In Situ Enzymatically Generated Photoswitchable Oxidase Mimetics and Their Application for Colorimetric Detection of Glucose Oxidase
Abstract
:1. Introduction
2. Results and Discussion
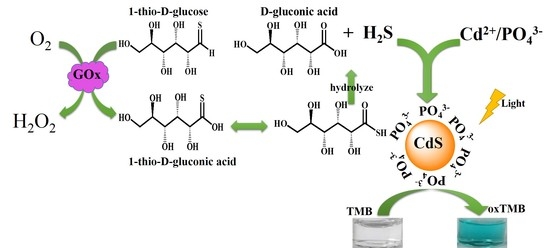
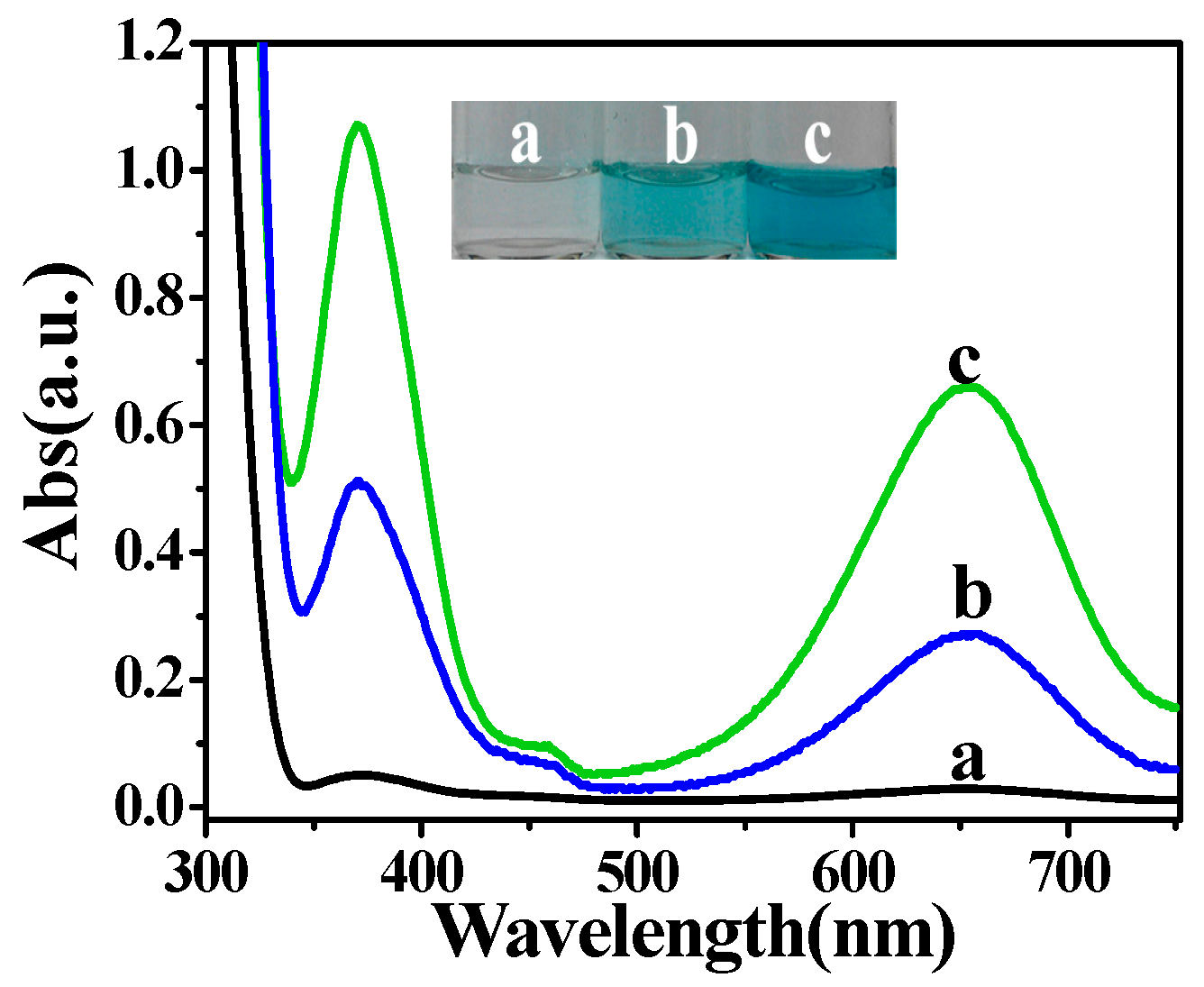
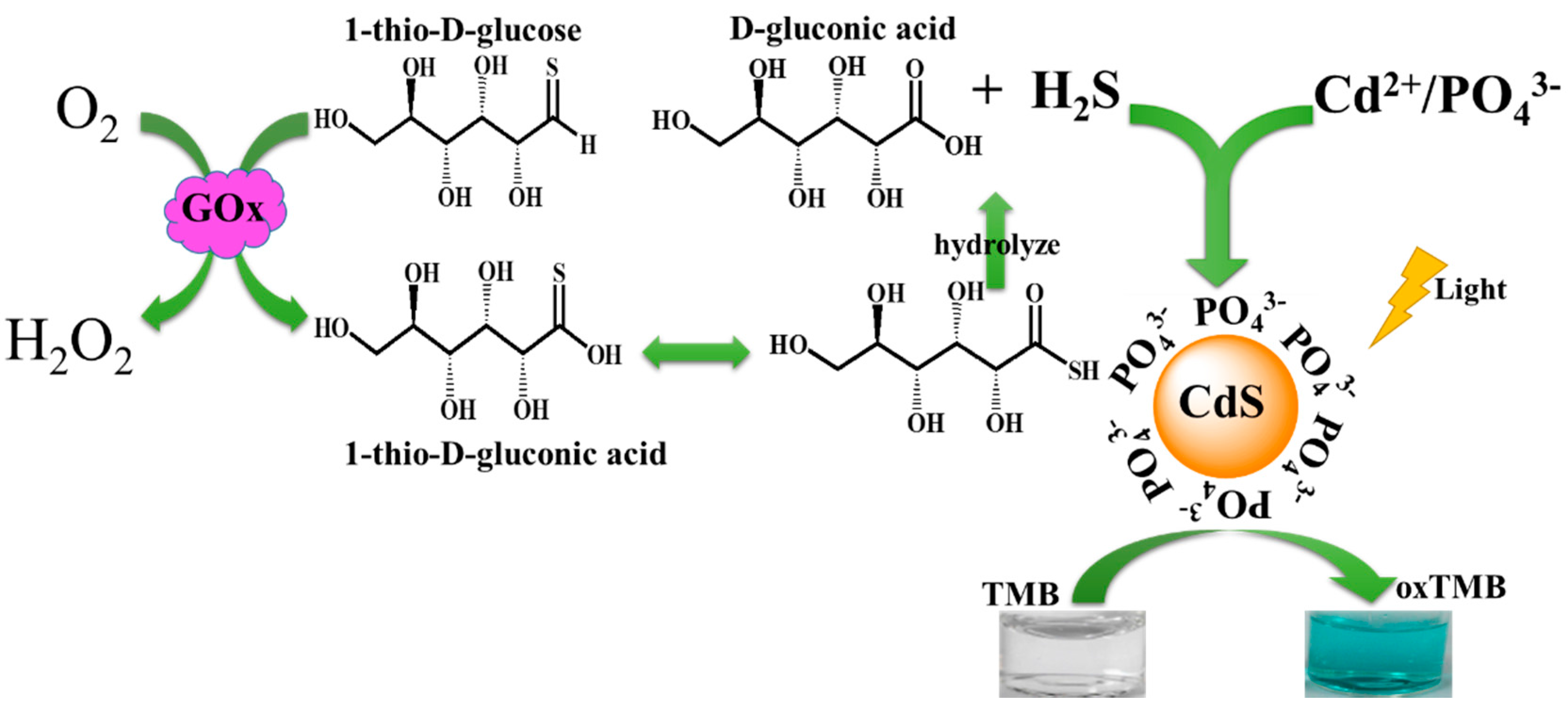
2.1. The Photoswitchable Oxidase Mimetics of PO43−-Capped CdS QDs Generated by GOx-Mediated Biocatalysis
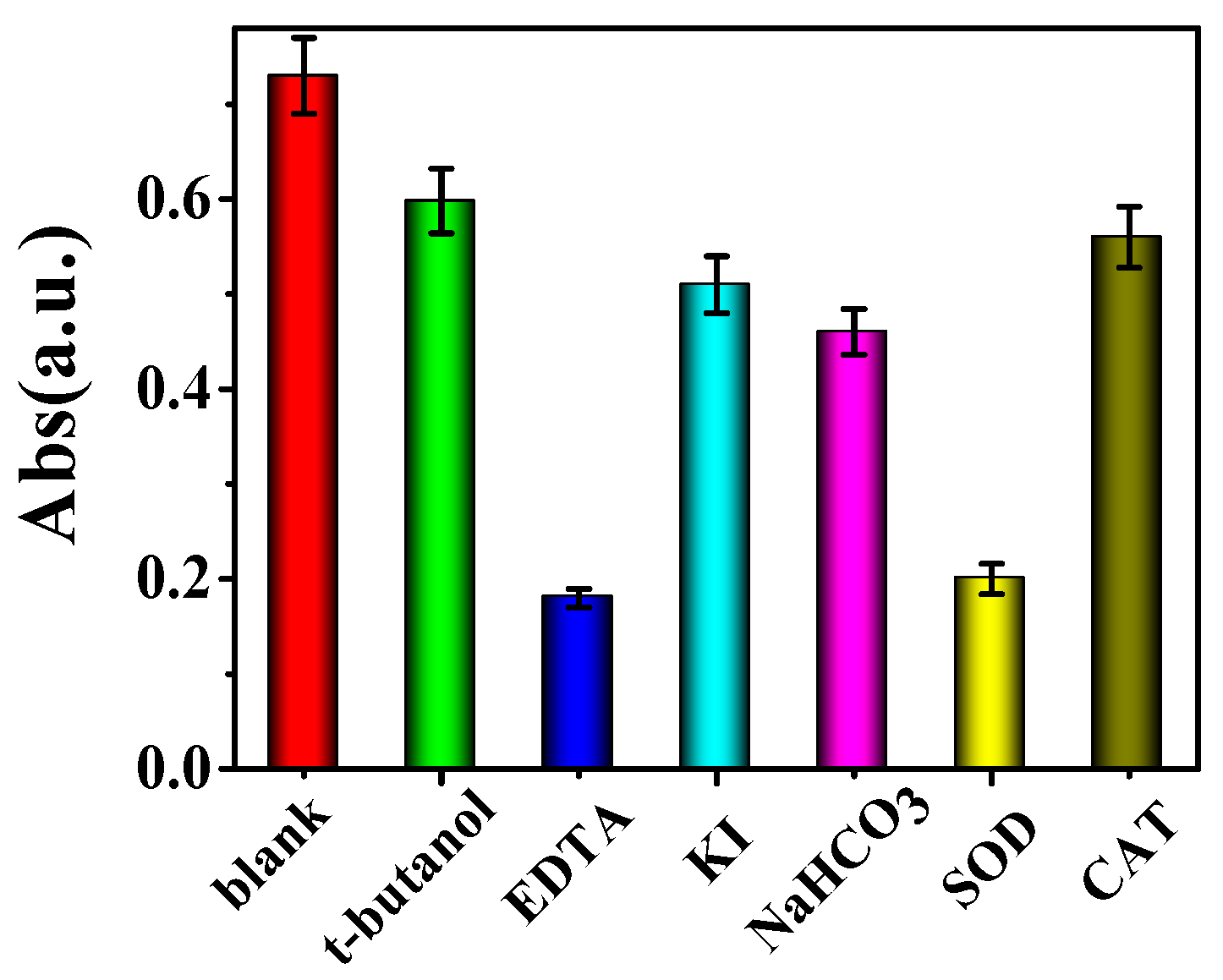
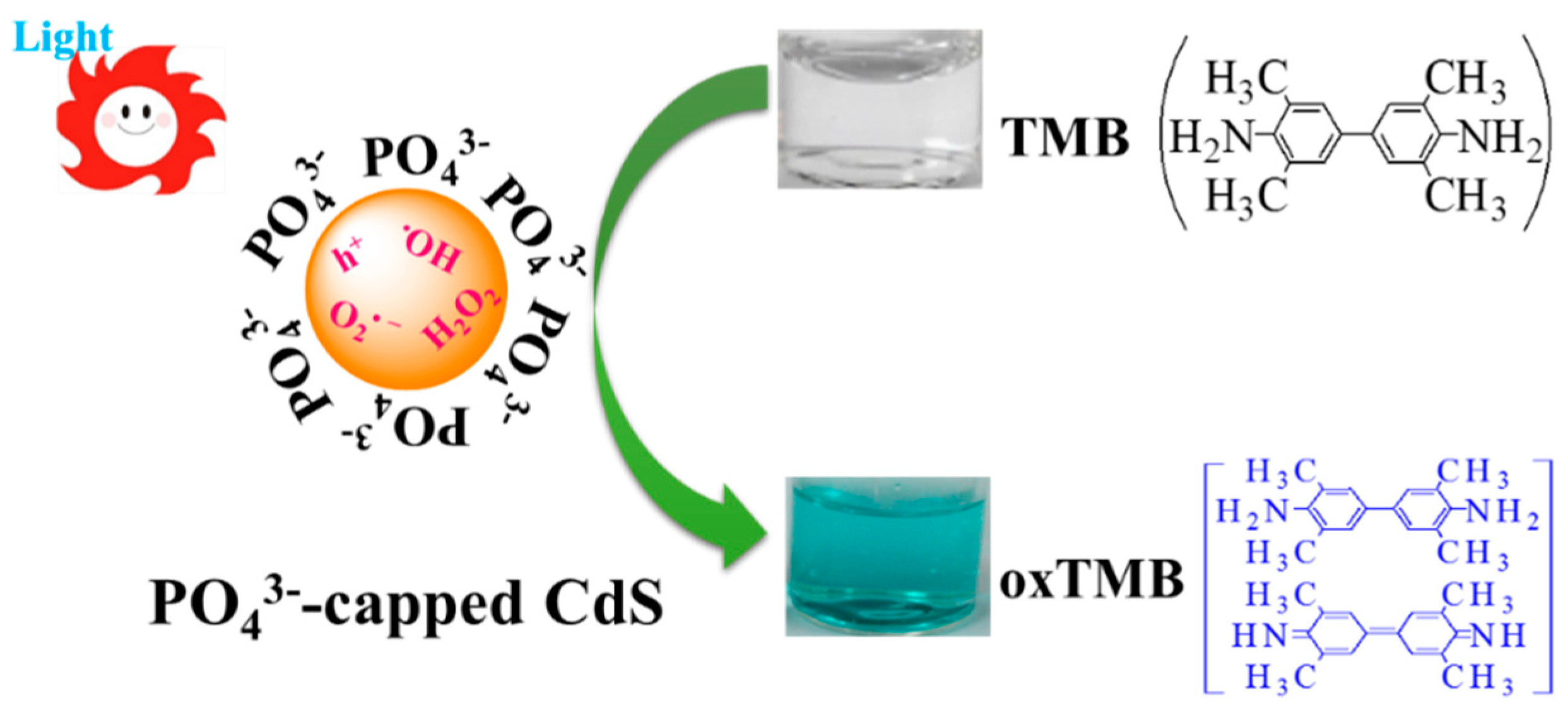
2.2. Mechanism of Photoswitchable Enzyme-Like Activity of PO43−-Capped CdS QDs
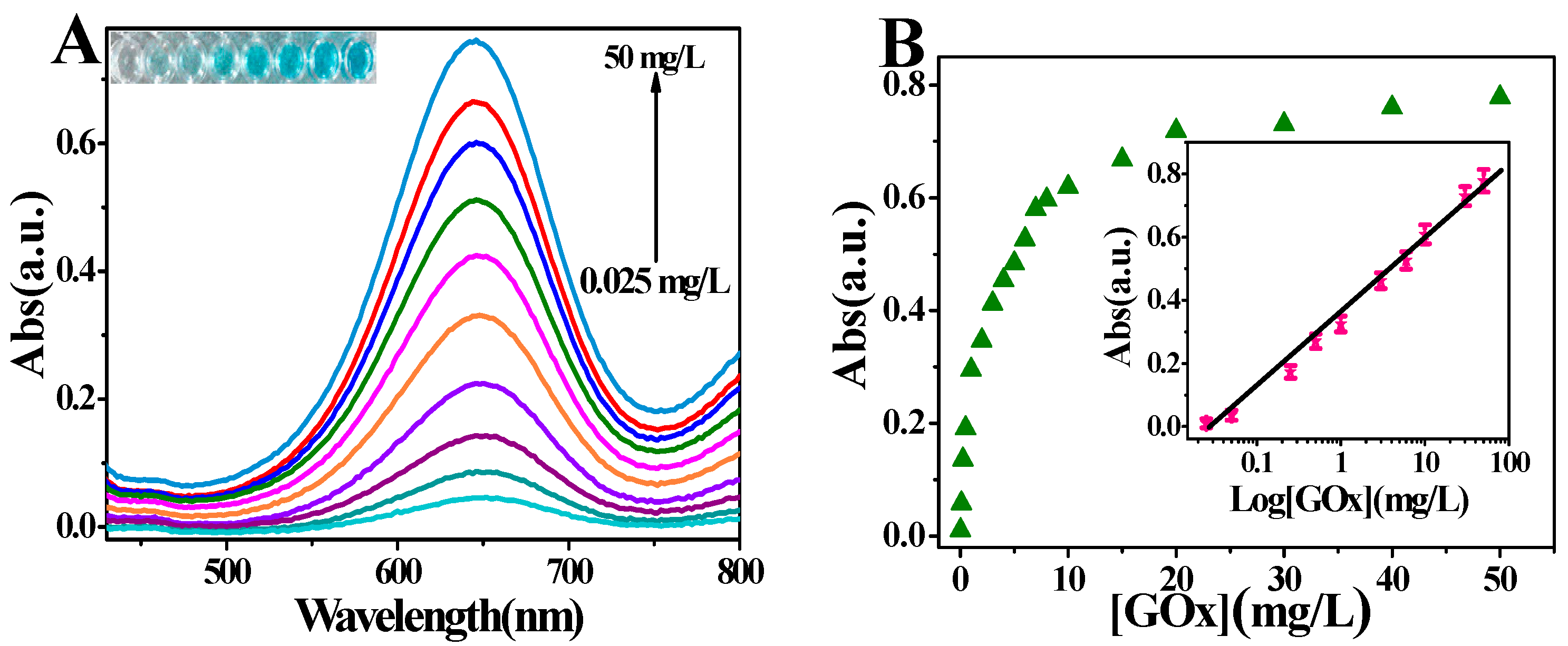
2.3. Probing the Activity of Glucose Oxidase Using PO43−-Capped CdS QDs
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Instrumentation
3.3. The Colorimetric Detection of Glucose Oxidase Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| GOx | glucose oxidase |
