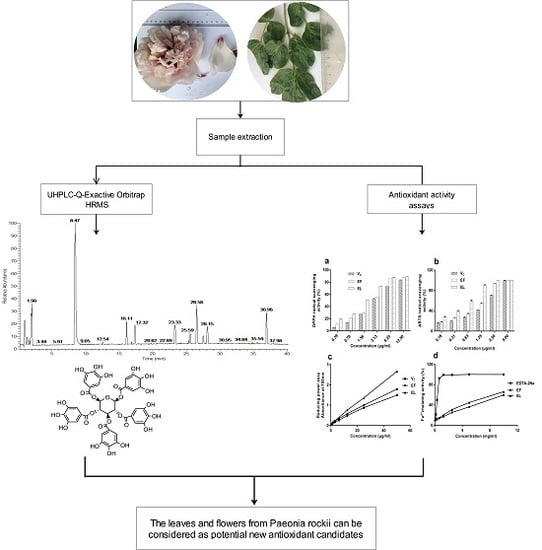
Identification of Chemical Composition of Leaves and Flowers from Paeonia rockii by UHPLC-Q-Exactive Orbitrap HRMS
Abstract
:1. Introduction
2. Results and Discussion
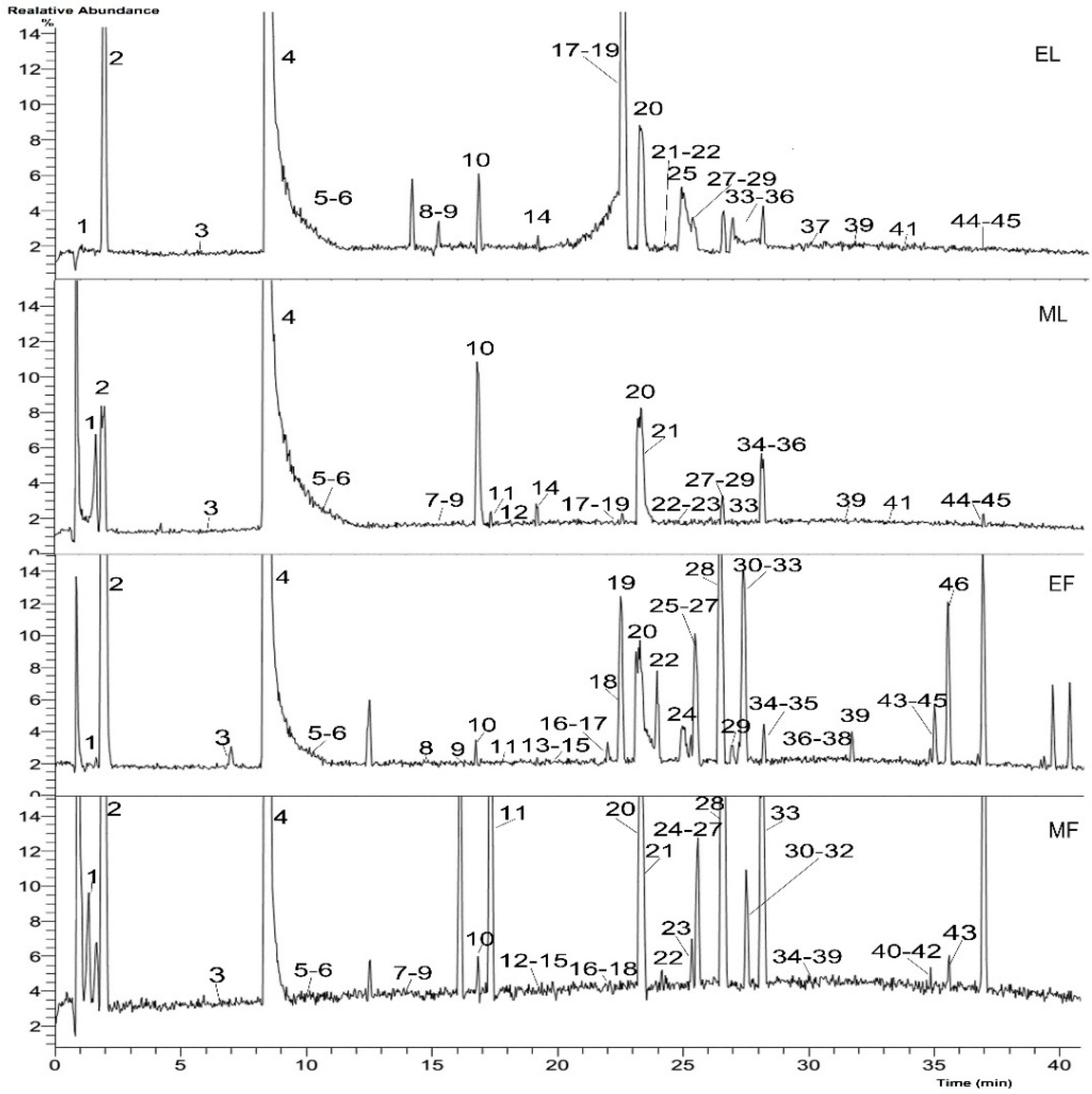
2.1. Identification of Chemical Compositions
2.1.1. Monoterpene Glycosides
2.1.2. Phenolic Acids
2.1.3. Tannins
2.1.4. Flavonoids
3. Material and Methods
3.1. Chemicals
3.2. Plant Material Collection and Sample Preparation
3.3. UHPLC-Q-Exactive Analysis
3.3.1. Liquid Chromatography
3.3.2. Mass Spectrometry
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yin, W.; Duan, W.; Liu, P.; Deng, R.; Ren, Y.; Zhao, S. GC-MS analysis of essential oil of Paeonia suffruticosa Andrews from ZhaoFen and RouFurong flowers in China. Natl. Sci. 2012, 4, 552–554. [Google Scholar]
- Picerno, P.; Mencherini, T.; Sansone, F.; del Gaudio, P.; Granata, I.; Porta, A.; Aquino, R.P. Screening of a polar extract of Paeonia rockii: Composition and antioxidant and antifungal activities. J. Ethnopharmacol. 2011, 138, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhou, Z.Q.; Lin, Q.B.; Pan, K.Y.; Li, M.Y. Phylogenetic analysis of Paeonia sect. Moutan (Paeoniaceae) based on multiple DNA fragments and morphological data. J. Syst. Evol. 2008, 46, 563–572. [Google Scholar]
- Wu, S.H.; Wu, D.G.; Chen, Y.W. Chemical constituents and bioactivities of plants from the genus Paeonia. Chem. Biodivers. 2010, 7, 90–104. [Google Scholar] [CrossRef] [PubMed]
- He, C.N.; Peng, Y.; Zhang, Y.C.; Xu, L.J.; Gu, J.; Xiao, P.G. Phytochemical and biological studies of paeoniaceae. Chem. Biodivers. 2010, 7, 805–838. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Y.; Raven, P.H.; Hong, D.Y. Flora of China; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2001; pp. 127–132. [Google Scholar]
- Liu, P.; Wang, Y.; Gao, J.; Lu, Z.; Yin, W.; Deng, R. Resveratrol trimers from seed cake of Paeonia rockii. Molecules 2014, 19, 19549–19556. [Google Scholar] [CrossRef] [PubMed]
