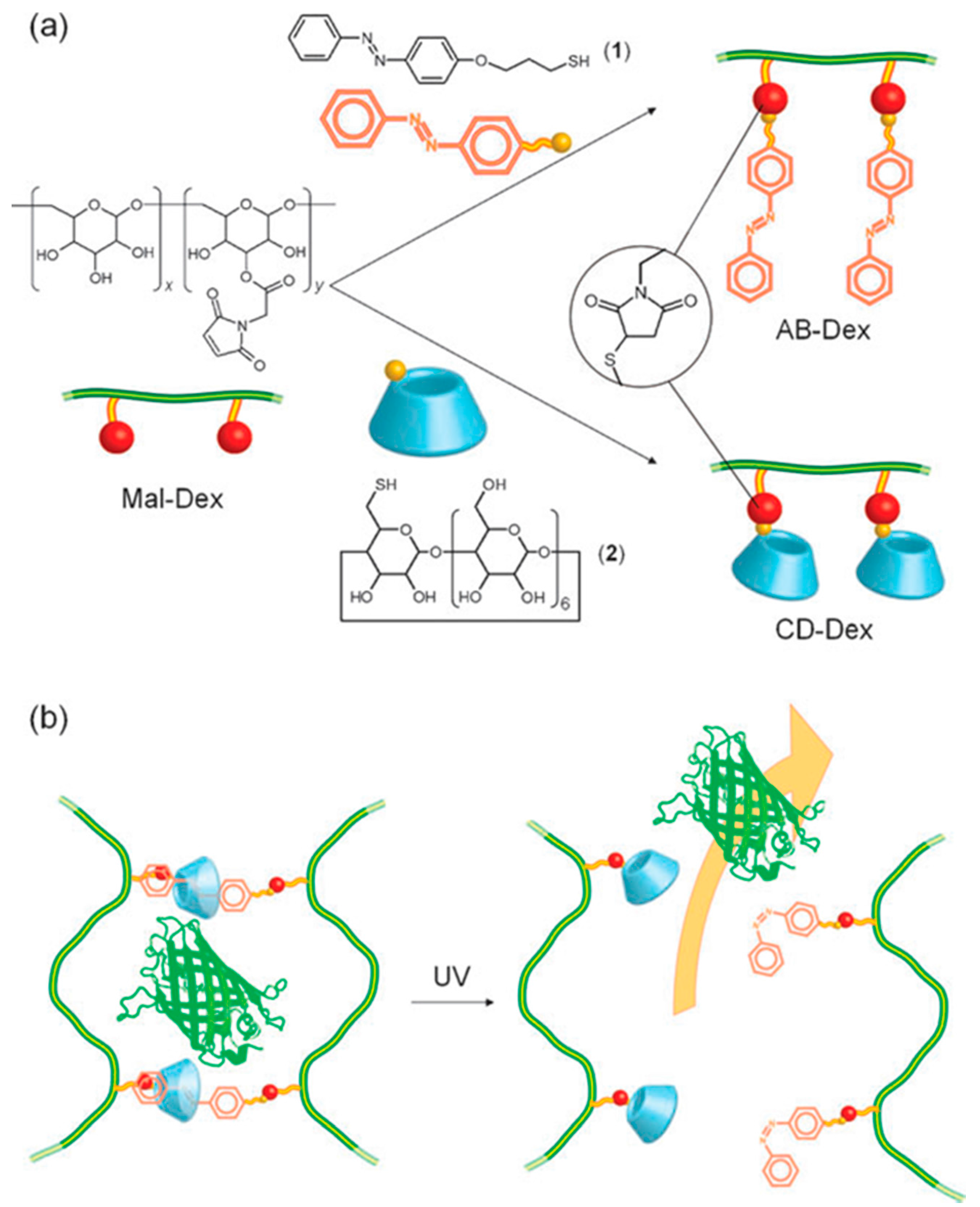
2.4. Light-Responsive Systems
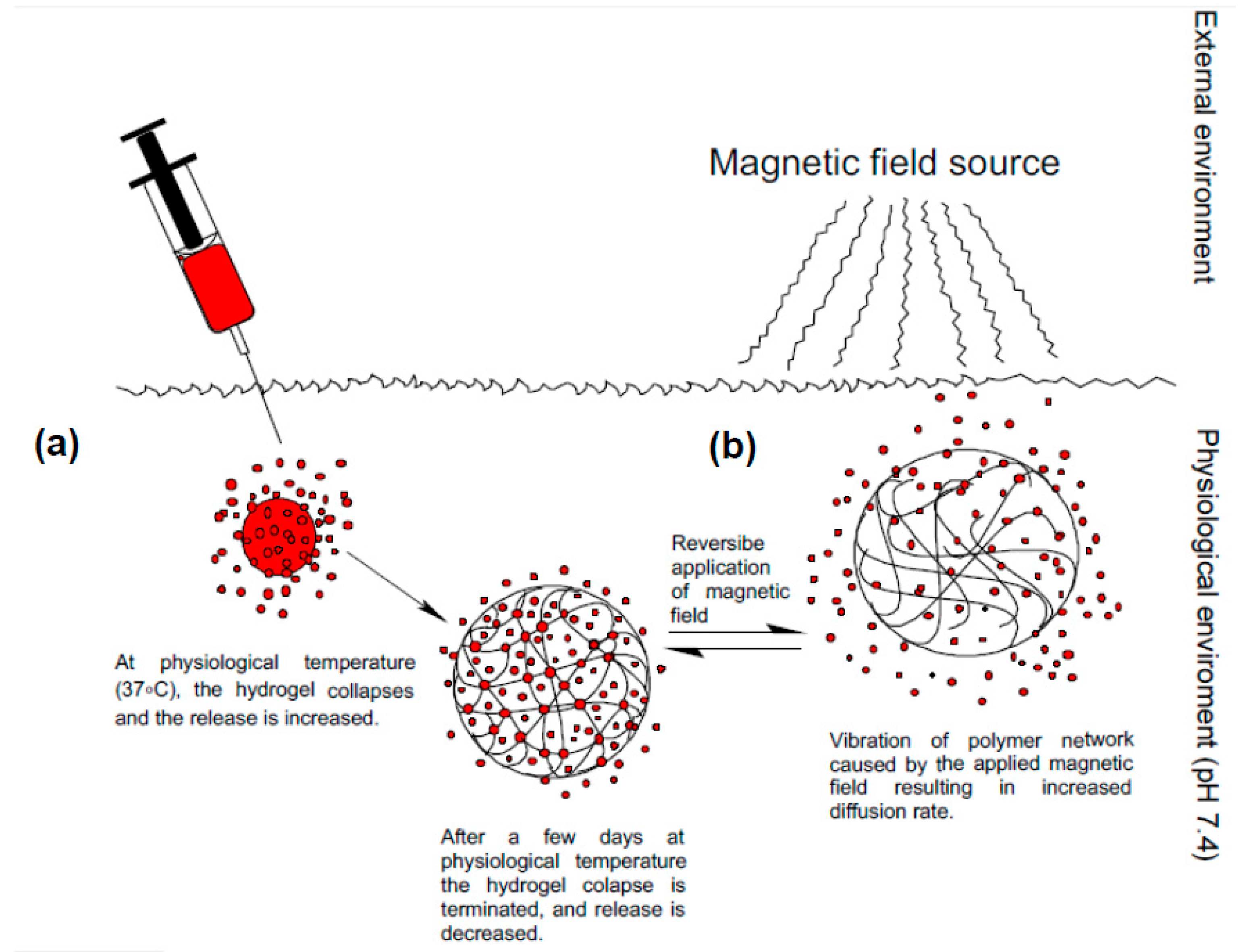
2.5. Ultrasound-Responsive Systems
2.6. Multi-Responsive Systems
3. Therapeutic Proteins and Peptides for Ocular Delivery
4. Current Progress in Stimuli-Responsive Ocular Delivery Systems
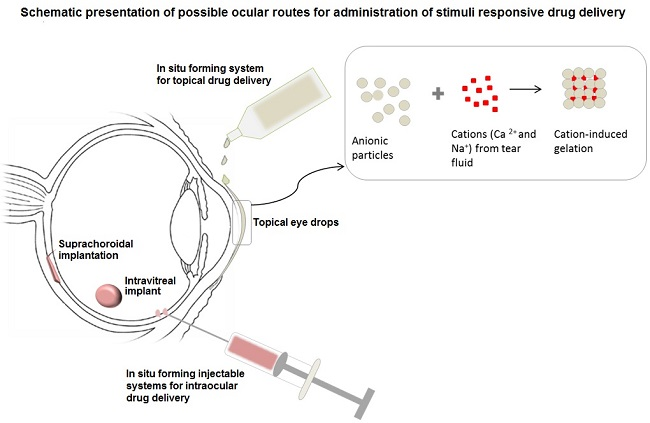
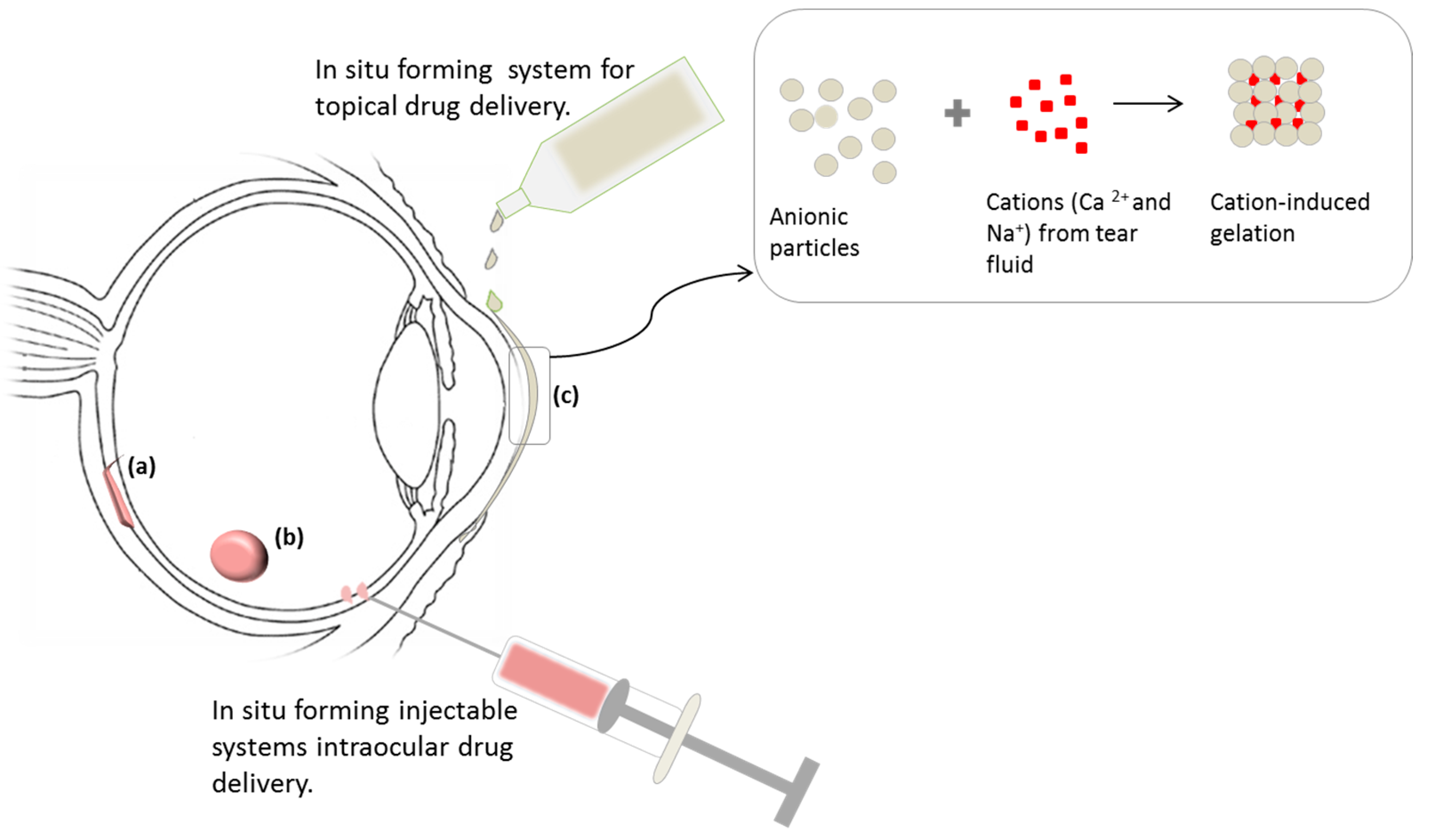
4.1. In Situ Forming Ophthalmic/Ocular Delivery Systems
4.2. Implantable Stimuli-Responsive Ocular Delivery Systems
5. Future Prospects
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Pisal, D.S.; Kosloski, M.P.; Balu-Iyer, S.V. Delivery of Therapeutic Proteins. J. Pharm. Sci. 2010, 99, 2557–2575. [Google Scholar] [CrossRef] [PubMed]
- Ratnaparkhi, M.P.; Chaudhari, S.P.; Pandya, V.A. Peptides And Proteins In Pharmaceuticals. Int. Curr. Pharm. Res. 2011, 3, 1–9. [Google Scholar]
- Koyamatsu, Y.; Hirano, T.; Kakizawa, Y.; Okano, F.; Takarada, T.; Maeda, M. pH-responsive release of proteins from biocompatible and biodegradable reverse polymer micelles. J. Control. Release 2014, 173, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Antosova, Z.; Mackova, M.; Kral, V.; Macek, T. Therapeutic application of peptides and proteins: Parenteral forever? Trends Biotechnol. 2009, 27, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.P.K.; Bansal, S.; Banik, A. Noninvasive routes of proteins and peptides drug delivery. Indian J. Pharm. Sci. 2011, 73, 367–375. [Google Scholar]
- Yellepeddi, V.K.; Palakurthi, S. Recent Advances in Ocular Drug Delivery. Adv. Drug Deliv. 2016, 32, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Meshram, S.; Thorat, S. Ocular in Situ Gels : Development, Evaluation and Advancements. Sch. Acad. J. Pharm. 2015, 4, 340–346. [Google Scholar]
- Vellonen, K.S.; Malinen, M.; Mannermaa, E.; Subrizi, A.; Toropainen, E.; Lou, Y.R.; Kidron, H.; Yliperttula, M.; Urtti, A. A critical assessment of in vitro tissue models for ADME and drug delivery. J. Control. Release 2014, 190, 94–114. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.P.; Kakkar, S. Nanotherapy for posterior eye diseases. J. Control. Release 2014, 193, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Gaudana, R.; Jwala, J.; Boddu, S.H.S.; Mitra, A.K. Recent perspectives in ocular drug delivery. Pharm. Res. 2009, 26, 1197–1216. [Google Scholar] [CrossRef] [PubMed]
- Yasin, M.N.; Svirskis, D.; Seyfoddin, A.; Rupenthal, I.D. Implants for drug delivery to the posterior segment of the eye: A focus on stimuli-responsive and tunable release systems. J. Control. Release 2014, 196, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Kompella, U.B.; Amrite, A.C.; Pacha Ravi, R.; Durazo, S.A. Nanomedicines for back of the eye drug delivery, gene delivery, and imaging. Prog. Retin. Eye Res. 2013, 36, 172–198. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Chiang, B.; Wu, X.; Prausnitz, M.R. Ocular delivery of macromolecules. J. Control. Release 2014, 190, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Oak, M.; Mandke, R.; Singh, J. Smart polymers for peptide and protein parenteral sustained delivery. Drug Discov. Today Technol. 2012, 9, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Cambre, J.N.; Sumerlin, B.S. Future perspectives and recent advances in stimuli-responsive materials. Prog. Polym. Sci. 2010, 35, 278–301. [Google Scholar] [CrossRef]
