DNA Cleavage and Condensation Activities of Mono- and Binuclear Hybrid Complexes and Regulation by Graphene Oxide
Abstract
:1. Introduction
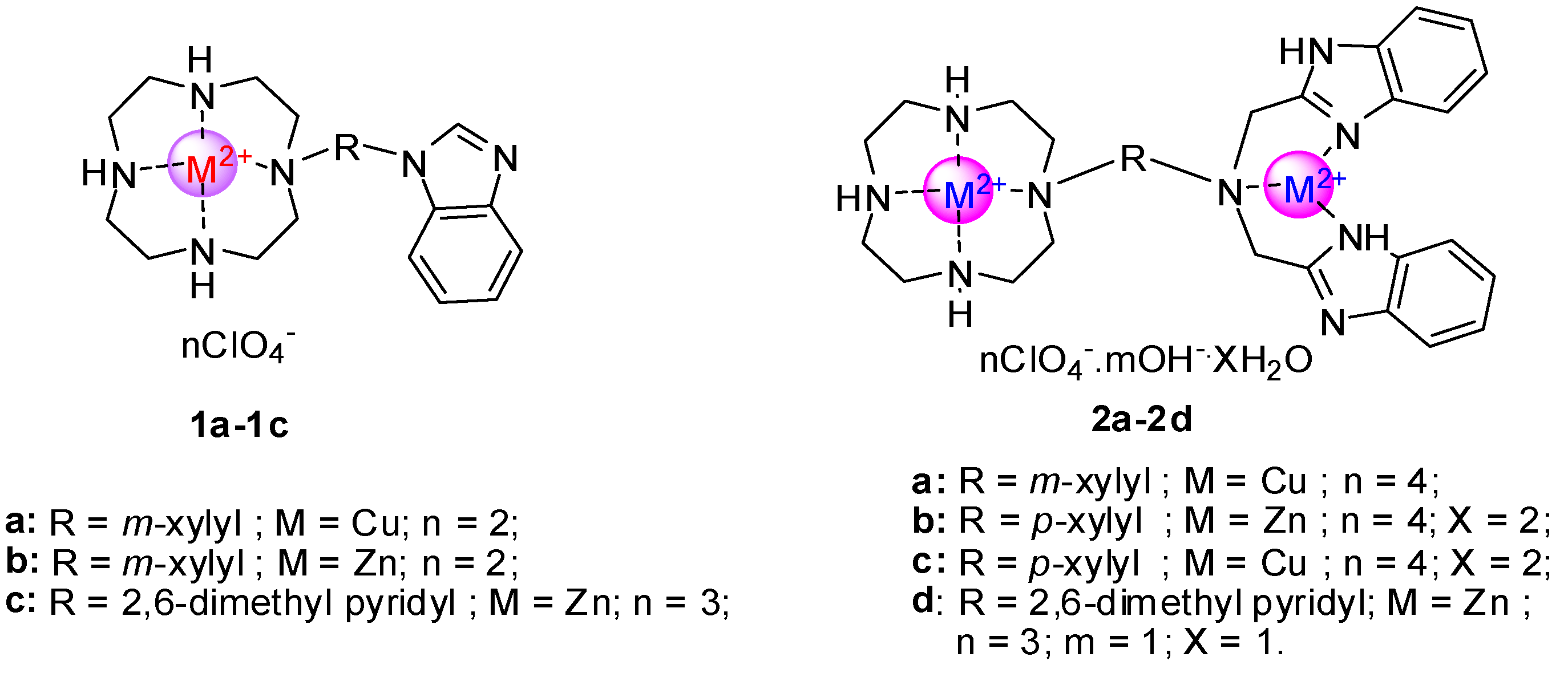
2. Results and Discussion
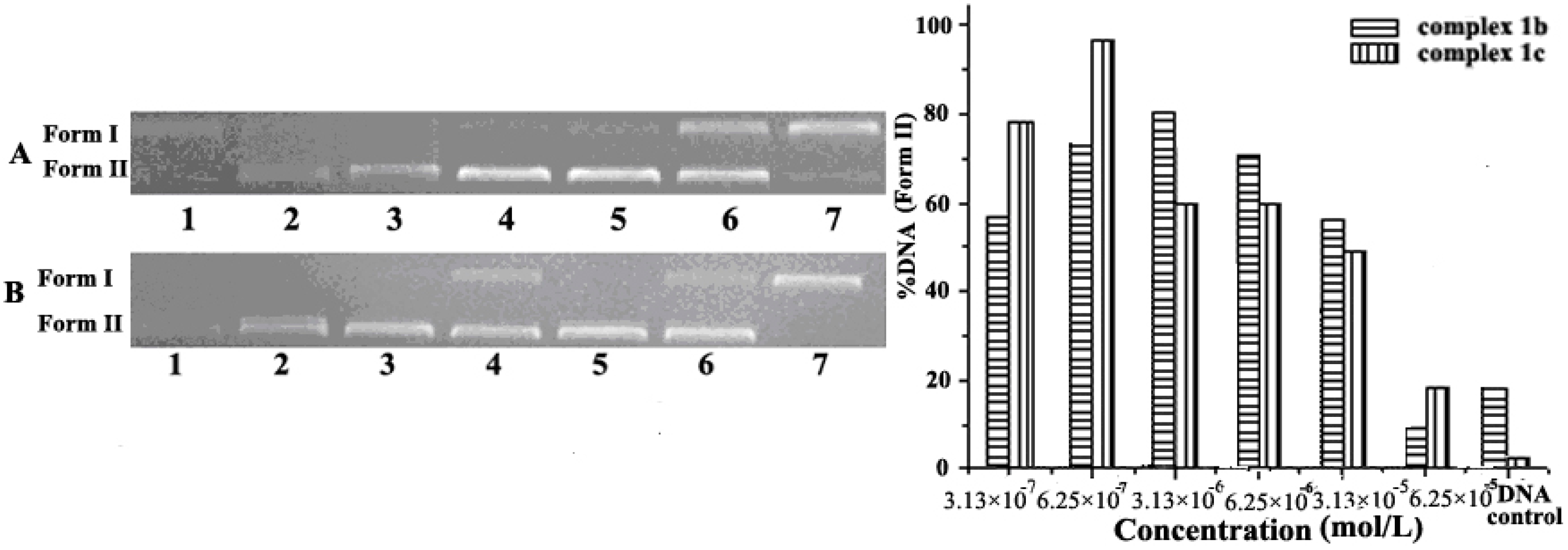
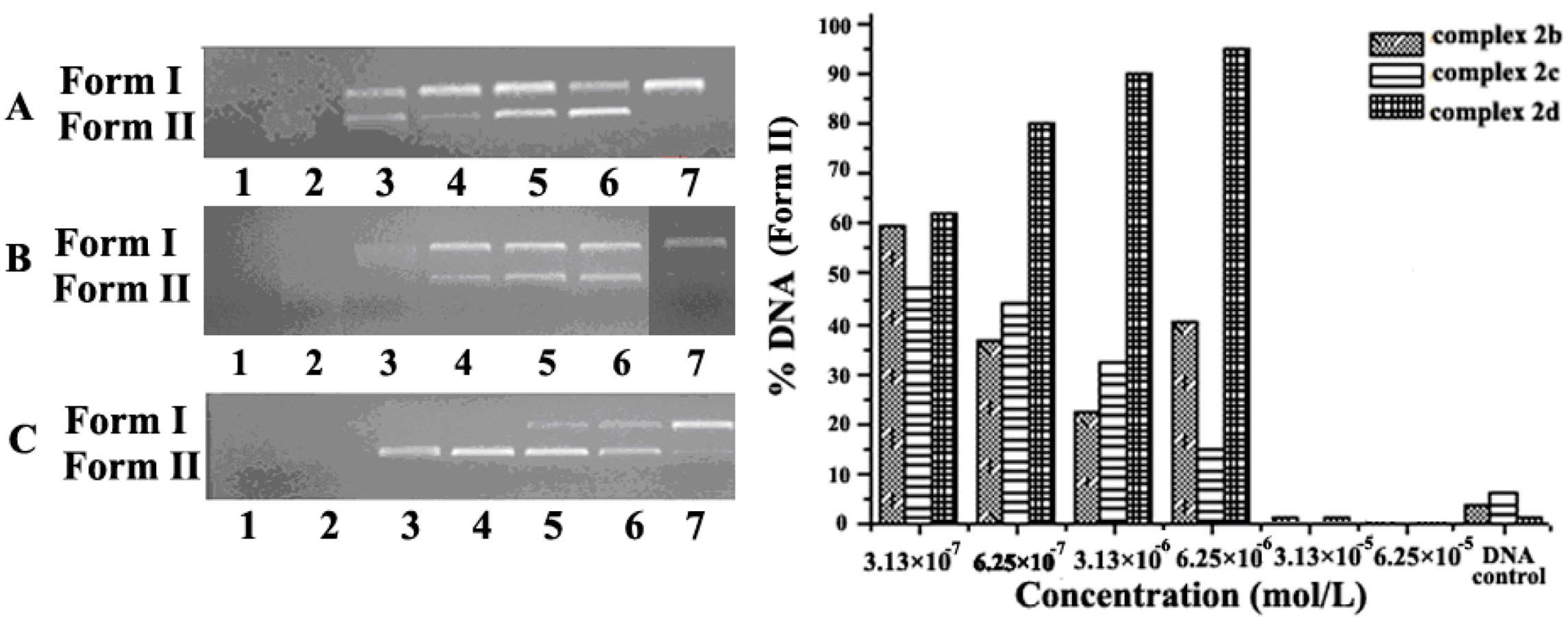
2.1. Concentration Effect of the Complex for DNA Cleavage and Condensation
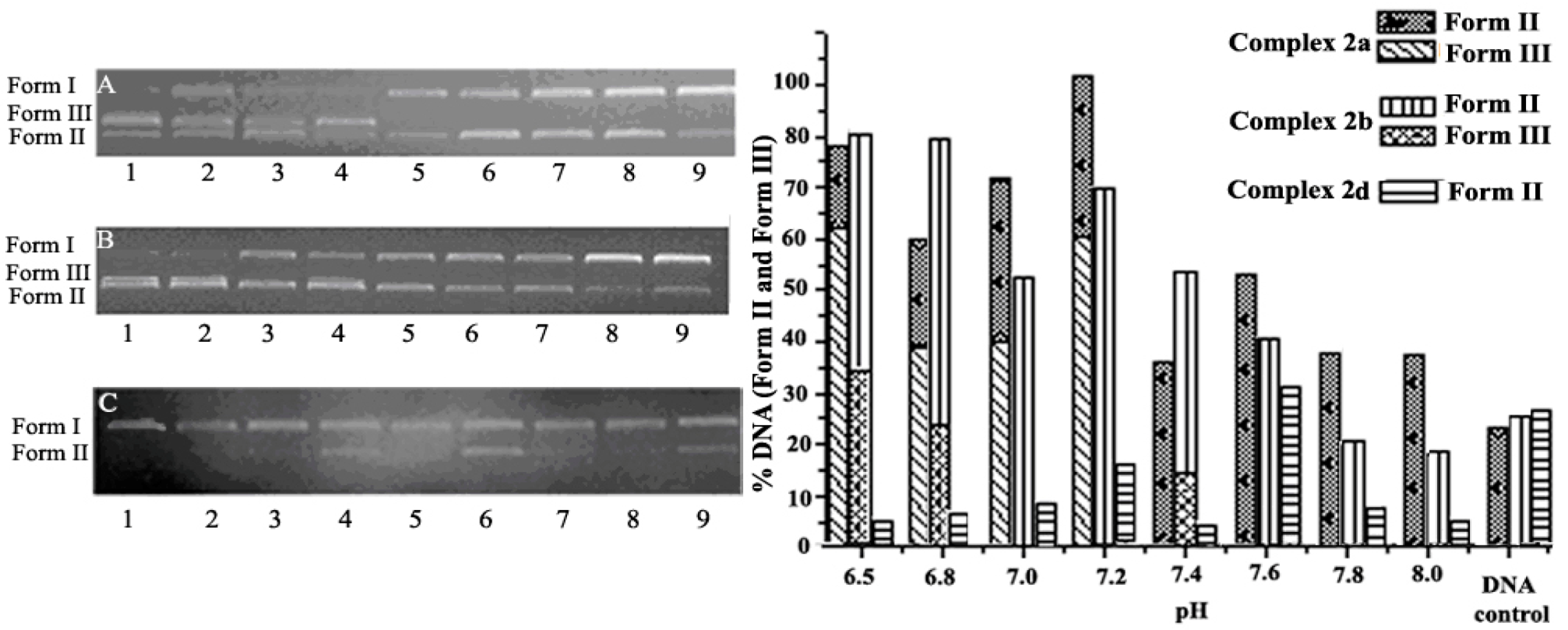
2.2. pH Effect on DNA Cleavage
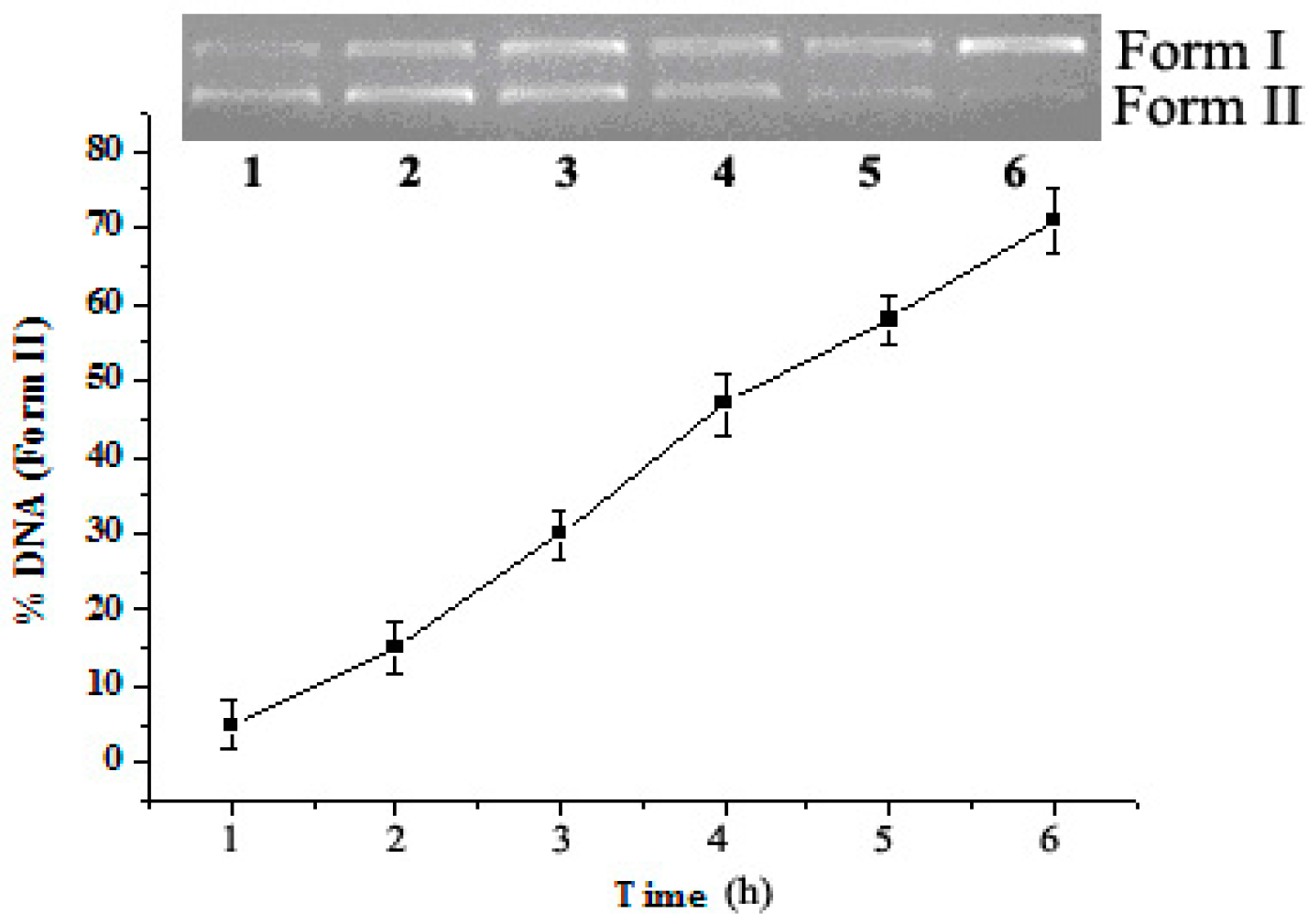
2.3. Reaction Time Effect on DNA Cleavage
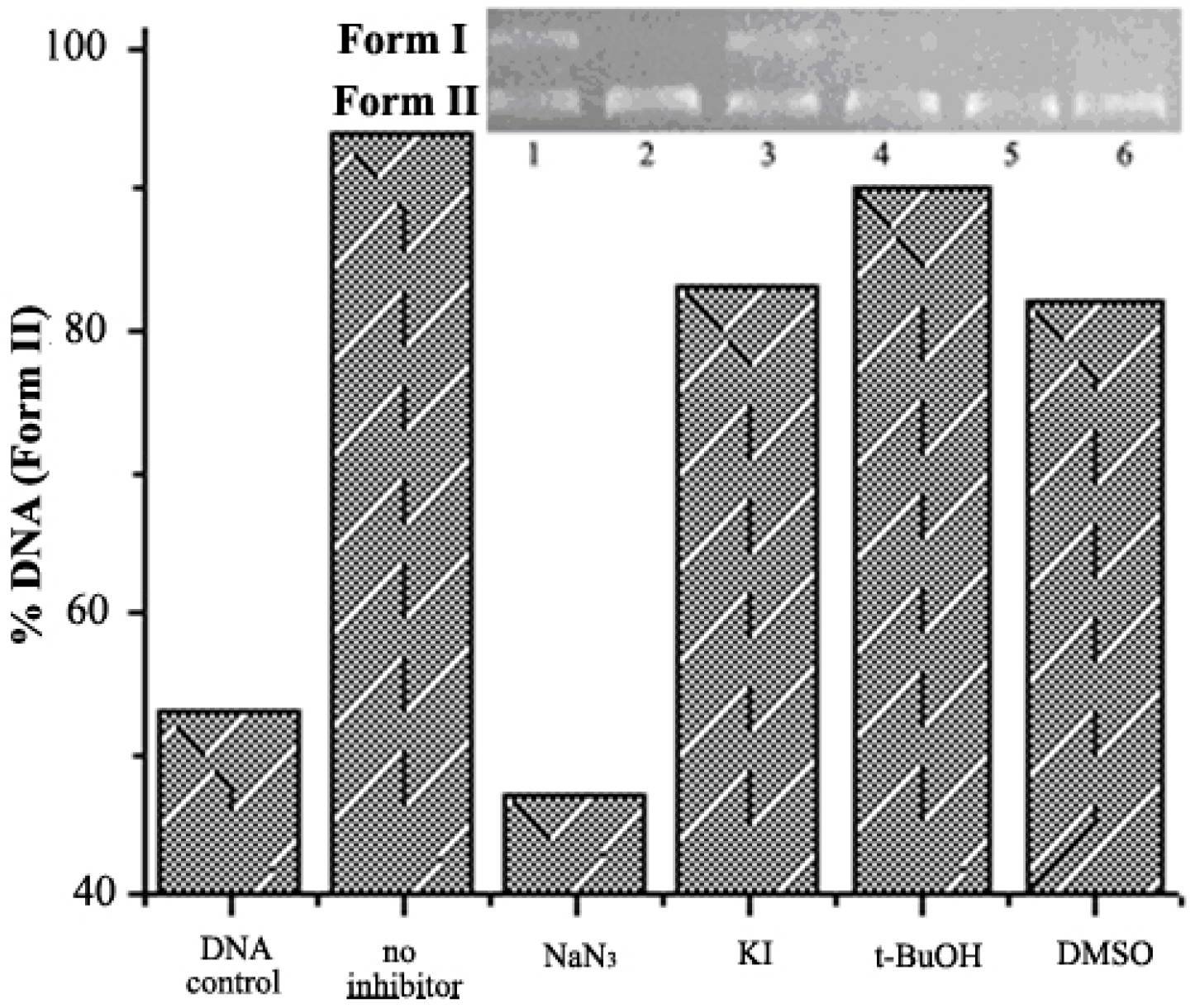
2.4. DNA Cleavage on the Presence of Typical Radical Scavengers
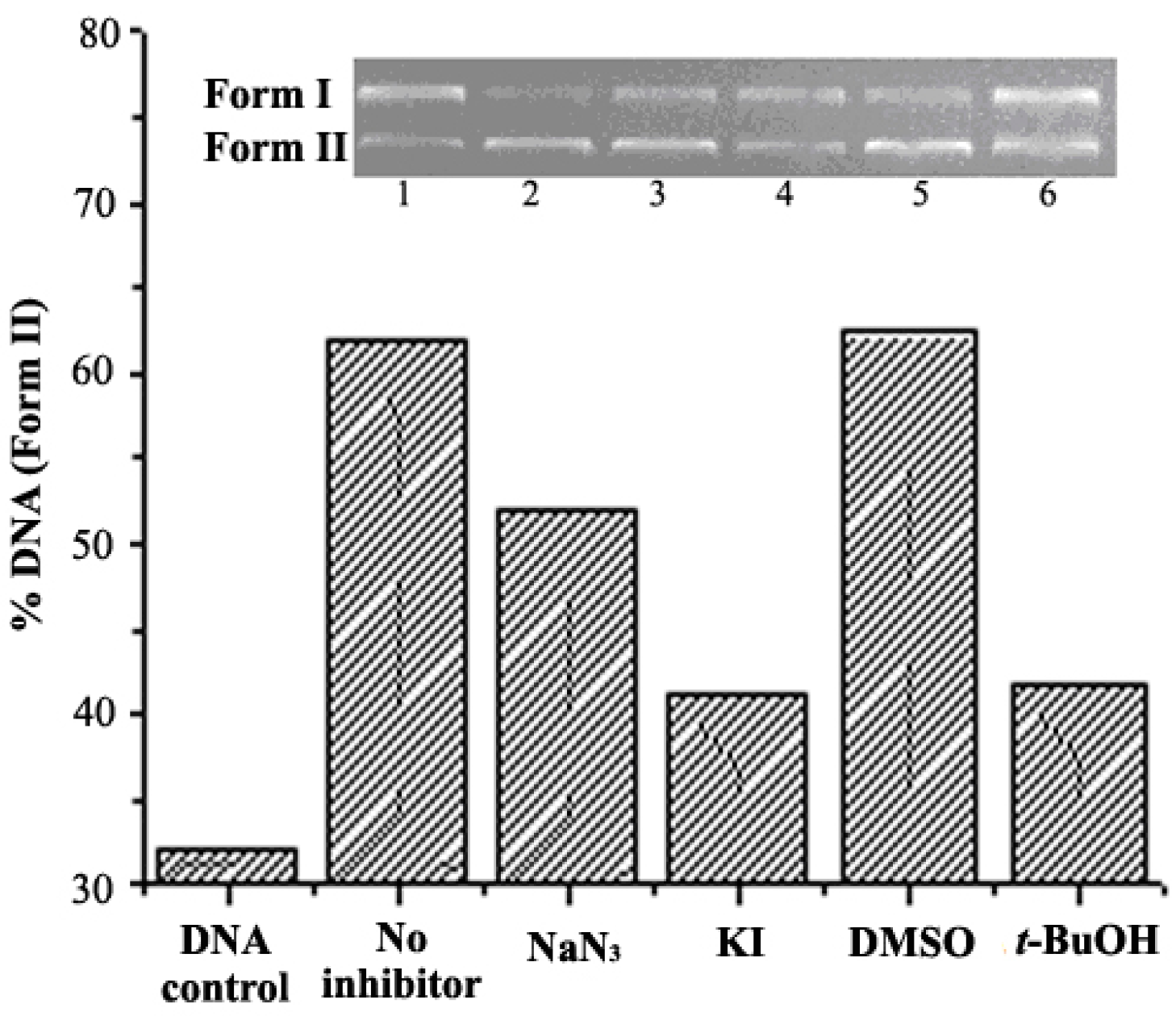
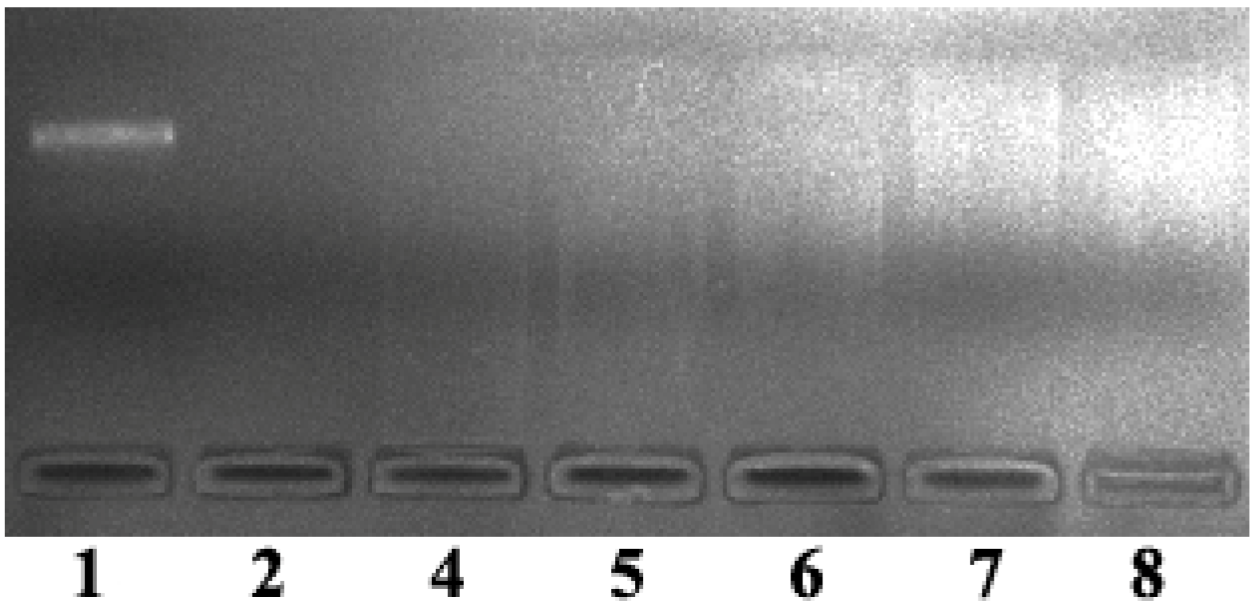
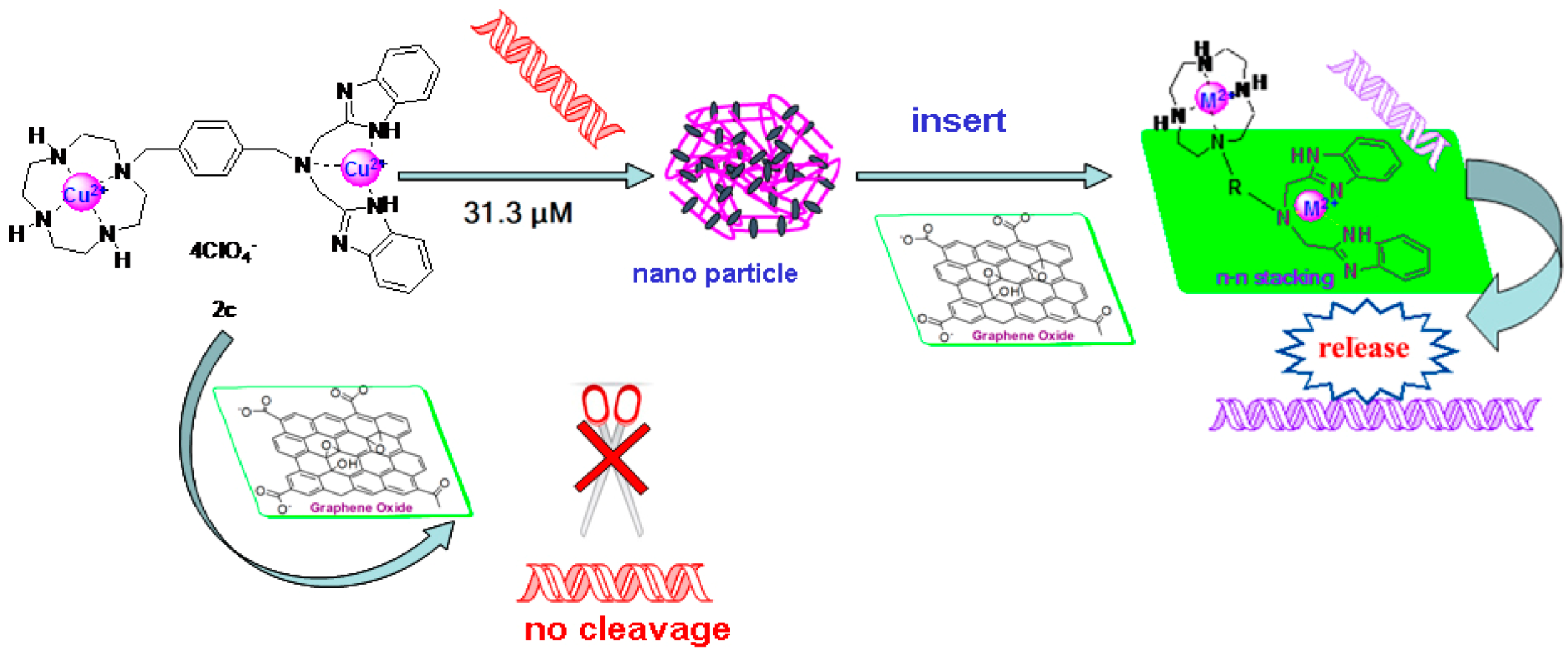
2.5. DNA Condenstion and Regulation by GO
2.6. Fluorescence Spectra of the Interaction between Complex and GO
2.7. Cytotoxicity
3. Experimental Section
3.1. Materials
3.2. Instrumentation
3.3. DNA Cleavage Experiments
3.4. DNA Condensation, Cleavage and Regulation by GO Experiments
3.5. Fluorescent Spectrum Analysis
3.6. Particle Size and ξ Potential Measurements
3.7. Cell Viability Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yu, L.; Li, F.Z.; Wu, J.Y.; Xie, J.Q.; Li, S.J. Development of the aza-crown ether metal complexes as artificial hydrolase. Inorg. Biochem. 