- Michalski, A.; Damoc, E.; Hauschild, J.P.; Lange, O.; Wieghaus, A.; Makarov, A.; Nagaraj, N.; Cox, J.; Mann, M.; Horning, S. Mass Spectrometry-based Proteomics Using Q Exactive, a High-performance Benchtop Quadrupole Orbitrap Mass Spectrometer. Mol. Cell. Proteom. 2011, 10. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Wu, M.; Chen, Y.; Zhang, Y.; Zhao, X.; Zheng, X. Revealing metabolomic variations in Cortex Moutan from different root parts using HPLC-MS method. Phytochem. Anal. PCA 2015, 26, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Li, Q.; Cai, H.; Tu, S.; Cai, B. Investigation of the Chemical Changes from Crude and Processed Paeoniae Radix Alba-Atractylodis Macrocephalae Rhizoma Herbal Pair Extracts by Using Q Exactive High-Performance Benchtop Quadrupole-Orbitrap LC-MS/MS. Evid. Based Complement. Altern. Med. eCAM 2014, 2014, 170959. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Gu, Z. Screening of bioactive compounds from moutan cortex and their anti-inflammatory activities in rat synoviocytes. Evid. Based Complement. Altern. Med. eCAM 2009, 6, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Cui, W.; Zhou, W.; Duan, J.-A.; Shang, E.; Tang, Y. Chemical fingerprinting and quantitative constituent analysis of Siwu decoction categorized formulae by UPLC-QTOF/MS/MS and HPLC-DAD. Chin. Med. 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Borras-Linares, I.; Herranz-Lopez, M.; Barrajon-Catalan, E.; Arraez-Roman, D.; Gonzalez-Alvarez, I.; Bermejo, M.; Fernandez Gutierrez, A.; Micol, V.; Segura-Carretero, A. Permeability Study of Polyphenols Derived from a Phenolic-Enriched Hibiscus sabdariffa Extract by UHPLC-ESI-UHR-Qq-TOF-MS. Int. J. Mol. Sci. 2015, 16, 18396–18411. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Liu, E.; Kojo, A.; Zhao, J.; Li, W.; Zhang, Y.; Wang, T.; Gao, X. Qualitative and quantitative analysis of Eclipta prostrata L. by LC/MS. Sci. World J. 2015, 2015, 980890. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.H.; Zhu, S.; Ge, Y.W.; He, Y.M.; Kazuma, K.; Wang, Z.; Yoshimatsu, K.; Komatsu, K. Monoterpene derivatives with anti-allergic activity from red peony root, the root of Paeonia lactiflora. Fitoterapia 2016, 108, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Nakamura, S.; Sugimoto, S.; Tsukioka, J.; Hinomaru, F.; Nakashima, S.; Matsumoto, T.; Ohta, T.; Fujimoto, K.; Yoshikawa, M.; et al. Constituents of flowers of Paeoniaceae plants, Paeonia suffruticosa and Paeonia lactiflora. Phytochem. Lett. 2015, 12, 98–104. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 2, 4, 5, 9, 10, 20, 22, 41, 43, 45, 46 are available from Ph.D. Rui Zeng.