- Ruel-Gariépy, E.; Leroux, J.-C. In situ-forming hydrogels—review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Lobel, E.; Trevgoda, A.; Peled, Y. A novel in situ-forming ophthalmic drug delivery system from alginates undergoing gelation in the eye. J. Control. Release 1997, 44, 201–208. [Google Scholar] [CrossRef]
- Gratieri, T.; Gelfuso, G.M.; Rocha, E.M.; Sarmento, V.H.; de Freitas, O.; Lopez, R.F.V. A poloxamer/chitosan in situ forming gel with prolonged retention time for ocular delivery. Eur. J. Pharm. Biopharm. 2010, 75, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Chen, W.; Huang, C.; Li, L.; Chen, C.; Li, W.; Wu, C. Development of a poloxamer analogs/carbopol-based in situ gelling and mucoadhesive ophthalmic delivery system for puerarin. Int. J. Pharm. 2007, 337, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Suzuki, S.; Kawasaki, N.; Endo, K.; Takahashi, A. In situ gelling xyloglucan formulations for sustained release ocular delivery of pilocarpine hydrochloride. Int. J. Pharm. 2001, 229, 29–36. [Google Scholar] [CrossRef]
- Brown, H.S.; Meltzer, G.; Merrill, R.C.; Fisher, M.; Ferré, C.; Place, V.A. Visual effects of pilocarpine in glaucoma comparative study of administration by eyedrops or by ocular therapeutic systems. Arch. Ophthalmol. 1976, 94, 1716–1719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Z.; Jo Lewis, P.; Chu, C.C. Fabrication and characterization of a smart drug delivery system: Microsphere in hydrogel. Biomaterials 2005, 26, 3299–3309. [Google Scholar] [CrossRef] [PubMed]
- Almeida, H.; Amaral, M.H.; Lobao, P.; Lobo, J.M.S. In situ gelling systems: A strategy to improve the bioavailability of ophthalmic pharmaceutical formulations. Drug Discov. Today 2014, 19, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Qi, H.; Chen, W.; Huang, C.; Su, C.; Li, W.; Hou, S. Preparation and evaluation of a Carbopol/HPMC-based in situ gelling ophthalmic system for puerarin. Yakugaku Zasshi 2007, 127, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Srividya, B.; Cardoza, R.M.; Amin, P.D. Sustained ophthalmic delivery of ofloxacin from a pH triggered in situ gelling system. J. Control. Release 2001, 73, 205–211. [Google Scholar] [CrossRef]
- Gan, L.; Gan, Y.; Zhu, C.; Zhang, X.; Zhu, J. Novel microemulsion in situ electrolyte-triggered gelling system for ophthalmic delivery of lipophilic cyclosporine A: in vitro and in vivo results. Int. J. Pharm. 2009, 365, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, S.K.; Saxena, P.; Rai, A. Stimuli sensitive hydrogels for ophthalmic drug delivery: A review. Int. J. Pharm. Investig. 2012, 2, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Horvát, G.; Gyarmati, B.; Berkó, S.; Szabó-Révész, P.; Szilágyi, B.Á.; Szilágyi, A.; Soós, J.; Sandri, G.; Bonferoni, M.C.; Rossi, S.; et al. Thiolated poly(aspartic acid) as potential in situ gelling, ocular mucoadhesive drug delivery system. Eur. J. Pharm. Sci. 2015, 67, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Kim, S.; Park, K. Issues in long-term protein delivery using biodegradable microparticles. J. Control. Release 2010, 146, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Al-tahami, K.; Singh, J. Smart Polymer Based Delivery Systems for Peptides and Proteins. Recent Pat. Drug Deliv. Formul. 2007, 1, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Ducat, E.; Deprez, J.; Gillet, A.; Noël, A.; Evrard, B.; Peulen, O.; Piel, G. Nuclear delivery of a therapeutic peptide by long circulating pH-sensitive liposomes: Benefits over classical vesicles. Int. J. Pharm. 