2016, 154, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, M.; Zhang, T.; Sun, H. DNA hydrolysis promoted by di- and multi-nuclear metal complexes. Coord. Chem. Rev. 2004, 248, 147–168. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, H.B.; Ji, L.N.; Mao, Z.W. Cheminform abstract: Insights into metalloenzyme microenvironments: Biomimetics metal complexes with a functional second coordination sphere. Chem. Soc. Rev. 2014, 42, 8360–8375. [Google Scholar] [CrossRef] [PubMed]
- Tirel, E.Y.; Bellamy, Z.; Adams, H.; Lebrun, V.; Duarte, F.; Williams, N.H. Catalytic Zinc Complexes for Phosphate Diester Hydrolysis. Angew. Chem. Int. Ed. 2014, 53, 8246–8250. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.M.; Berghot, M.A.; Gouda, M.A. Synthesis and study of some new 1,3-isoindoledione derivatives as potential antibacterial agents. Eur. J. Med. Chem. 2010, 45, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Alp, M.; Göker, H.; Brun, R.; Yıldız, S. Synthesis and antiparasitic and antifungal evaluation of 2′-arylsubstituted-1H, 1′H-[2,5′]bisbenzimidazolyl-5-carboxamidines. Eur. J. Med. Chem. 2009, 44, 2002–2008. [Google Scholar] [CrossRef] [PubMed]
- Jean-François, B.; Frédéric, D.; Jérôme, F.; Jean, L.; Jérôme, G.; Philippe, M.; Laurence, Q.; Eric, A.; Tom, G.; Peggy, J. Selection of a Respiratory Syncytial Virus Fusion Inhibitor Clinical Candidate, Part 1: Improving the Pharmacokinetic Profile Using the Structure-Property Relationship. J. Med. Chem. 2007, 50, 4572–4584. [Google Scholar]