| Peak | TR (min) | Formula | m/z Calculated | m/z Experimental | Error (ppm) | MS/MS Fragments | Proposed Compound | Sample |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.55 | C13H16O10 | 331.06597 | 331.06766 | 5.095 | 169, 125 | 1-O-Galloyl glucose | EF, EL, MF, ML |
| 2 | 2.00 | C7H6O5 | 169.01315 | 169.01328 | 0.771 | 125, 97 | Gallic acid | EF, EL, MF, ML |
| 3 | 6.99 | C7H6O3 | 137.02332 | 137.02327 | −0.37 | 93 | Hydroxybenzoic acid | EF, EL, MF, ML |
| 4 | 8.98 | C8H8O5 | 183.0288 | 183.02907 | 1.476 | 168, 124 | Methyl gallate | EF, EL, MF, ML |
| 5 | 11.14 | C23H28O12 | 495.1497 | 495.15182 | 4.276 | 465, 345, 281, 195, 165, 137 | Oxypaeoniflorin a | EF, EL, MF, ML |
| 6 | 11.25 | C20H20O14 | 483.07693 | 483.07922 | 2.288 | 331, 313, 169, 125 | Digalloyl glucose | EL, ML |
| 7 | 14.72 | C27H30O17 | 625.13993 | 625.14282 | 2.894 | 462, 301, 299 | Quercetin-3,7-diglucoside | MF, ML |
| 8 | 14.99 | C27H24O18 | 635.08789 | 635.09076 | 2.879 | 465, 313, 169, 125 | Trigalloyl glucose | EF, EL, MF, ML |
| 9 | 15.15 | C9H10O5 | 197.04445 | 197.04492 | 2.386 | 169, 152, 125 | Ethyl gallate | EF, EL, MF, ML |
| 10 | 16.83 | C23H28O11 | 479.15479 | 479.15692 | 4.449 | 327, 195, 165, 121 | Paeoniflorin a | EF, EL, MF, ML |
| 11 | 17.15 | C27H30O16 | 609.14501 | 609.14795 | 4.825 | 447, 446, 285, 283 | Kaempferol-3,7-di-O-glucoside | EF, EL, MF, ML |
| 12 | 18.22 | C28H32O17 | 639.15558 | 639.15863 | 3.054 | 476, 315, 313 | Isorhamnetin-3,7-di-O-glucoside | MF, ML |
| 13 | 19.00 | C23H28O12 | 495.1497 | 495.15189 | 4.418 | 465, 345, 281, 195, 151, 137 | Oxypaeoniflorin isomer | EF, MF, |
| 14 | 19.20 | C34H28O22 | 787.09885 | 787.10205 | 4.067 | 617, 465, 313, 169, 125 | Tetragalloylglucose | EF, EL, MF, ML |
| 15 | 20.65 | C23H28O12 | 495.1497 | 495.15195 | 4.539 | 465, 345, 281, 195, 151, 137 | Oxypaeoniflorin isomer | EF, MF |
| 16 | 21.61 | C21H20O12 | 463.08710 | 463.08923 | 4.594 | 301, 257, 151 | Quercetin-7-O-glucoside | EF, MF |
| 17 | 21.89 | C28H24O16 | 615.09806 | 615.10052 | 3.998 | 463, 301, 169, 151, 125 | Quercetin galloylglucoside | EF, EL, MF, ML |