2011, 420, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, D.S. Biodegradable and pH-sensitive polymersome with tuning permeable membrane for drug delivery carrier. Chem. Commun. 2010, 46, 4481–4483. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.C.; Lin, Y.W.; Huang, Y.F.; Chuang, C.K.; Chern, C.S. Polymer vesicles containing small vesicles within interior aqueous compartments and pH-responsive transmembrane channels. Angew. Chemie Int. Ed. 2008, 47, 1875–1878. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, I.M. Enhanced pH-responsive carrier system based on alginate and chemically modified carboxymethyl chitosan for oral delivery of protein drugs: Preparation and in-vitro assessment. Carbohydr. Polym. 2010, 80, 1125–1136. [Google Scholar] [CrossRef]
- Gong, R.; Li, C.; Zhu, S.; Zhang, Y.; Du, Y.; Jiang, J. A novel pH-sensitive hydrogel based on dual crosslinked alginate/N-α-glutaric acid chitosan for oral delivery of protein. Carbohydr. Polym. 2011, 85, 869–874. [Google Scholar] [CrossRef]
- Schmucker, C.; Loke, Y.K.; Ehlken, C.; Agostini, H.T.; Hansen, L.L.; Antes, G.; Lelgemann, M. Intravitreal bevacizumab (Avastin) versus ranibizumab (Lucentis) for the treatment of age-related macular degeneration: A safety review. Br. J. Ophthalmol. 2011, 95, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Huth, U.S.; Schubert, R.; Peschka-Süss, R. Investigating the uptake and intracellular fate of pH-sensitive liposomes by flow cytometry and spectral bio-imaging. J. Control. Release 2006, 110, 490–504. [Google Scholar] [CrossRef] [PubMed]
- Garbern, J.C.; Hoffman, A.S.; Stayton, P.S. Injectable pH- and temperature-responsive poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers for delivery of angiogenic growth factors. Biomacromolecules 2010, 11, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Kang Derwent, J.J.; Mieler, W.F. Thermoresponsive hydrogels as a new ocular drug delivery platform to the posterior segment of the eye. Trans. Am. Ophthalmol. Soc. 2008, 106, 204–206. [Google Scholar]
- Kumar, S.; Haglund, B.O.; Himmelstein, K.J. In Situ -Forming Gels for Ophthalmic Drug Delivery. J. Ocul. Pharmacol. Ther. 1994, 10, 47–56. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, C.; Shen, W.; Cheng, Z.; Yu, L.L.; Ping, Q. Poly(N-isopropylacrylamide)-chitosan as thermosensitive in situ gel-forming system for ocular drug delivery. J. Control. Release 2007, 120, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Bawa, P.; Pillay, V.; Choonara, Y.E.; du Toit, L.C. Stimuli-responsive polymers and their applications in drug delivery. Biomed. Mater. 2009, 4, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive polymers for biomedical applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Ninawe, P.R.; Hatziavramidis, D.; Parulekar, S.J. Delivery of drug macromolecules from thermally responsive gel implants to the posterior eye. Chem. Eng. Sci. 2010, 65, 5170–5177. [Google Scholar] [CrossRef]
- Thornton, P.D.; Mart, R.J.; Webb, S.J.; Ulijn, R.V. Enzyme-responsive hydrogel particles for the controlled release of proteins: designing peptide actuators to match payload. Soft Matter 2008, 4, 821. [Google Scholar] [CrossRef]
- Aimetti, A.A.; Tibbitt, M.W.; Anseth, K.S. Human Neutrophil Elastase Responsive Delivery from Poly( ethylene glycol) Hydrogels. Biomacromolecules 2009, 10, 1484–1489. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Matsusaki, M.; Kida, T.; Akashi, M. Enzyme-responsive release of encapsulated proteins from biodegradable hollow capsules. Biomacromolecules 2006, 7, 2715–2718. [Google Scholar] [CrossRef] [PubMed]