- Yunsong, T.; Bouska, J.J.; Ellis, P.A.; Johnson, E.F.; Joel, L.; Xuesong, L.; Marcotte, P.A.; Olson, A.M.; Osterling, D.J.; Magdalena, P. Synthesis and evaluation of a new generation of orally efficacious benzimidazole-based poly(ADP-ribose) polymerase-1 (PARP-1) inhibitors as anticancer agents. J. Med. Chem. 2009, 52, 6803–6813. [Google Scholar]
- Christine, L.S.; Anne, B.; John, M.; Hendrik, V.D.B.; Mekala, G.; Stephen, N. New mustard-linked 2-aryl-bisbenzimidazoles with antiproliferative activity. Org. Biomol. Chem. 2006, 4, 1305–1312. [Google Scholar]
- Li, S.; Xie, J.Q.; Xiang, Q.X.; Quan, X.J.; Xu, J.Q. Synthesis, characterisation and molecular recognition of novel Zn(II) macrocyclic complexes with imidazole or benzimidazole pendants. J. Chem. Res. 2014, 38, 102–107. [Google Scholar] [CrossRef]
- Widom, J.; Baldwin, R.L. Cation-induced toroidal condensation of DNA☆: Studies with Co3+ (NH3)6. J. Mol. Biol. 1981, 144, 431–453. [Google Scholar] [CrossRef]
- Liang, L.; Hang, Z.; Meng, X.; Yin, J.; Li, D.; Liu, C. Dinuclear metal(II) complexes of polybenzimidazole ligands as carriers for DNA delivery. Biomaterials 2010, 31, 1380–1391. [Google Scholar]