| 18 | 22.29 | C30H32O15 | 631.16575 | 631.16864 | 4.584 | 613, 491, 479, 399, 313, 271, 211, 169, 125, 121 | Galloylpaeoniflorin | EF, EL, MF, ML |
| 19 | 22.50 | C15H12O9 | 335.03976 | 335.04150 | 5.198 | 183, 168, 124 | Methyl digallate | EF, EL, ML |
| 20 | 23.15 | C41H32O26 | 939.10981 | 939.11316 | 3.570 | 787, 635, 617, 465, 447, 313, 295, 169, 125 | Pentagalloyl glucose | EF, EL, MF, ML |
| 21 | 23.60 | C21H20O11 | 447.09219 | 447.0943 | 4.724 | 285 | Luteolin-7-O-glucoside a | EL, MF, ML |
| 22 | 24.10 | C21H20O12 | 463.08710 | 463.08914 | 4.400 | 301, 300, 271, 255, 179, 151 | Quercetin-3-O-glucoside a | EF, EL, MF, ML |
| 23 | 24.95 | C27H30O15 | 593.15010 | 593.15308 | 2.984 | 431, 269 | Apigenin diglucoside | MF, ML |
| 24 | 25.31 | C23H28O11 | 479.15479 | 479.15707 | 4.637 | 449, 327, 195, 183, 151, 139, 121 | Paeoniflorin isomer | EF, MF, |
| 25 | 25.47 | C21H20O11 | 447.09219 | 447.09412 | 4.322 | 285, 284, 257, 151 | Kaempferol-7-O-glucoside | EF, EL, MF |
| 26 | 25.56 | C30H32O14 | 615.17083 | 615.17334 | 4.007 | 585, 477, 447, 431, 281, 239, 179, 137, 93 | Mudanpioside H | EF, MF |
| 27 | 25.65 | C28H24O15 | 599.10315 | 599.10571 | 4.279 | 447, 313, 285, 284, 169, 151, 125 | Kaempferol galloylglucoside | EF, EL, MF, ML |
| 28 | 26.52 | C21H20O10 | 431.09727 | 431.09882 | 3.588 | 269, 268 | Apigenin-7-O-glucoside | EF, EL, MF, ML |
| 29 | 26.93 | C48H34O30 | 1091.12077 | 1091.12451 | 3.432 | 939, 787, 769, 635, 617, 465, 447, 431, 295, 169, 125, 123 | 6-O-(m-Galloyl)galloyl-1,2,3,4-tetragalloylglucose | EL, ML |
| 30 | 27.04 | C22H22O12 | 477.10275 | 477.10483 | 2.078 | 357, 315, 299, 287, 271, 169, 151 | Isorhamnetin7-O-glucoside | EF, MF |
| 31 | 27.22 | C23H28O11 | 479.15479 | 479.15688 | 4.324 | 449, 327, 195, 183, 151, 139, 121 | Paeoniflorin isomer | EF, MF |
| 32 | 27.30 | C27H30O15 | 593.15010 | 593.25183 | 2.734 | 431, 285 | Kaepferol glucosyl rhamnoside | MF |
| 33 | 27.40 | C21H20O11 | 447.09219 | 447.09396 | 3.964 | 285, 284, 255, 227, 179, 151 | Astragalin | EF, EL, MF, ML |
| 34 | 28.10 | C27H30O14 | 577.15518 | 577.15741 | 3.861 | 431, 413, 269 | Apigenin rhamnoglucoside | EF, EL, MF, ML |
| 35 | 28.19 | C22H22O12 | 477.10275 | 477.10468 | 4.040 | 357, 314, 285, 271, 257, 243, 151 | Isorhamnetin-3-O-glucoside | EF, EL, MF, ML |