- Yagai, S.; Kitamura, A. Recent advances in photoresponsive supramolecular self-assemblies. Chem. Soc. Rev. 2008, 37, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Shum, P.; Kim, J.M.; Thompson, D.H. Phototriggering of liposomal drug delivery systems. Adv. Drug Deliv. Rev. 2001, 53, 273–284. [Google Scholar] [CrossRef]
- Peng, K.; Tomatsu, I.; Kros, A. Light controlled protein release from a supramolecular hydrogel. Chem. Commun. 2010, 46, 4094–4096. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Sefah, K.; Bamrungsap, S.; Chang, H.T.; Tan, W. Selective photothermal therapy for mixed cancer cells using aptamer-conjugated nanorods. Langmuir 2008, 24, 11860–11865. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Trondoli, A.C.; Zhu, G.; Chen, Y.; Chang, Y.J.; Liu, H.; Huang, Y.F.; Zhang, X.; Tan, W. Near-infrared light-responsive core-shell nanogels for targeted drug delivery. ACS Nano 2011, 5, 5094–5099. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Cai, T.; Wang, Y.; Wang, H.; Ji, J. Light-Responsive Polyion Complex Micelles with Switchable Surface Charge for Efficient Protein Delivery. ACS Macro Lett. 2014, 3, 679–683. [Google Scholar] [CrossRef]
- Zhao, H.; Sterner, E.S.; Coughlin, E.B.; Theato, P. O-Nitrobenzyl alcohol derivatives: Opportunities in polymer and materials science. Macromolecules 2012, 45, 1723–1736. [Google Scholar] [CrossRef]
- Mulvagh, S.L.; DeMaria, A.N.; Feinstein, S.B.; Burns, P.N.; Kaul, S.; Miller, J.G.; Monaghan, M.; Porter, T.R.; Shaw, L.J.; Villanueva, F.S. Contrast echocardiography: current and future applications. J. Am. Soc. Echocardiogr. 2000, 13, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-Z.; Du, L.-N.; Lu, C.-T.; Jin, Y.-G.; Ge, S.-P. Potential and problems in ultrasound-responsive drug delivery systems. Int. J. Nanomedicine 2013, 8, 1621–1633. [Google Scholar] [PubMed]
- Wang, J.; Pelletier, M.; Zhang, H.; Xia, H.; Zhao, Y. High-frequency ultrasound-responsive block copolymer micelle. Langmuir 2009, 25, 13201–13205. [Google Scholar] [CrossRef] [PubMed]
- Suslick, K.S. Sonochemistry. Science 1990, 247, 1439. [Google Scholar] [CrossRef] [PubMed]
- Casolaro, M.; Casolaro, I.; Bottari, S.; Del Bello, B.; Maellaro, E.; Demadis, K.D. Long-term doxorubicin release from multiple stimuli-responsive hydrogels based on α-amino-acid residues. Eur. J. Pharm. Biopharm. 2014, 88, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Wang, K.; Du, S.; Chen, L.; Nie, J.; Zhang, W. Design of genipin-crosslinked microgels from concanavalin A and glucosyloxyethyl acrylated chitosan for glucose-responsive insulin delivery. Carbohydr. Polym. 2014, 103, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Zakharchenko, S.; Puretskiy, N.; Stoychev, G.; Stamm, M.; Ionov, L. Temperature controlled encapsulation and release using partially biodegradable thermo-magneto-sensitive self-rolling tubes. Soft Matter 2010, 6, 2633–2636. [Google Scholar] [CrossRef]
- Scott, I.U.; Karp, C.L.; Nuovo, G.J. Human papillomavirus 16 and 18 expression in conjunctival intraepithelial neoplasia. Ophthalmology 2002, 109, 542–547. [Google Scholar] [CrossRef]
- Nemet, A.Y.; Sharma, V.; Benger, R. Interferon α2b treatment for residual ocular surface squamous neoplasia unresponsive to excision, cryotherapy and mitomycin-C. Clin. Exp. Ophthalmol. 2006, 34, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Kawahara, M.; Nakata, K.; Nishida, T. Restoration of corneal epithelial barrier function and wound healing by substance P and IGF-1 in rats with capsaicin-induced neurotrophic keratopathy. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2937–2940. [Google Scholar] [CrossRef]