- Jun, Y.; Xianggao, M.; Shibing, Z.; Dan, Z.; Li, W.; Changlin, L. The effect of a nuclear localization sequence on transfection efficacy of genes delivered by cobalt(II)–polybenzimidazole complexes. Biomaterials 2012, 33, 7884–7894. [Google Scholar]
- Liu, G.; Choi, K.Y.; Bhirde, A.; Swierczewska, M.; Yin, J.; Lee, S.W.; Park, J.H.; Hong, J.I.; Xie, J.; Niu, G. Sticky nanoparticles: A platform for siRNA delivery by a bis(zinc(II) dipicolylamine)-functionalized, self-assembled nanoconjugate. Chem. Int. Ed. 2012, 51, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Yu, Z.; Hazeldine, S.T.; Firestine, S.M.; David, O. Cyclam-based polymeric copper chelators for gene delivery and potential PET imaging. Biomacromolecules 2012, 13, 3220–3227. [Google Scholar]
- Choi, K.Y.; Silvestre, O.F.; Huang, X.; Min, K.H.; Howard, G.P.; Hida, N.; Jin, A.J.; Carvajal, N.; Sang, W.L.; Hong, J.I. Versatile RNA interference nanoplatform for systemic delivery of RNAs. ACS Nano 2015, 8, 4559–4570. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.; Hua, T.; Shan, D.; Liu, X.; Xu, P.; Qiao, R.; Zhao, Y. Controllable DNA Condensation-Release Induced by Simple Azaheterocyclic-Based Metal Complexes. J. Phys. Chem. B 2011, 115, 13350–13354. [Google Scholar]