| 36 | 28.80 | C28H24O14 | 583.10823 | 583.11066 | 2.428 | 431, 313, 269, 169, 125 | Apigenin galloylglucoside isomer | EF, EL, MF, ML |
| 37 | 30.36 | C30H32O13 | 599.17592 | 599.17865 | 4.561 | 477, 447, 431, 285, 281, 239, 179, 169, 149, 137, 121, 93 | Benzoyloxypaeoniflorin | EF, MF |
| 38 | 31.06 | C28H24O14 | 583.10823 | 583.11072 | 2.488 | 431, 313, 269, 169, 125 | Apigenin galloylglucoside isomer | EF, MF |
| 39 | 31.76 | C30H32O13 | 599.17592 | 599.17847 | 4.260 | 477, 447, 195,165, 137, 121, 93 | Mudanpioside C | EF, EL, MF, ML |
| 40 | 32.82 | C28H24O14 | 583.10823 | 583.11108 | 2.848 | 432, 431, 269, 268, 169, 125 | Apigenin galloylglucoside isomer | EF, MF |
| 41 | 32.89 | C15H10O6 | 285.03936 | 285.04095 | 5.562 | 257, 175, 151, 133 | Luteolin a | EF, EL, MF, ML |
| 42 | 33.64 | C16H12O7 | 315.04993 | 315.05185 | 6.097 | 283, 255, 227, 211 | Isorhamnetin isomer | EF, MF |
| 43 | 35.00 | C15H10O6 | 285.03936 | 285.04095 | 5.562 | 257, 229, 151 | Kaempferol a | EF, MF |
| 44 | 35.40 | C31H34O14 | 629.18648 | 629.18927 | 2.788 | 449, 347, 165, 121 | Mudanpioside B | EF, EL, MF, ML |
| 45 | 35.52 | C15H10O5 | 269.04445 | 269.04602 | 5.836 | 225, 159, 151, 117, 107 | Apigenin a | EF, EL, MF, ML |
| 46 | 35.88 | C16H12O7 | 315.04993 | 315.18200 | 6.002 | 300, 271, 151 | Isorhamnetin a | EF |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Kuang, G.; Chen, X.; Zeng, R. Identification of Chemical Composition of Leaves and Flowers from Paeonia rockii by UHPLC-Q-Exactive Orbitrap HRMS. Molecules 2016, 21, 947. https://doi.org/10.3390/molecules21070947
Li J, Kuang G, Chen X, Zeng R. Identification of Chemical Composition of Leaves and Flowers from Paeonia rockii by UHPLC-Q-Exactive Orbitrap HRMS. Molecules. 2016; 21(7):947. https://doi.org/10.3390/molecules21070947
Chicago/Turabian StyleLi, Jinhua, Gang Kuang, Xiaohu Chen, and Rui Zeng. 2016. "Identification of Chemical Composition of Leaves and Flowers from Paeonia rockii by UHPLC-Q-Exactive Orbitrap HRMS" Molecules 21, no. 7: 947. https://doi.org/10.3390/molecules21070947
APA StyleLi, J., Kuang, G., Chen, X., & Zeng, R. (2016). Identification of Chemical Composition of Leaves and Flowers from Paeonia rockii by UHPLC-Q-Exactive Orbitrap HRMS. Molecules, 21(7), 947. https://doi.org/10.3390/molecules21070947