- Nishida, T. The role of fibronectin in corneal wound healing explored by a physician-scientist. Jpn. J. Ophthalmol. 2012, 56, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.D.; Skubitz, A.P.; Furcht, L.T. Type IV collagen and corneal epithelial adhesion and migration. Effects of type IV collagen fragments and synthetic peptides on rabbit corneal epithelial cell adhesion and migration in vitro. Invest. Ophthalmol. Vis. Sci. 1991, 32, 2766–2773. [Google Scholar] [PubMed]
- Kymionis, G.D.; Bouzoukis, D.I.; Diakonis, V.F.; Siganos, C. Treatment of chronic dry eye: Focus on cyclosporine. Clin. Ophthalmol. 2008, 2, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Kunert, K.S.; Tisdale, A.S.; Gipson, I.K. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002, 120, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Koyasu, S. Mechanisms of action of cyclosporine. Immunopharmacology 2000, 47, 119–125. [Google Scholar] [CrossRef]
- Bakri, S.J.; Snyder, M.R.; Reid, J.M.; Pulido, J.S.; Ezzat, M.K.; Singh, R.J. Pharmacokinetics of Intravitreal Ranibizumab (Lucentis). Ophthalmology 2007, 114, 2179–2182. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, V.; Agrahari, V.; Hung, W.-T.; Christenson, L.; Mitra, A.K. Composite Nanoformulation Therapeutics for Long Term Ocular Delivery of Macromolecules. Mol. Pharm. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, R.E.; Marfurt, C.F. Topical substance P and corneal epithelial wound closure in the rabbit. Invest. Ophthalmol. Vis. Sci. 1997, 38, 388–395. [Google Scholar] [PubMed]
- Nelson, J.D.; Gordon, J.F. Topical fibronectin in the treatment of keratoconjunctivitis sicca. Chiron Keratoconjunctivitis Sicca Study Group. Am. J. Ophthalmol. 1992, 114, 441–447. [Google Scholar] [CrossRef]
- McCulley, J.P.; Horowitz, B.; Husseini, Z.M.; Horowitz, M. Topical fibronectin therapy of persistent corneal epithelial defects. Fibronectin Study Group. Trans. Am. Ophthalmol. Soc. 1993, 91, 367–390. [Google Scholar] [PubMed]
- Finger, P.T.; Sedeek, R.W.; Chin, K.J. Topical Interferon Alfa in the Treatment of Conjunctival Melanoma and Primary Acquired Melanosis Complex. Am. J. Ophthalmol. 2008, 145, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, S.G.; Boulton, M.E. Consequences of oxidative stress in age-related macular degeneration. Mol. Aspects Med. 2012, 33, 399–417. [Google Scholar] [CrossRef] [PubMed]
- Gantyala, S.P.; Shekhar, H.; Vanathi, M.; Sinha, R.; Titiyal, J.S. Interferons in Ophthalmology Current Status and Advancing Trends. Delhi J. Ophthalmol. 2013, 23, 295–297. [Google Scholar] [CrossRef]
- Huerva, V.; Mangues, I. Treatment of conjunctival squamous neoplasias with interferon alpha 2ab. J. Fr. Ophtalmol. 2008, 31, 317–325. [Google Scholar] [CrossRef]