- Huang, X.; Dong, X.; Xue, L.; Meng, X.; Dan, Z.; Liu, C. Metal-polybenzimidazole complexes as a nonviral gene carrier: Effects of the DNA affinity on gene delivery. J. Inorg. Biochem. 2013, 129, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ma, C.; Xu, P.; Gao, Y.; Zhang, J.; Qiao, R.; Zhao, Y. Effective and Reversible DNA Condensation Induced by Simple Cyclic/rigid Polyamine Containing Carbonyl Moiety. J. Phys. Chem. B 2013, 117, 7857–7867. [Google Scholar] [CrossRef] [PubMed]
- Min, S.S.; Kwon, Y.J. Stimuli-responsive polymers and nanomaterials for gene delivery and imaging applications. Adv. Drug Deliv. Rev. 2012, 64, 1046–1059. [Google Scholar]
- Cai, X.; Li, Y.; Yue, D.; Yi, Q.; Li, S.; Shi, D.; Gu, Z. Reversible PEGylation and Schiff-base linkedimidazole modification of polylysine for high-performance gene delivery. J. Mater. Chem. B 2015, 3, 1507–1517. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, C.; Wu, C.; Zhou, X.; Lin, M.; Wu, X.; Xin, X.; Chen, X.; Xu, L.; Liu, H. Nuclease Activity and Cytotoxicity Enhancement of the DNA Intercalators via Graphene Oxide. J. Phys. Chem. C 2015, 116, 15839–15846. [Google Scholar] [CrossRef]
- Ren, H.; Chong, W.; Zhang, J.; Zhou, X.; Xu, D.; Jing, Z.; Guo, S.; Zhang, J. DNA cleavage system of nanosized graphene oxide sheets and copper ions. ACS Nano 2010, 4, 7169–7174. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Ran, N.; Singha, K.; Oh, I.K.; Kim, W. Graphene Oxide-Polyethylenimine Nanoconstruct as a Gene Delivery Vector and Bioimaging Tool. J. Bioconjug. Chem. 2011, 22, 2558–2567. [Google Scholar] [CrossRef] [PubMed]