- Shen, J.; Deng, Y.; Jin, X.; Ping, Q.; Su, Z.; Li, L. Thiolated nanostructured lipid carriers as a potential ocular drug delivery system for cyclosporine A: Improving in vivo ocular distribution. Int. J. Pharm. 2010, 402, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Rauck, B.M.; Friberg, T.R.; Medina Mendez, C.A.; Park, D.; Shah, V.; Bilonick, R.A.; Wang, Y. Biocompatible reverse thermal gel sustains the release of intravitreal bevacizumab in vivo. Investig. Ophthalmol. Vis. Sci. 2013, 55, 469–470. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Tong, Z.; Yang, D.; Nie, J. Glucose-responsive insulin delivery microhydrogels from methacrylated dextran/concanavalin A: preparation and in vitro release study. Carbohydr. Polym. 2012, 89, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Velez-montoya, R.; Oliver, S.C.N.; Olson, J.L.; Fine, S.L.; Mandava, N.; Quiroz-mercado, H. Age-Related Macular Degeneration: Today’s and Future Treatments. J. Ret. Vit. Dis. 2013, 33, 1487–1502. [Google Scholar]
- Kute, R.B.; Gondkar, P.R.; Saudagar, S.B. Ophthalmic In-situ Gel: An Overview. World J. Pharm. Pharm. Sci. 2015, 4, 549–568. [Google Scholar]
- Sieg, J.W.; Robinson, J.R. Mechanistic studies on transcorneal permeation of fluorometholone. J. Pharm. Sci. 1981, 70, 1026–1029. [Google Scholar] [CrossRef] [PubMed]
- Zarbin, M.A.; Rosenfeld, P.J. Pathway-based therapies for age-related macular degeneration: an integrated survey of emerging treatment alternatives. Retina 2010, 30, 1350–1367. [Google Scholar] [CrossRef] [PubMed]
- Athanasakis, K.; Fragoulakis, V.; Tsiantou, V.; Masaoutis, P.; Maniadakis, N.; Kyriopoulos, J. Cost-effectiveness analysis of ranibizumab versus verteporfin photodynamic therapy, pegaptanib sodium, and best supportive care for the treatment of age-related macular degeneration in Greece. Clin. Ther. 2012, 34, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Qin, G.; Li, X.; Lv, H.; Qian, Z.; Yu, L. The PEG-PCL-PEG Hydrogel as an Implanted Ophthalmic Delivery System after Glaucoma Filtration Surgery; a Pilot Study. Med. hypothesis, Discov. Innov. Ophthalmol. 2014, 3, 3–8. [Google Scholar]
- Liu, Z.; Li, J.; Nie, S.; Liu, H.; Ding, P.; Pan, W. Study of an alginate/HPMC-based in situ gelling ophthalmic delivery system for gatifloxacin. Int. J. Pharm. 2006, 315, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Shastri, D.; Patel, L.; Parikh, R. Studies on In situ Hydrogel: A Smart Way for Safe and Sustained Ocular Drug Delivery. J. Young Pharm. 2010, 2, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Maurice, D.M.; Mishima, S. Ocular Pharmacokinetics; Springer: Berlin & Heidelberg, Germany, 1984; pp. 19–116. [Google Scholar]
- Kuno, N.; Fujii, S. Recent Advances in Ocular Drug Delivery Systems. Polymers 2011, 3, 193–221. [Google Scholar] [CrossRef]
- Schoenwald, R. Textbook of Ocular Pharmacology; Lippincott-Raven: Philadelphia, PA, USA, 1997; pp. 119–138. [Google Scholar]
- Xie, B.; Jin, L.; Luo, Z.; Yu, J.; Shi, S.; Zhang, Z.; Shen, M.; Chen, H.; Li, X.; Song, Z. An injectable thermosensitive polymeric hydrogel for sustained release of Avastin1 to treat posterior segment disease. Int. J. Pharm. 2015, 490, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, L.C.; Carmichael, T.; Govender, T.; Kumar, P.; Choonara, Y.E.; Pillay, V. In vitro, in vivo, and in silico evaluation of the bioresponsive behavior of an intelligent intraocular implant. Pharm. Res. 2014, 31, 607–634. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Shih, J.; Lo, R.; Saati, S.; Agrawal, R.; Humayun, M.S.; Tai, Y.; Meng, E. An electrochemical intraocular drug delivery device. Sens. Actuators 2008, 143, 41–48. [Google Scholar] [CrossRef]
- Lo, R.; Li, P.; Saati, S.; Agrawal, R.; Humayun, S.; Meng, E. A refillable microfabricated drug delivery device for treatment of ocular diseases. Lab Chip 2008, 8, 1027–1030. [Google Scholar] [CrossRef] [PubMed]
- Haghjou, N.; Soheilian, M.; Abdekhodaie, M.J. Sustained release intraocular drug delivery devices for treatment of uveitis. J. Ophthalmic Vis. Res. 2011, 6, 317–319. [Google Scholar] [PubMed]
- Parlato, M.; Johnson, A.; Hudalla, G.A.; Murphy, W.L. Adaptable poly(ethylene glycol) microspheres capable of mixed-mode degradation. Acta Biomater. 2013, 9, 9270–9280. [Google Scholar] [CrossRef] [PubMed]
- Oubraham, H.; Cohen, S.Y.; Samimi, S.; Marotte, D.; Bouzaher, I.; Bonicel, P.; Fajnkuchen, F.; Tadayoni, R. Inject and extend dosing versus dosing as needed: A comparative retrospective study of ranibizumab in exudative age-related macular degeneration. Retina 2011, 31, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.S.; Schwartz, S.D.; Hubschman, J.-P. Age-related macular degeneration: Current and novel therapies. Maturitas 2010, 66, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Juzenas, P.; Juzeniene, A.; Kaalhus, O.; Iani, V.; Moan, J. Noninvasive fluorescence excitation spectroscopy during application of 5-aminolevulinic acid in vivo. Photochem. Photobiol. Sci. 2002, 1, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Malec, J.; Dot, D.; Cejková, J.; Br, B. Decreased expression of antioxidant enzymes in the conjunctival epithelium of dry eye (Sjögren’s syndrome) and its possible contribution to the development of ocular surface oxidative injuries. Histol. Histopathol. 2008, 23, 1477–1483. [Google Scholar]
| Model Drug | Polymer | Stimuli | Objective | Reference |
|---|---|---|---|---|
| Timolol Maleate | Poloxamer and Chitosan | Temperature | Improved mechanical and mucoadhesive properties and improved retention time for the treatment of ocular diseases. | [18] |
| Puerarin | Poloxamer and Carbopol | Temperature | Maintain sufficient drug concentration at the precorneal area. | [19] |
| Pilocarpine Hydrochloride | Xyloglucan | Temperature | Sustained ocular delivery. | [20,21] |
| Ovalbumin | Poly(N-isopropylacrylamide) | Temperature | Prolonged, externally controlled delivery of actives over a specific period of time. | [22] |
| Timolol Maleate | Poly(N-isopropylacrylamide) and Chitosan | Temperature | Improved bioavailability and efficacy. | [23] |
| Peurarin | Carbopol and Methylcellulose | Temperature and pH | Improved gel strength, increased precorneal residence time and bioavailability. | [24] |
| Ofloxacin | Polyacrylic acid and HPMC | pH | To provide sustained release of the drug during treatment. | [25] |
| Cyclosporine A | Gellan gum | Electrolytes | Increased drug loading capacity. | [26] |
| Gatifloxacin | Alginate and HPMC | Ions | Enhanced ocular bioavailability and patient compliance. | [27] |
| Diclofenac Sodium | Thiolated poly(aspartic acid) | Oxidation | Prolonged residence time and reduced administration frequency. | [28] |
| Stimuli | Model Protein/Peptide | Responsive Moiety | Mechanism | Delivery System | Reference |
|---|---|---|---|---|---|
| pH | Print 3G | Cholesterylhemisuccinate (CHEMS) | CHEMS possesses an inverted cone shape at physiological pH. Under acidic conditions, the carboxylic acid group becomes protonated and CHEMS loses the shape releasing the protein. This transition occurs within endosome where pH is decreased during endocytosis. | Liposomes | [23,36,37] |
| pH | BSA | PLGA-carboxyl group | Undergoes deprotonation at neutral or basic pH which leads to release of the protein. | Micelles | [38,39] |