- Kris, E.; Rolf, E.; Zonghoon, L.; Nasim, A.; Will, G.; Alex, Z. Determination of the Local Chemical Structure of Graphene Oxide and Reduced Graphene Oxide. Adv. Mater. 2010, 22, 4467–4472. [Google Scholar]
- Yu, H.; Lu, Q.S.; Ji, Z.; Zhang, Z.W.; Yu, Z.; Chen, S.Y.; Li, K.; Tan, X.Y.; Lin, H.H.; Yu, X.Q. DNA cleavage by novel copper(II) complex and the role of β-cyclodextrin in promoting cleavage. Bioorg. Med. Chem. 2008, 16, 1103–1110. [Google Scholar]
- Zhao, Y.M.; Zhu, J.H.; He, W.J.; Yang, Z.; Zhu, Y.G.; Li, Y.Z.; Zhang, J.F.; Guo, Z.J. Oxidative DNA Cleavage Promoted by Multinuclear Copper Complexes: Activity Dependence on the Complex Structure. J. Chem. Eur. 2006, 12, 6621–6629. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xie, J.; Feng, F. Copper and zinc complexes of a diaza-crown ether as artificial nucleases for the efficient hydrolytic cleavage of DNA. New J. Chem. 2015, 39, 5654–5660. [Google Scholar] [CrossRef]
- Chitrapriya, N.; Wang, W.; Jang, Y.J.; Kim, S.K.; Kim, J.H. Ligand effect and cooperative role of metal ions on the DNA cleavage efficiency of mono and binuclear Cu(II) macrocyclic ligands complexes. J. Inorg. Biochem. 2014, 140, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.X.; Ji, Z.; Liu, P.Y.; Xia, C.Q.; Zhou, Z.Y.; Xie, R.G.; Yu, X.Q. Dinuclear macrocyclic polyamine zinc(ii) complexes: syntheses, characterization and their interaction with plasmid DNA. J. Inorg. Biochem. 2007, 2, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Young, M.J.; Wahnon, D.; Hynes, R.C.; Chin, J. Reactivity of copper(II) hydroxides and copper(II) alkoxides for cleaving an activated phosphate diester. J. Am. Chem. Soc. 2002, 117, 9441–9447. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.X.; Xiang, Q.X.; Zhang, L.Q.; Zhou, C.H.; Xie, J.Q.; Lan, Y.; Li, F.Z. The synthesis and activities of novel mononuclear or dinuclear cyclen complexes bearing azole pendants as antibacterial and antifungal agents. Eur. J. Med. Chem. 2014, 84, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yu, Z.; Zhang, J.; Zhang, D.W.; Lu, Q.S.; Lu, J.L.; Chen, S.Y.; Lin, H.H.; Yu, X.Q. DNA binding and photocleavage study of novel anthracene-appended macrocyclic polyamines. Org. Biomol. Chem. 2009, 7, 2278–2285. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, J.; Wang, M.Q.; Zhang, D.W.; Lu, Q.S.; Huang, Y.; Lin, H.H.; Yu, X.Q. DNA binding and cleavage activity of macrocyclic polyamines bearing mono- or bis-acridine moieties. Eur. J. Med. Chem. 2010, 45, 5302–5308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Yang, H.J.; Shen, G.X.; Cheng, P.; Zhang, J.Y.; Guo, S.W. Reduction of graphene oxide via L-ascorbic acid. Chem. Commun. 