| Enzyme (Human Neutrophil Elastase) | Anti-inflammatory proteins/peptides | HNE-sensitive peptides (Aminobutyric acid, Novarline, Norleucine) | HNE diffuses into the hydrogel and splits the substrate releasing the therapeutic agent into the site of action. | Hydrogel | [17] |
| Enzyme (Chitosanase) | Fluorescein isothiocyanate-labeled albumin (FITC-albumin) | Chitosan | Enzymatic degradation weakens the capsule wall releasing the protein and the capsule is destroyed over time. Degradation is specific to chitosanase. | Hollow capsules | [40] |
| Dual Stimuli (pH, Glucose) | Insulin | Concanavalin A, N-(2-(dimethylamino) ethyl)-methacrylamide | Decrease in pH causes ionization of the amino groups causing swelling. Concanavalin A binds glucose, reducing crosslinking density in mycrohydrogels and increasing hydrophilicity leading to swelling then release of Insulin. | Microhydrogel | [41] |
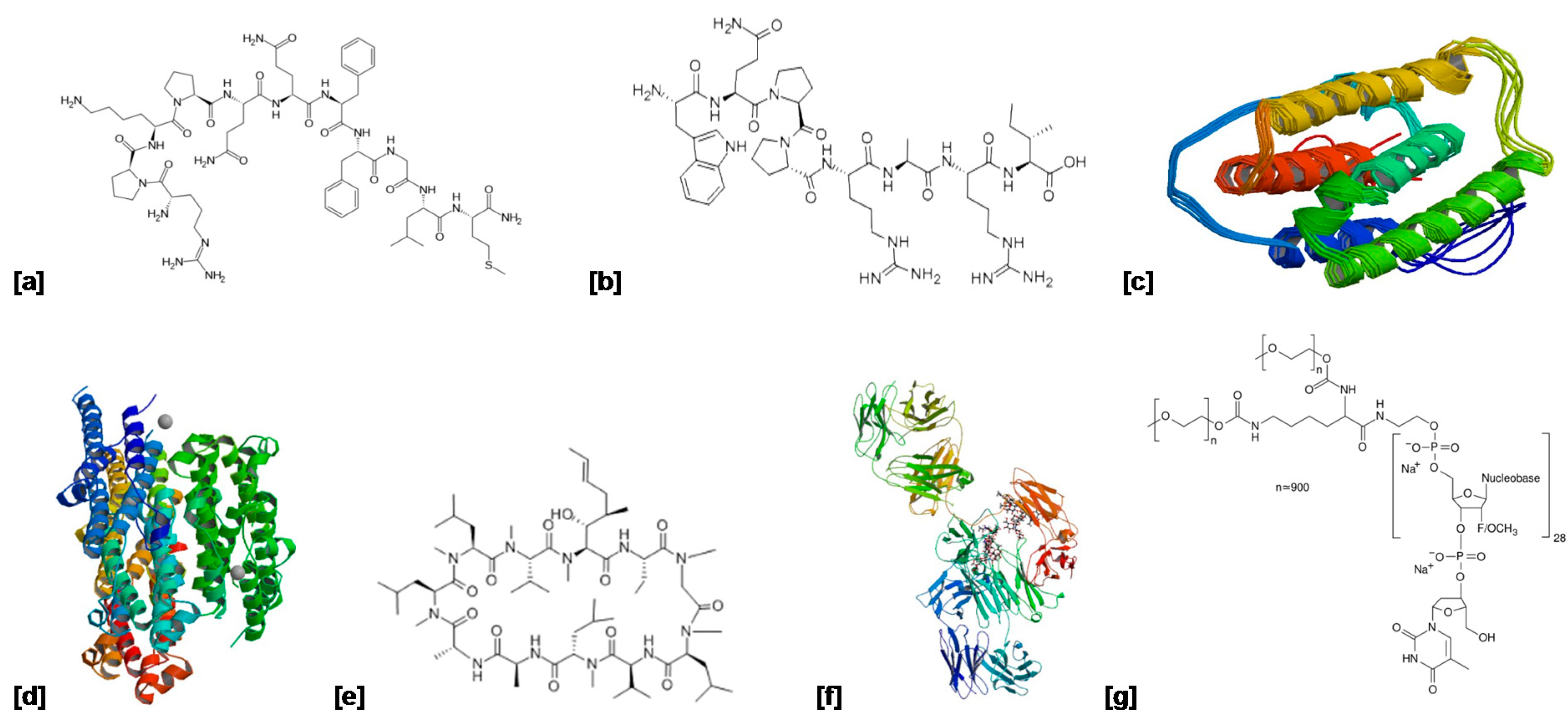
| Protein/Peptide | Indication | Mechanism of Action | Delivery System | Molecular Weight | Structure (Figure 4) | Reference |
|---|---|---|---|---|---|---|
| Substance P | Corneal injury | Stimulates corneal epithelial cell migration and proliferation. | Topical eye drops | ~13 kDa | (a) | [73] |
| Fibronectin | Persistent corneal epithelial defect | Functions in corneal re-epithelialization by providing a provisional matrix for epithelial cell adhesion and migration. | Topical eye drops | 220 kDa | (b) | [74,75] |
| Interferon α2a | Herpes simplex, keratitis, Macular edema and AMD | Immunomodulatory agent. | Injection (Roferon A®) | 19 kDa | (c) | [76] |
| Interferon α2b | Ocular surface squamous neoplasia, Conjunctival melanoma | Immunomodulatory agent. | Topical drops and subconjunctival injection (Intron A®) | ~19 kDa | (d) | [77,78,79] |
| Cyclosporine A | Keratoconjunctivitis sicca and dry eye disease | Immunosuppressive agent that inhibits activation of T lymphocytes. | Eye drops (Restasis®) | ~12 kDa | (e) | [26,80] |
| Bevacizumab | Age-related macular degeneration | Vascular endothelial growth factor inhibitor. Regulates angiogenesis. | Intravitreal injection (Avastin®) | 149 kDa | (f) | [81] |
| Pegaptinib | Age-related macular degeneration | Selective antagonist of the 165 isoform of vascular endothelial growth factor–A. | Intravitreal injection (Macugen®) | ~50 kDa | (g) | [82,83] |
| Bioactive | Polymers | Routes of Administration | Reference |
|---|---|---|---|
| VEGF inhibitors | PNIPAAm | Intravitreal (injection) | [40] |
| Fluocinolone acetonide | Polyimide | Intravitreal (implant) | [86] |
| Fuconazole | Gellan gum, carragenan | Topical | [87] |
| Bevacizumab | Poly (ethylene glycol)-poly (ε-caprolactone)-poly (ethylene glycol) | Intracameral | [88] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahlumba, P.; Choonara, Y.E.; Kumar, P.; Du Toit, L.C.; Pillay, V. Stimuli-Responsive Polymeric Systems for Controlled Protein and Peptide Delivery: Future Implications for Ocular Delivery. Molecules 2016, 21, 1002. https://doi.org/10.3390/molecules21081002
Mahlumba P, Choonara YE, Kumar P, Du Toit LC, Pillay V. Stimuli-Responsive Polymeric Systems for Controlled Protein and Peptide Delivery: Future Implications for Ocular Delivery. Molecules. 2016; 21(8):1002. https://doi.org/10.3390/molecules21081002
Chicago/Turabian StyleMahlumba, Pakama, Yahya E. Choonara, Pradeep Kumar, Lisa C. Du Toit, and Viness Pillay. 2016. "Stimuli-Responsive Polymeric Systems for Controlled Protein and Peptide Delivery: Future Implications for Ocular Delivery" Molecules 21, no. 8: 1002. https://doi.org/10.3390/molecules21081002