2010, 46, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Zhang, J.; Yang, W.H.; Dai, Z.H.; Zhu, W.; Yu, X.Q. Biodegradable cross-linked poly(amino alcohol esters) based on LMW PEI for gene delivery. Mol. BioSyst. 2011, 7, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1a–1c are available from the authors.








© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Dai, M.; Zhang, C.; Jiang, B.; Xu, J.; Zhou, D.; Gu, Z. DNA Cleavage and Condensation Activities of Mono- and Binuclear Hybrid Complexes and Regulation by Graphene Oxide. Molecules 2016, 21, 920. https://doi.org/10.3390/molecules21070920
Li S, Dai M, Zhang C, Jiang B, Xu J, Zhou D, Gu Z. DNA Cleavage and Condensation Activities of Mono- and Binuclear Hybrid Complexes and Regulation by Graphene Oxide. Molecules. 2016; 21(7):920. https://doi.org/10.3390/molecules21070920
Chicago/Turabian StyleLi, Shuo, Mingxing Dai, Chunping Zhang, Bingying Jiang, Junqiang Xu, Dewen Zhou, and Zhongwei Gu. 2016. "DNA Cleavage and Condensation Activities of Mono- and Binuclear Hybrid Complexes and Regulation by Graphene Oxide" Molecules 21, no. 7: 920. https://doi.org/10.3390/molecules21070920