| CdS QDs | cadmium sulfide quantum dots |
| TMB | 3,3′,5,5′-tetramethylbenzydine |
| NPs | Nanoparticles |
| h+ | photo-generated holes |
| •OH | hydroxyl radical |
| O2•− | superoxide anion |
| HRP | horseradish peroxidase |
| SOD | superoxide dismutase |
| CAT | Catalase |
| PDDA | poly dimethyl diallyl ammonium chloride |
| TGA | thioglycolic acid |
| CS | Chitosan |
References
- Wei, H.; Wang, E. Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal. Chem. 2008, 80, 2250–2254. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Jv, Y.; Li, B.X.; Cao, R. Positively-charged gold nanoparticles as peroxidiase mimic and their application in hydrogen peroxide and glucose detection. Chem. Commun. 2010, 46, 8017–8019. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wu, X.; Liu, J.; Hu, X.; Zhang, K.; Hou, S.; Xie, S. Design of AgM bimetallic alloy nanostructures (M = Au, Pd, Pt) with tunable morphology and peroxidase-like activity. Chem. Mater. 2010, 22, 2988–2994. [Google Scholar] [CrossRef]
- Mu, J.S.; Wang, Y.; Zhao, M.; Zhang, L. Intrinsic peroxidase-like activity and catalase-like activity of Co3O4 nanoparticles. Chem. Commun. 2012, 48, 2540–2542. [Google Scholar] [CrossRef] [PubMed]
- André, R.; Natálio, F.; Humanes, M.; Leppin, J.; Heinze, K.; Wever, R.; Schröder, H.C.; Müller, W.E.G.; Tremel, W. V2O5 nanowires with an intrinsic peroxidase-like activity. Adv. Funct. Mater. 2011, 21, 501–509. [Google Scholar] [CrossRef]
- Shi, W.B.; Wang, Q.L.; Long, Y.J.; Cheng, Z.L.; Chen, S.H.; Zheng, H.Z. Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem. Commun. 2011, 47, 6695–6697. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, X.; Zhao, C.; Qu, K.; Ren, J.; Qu, X. Label-free colorimetric detection of single nucleotide polymorphism by using single-walled carbon nanotube intrinsic peroxidase-like activity. Chem. Eur. J. 2010, 16, 3617–3621. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.J.; Han, Z.D.; Zhu, J.J. Helical carbon nanotubes: Intrinsic peroxidase catalytic activity and its application for biocatalysis and biosensing. Chem. Eur. J. 2011, 17, 9377–9384. [Google Scholar] [CrossRef] [PubMed]
- Asati, A.; Santra, S.; Kaittanis, C.; Nath, S.; Perez, J.M. Oxidase-like activity of polymer-coated cerium oxide nanoparticles. Angew. Chem. Int. Ed. 2009, 48, 2308–2312. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Wu, Q.; Shan, Z.; Huang, X.M. BSA-stabilized Au clusters as peroxidase mimetics for use in xanthine detection. Biosen. Bioelectron. 2011, 26, 3614–3619. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Jin, L.Y.; Dong, Y.M.; Wu, X.M.; Li, Z.J. Intrinsic enzyme mimicking activity of gold nanoclusters upon visible light triggering and its application for colorimetric trypsin detection. Biosens. Bioelectron. 2015, 64, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, M.; Kros, A.; Lutterman, J.; Nolte, R.; Jansen, J. A percutaneous device as model to study the in vivo performance of implantable amperometric glucose sensors. J. Mater. Sci. Mater. Med. 2001, 12, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Karmali, K.; Karmali, A.; Teixeira, A.; Marcelo Curtob, M.J. Assay for glucose oxidase from aspergillus niger and penicillium amagasakiense by fourier transform infrared spectroscopy. Anal. Biochem. 2004, 333, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Saa, L.; Pavlov, V. Enzymatic growth of quantum dots: Applications to probe glucose oxidase and horseradish peroxidase and sense glucose. Small 2012, 8, 3449–3455. [Google Scholar] [CrossRef] [PubMed]
- Malashikhina, N.; Garai-Ibabe, G.; Pavlov, V. Unconventional application of conventional enzymatic substrate: First fluorogenic immunoassay based on enzymatic formation of quantum dots. Anal. Chem. 2013, 85, 6866–6870. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Sakata, T.; Mori, H.; Yoneyama, H. Preparation of Monodisperse CdS Nanocrystals by size selective photocorrosion. J. Phys. Chem. 1996, 100, 13781–13785. [Google Scholar] [CrossRef]
- Yu, W.W.; Qu, L.H.; Guo, W.Z.; Peng, X.G. Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem. Mater. 2003, 15, 2854–2860. [Google Scholar] [CrossRef]
- Jin, L.Y.; Dong, Y.M.; Wu, X.M.; Cao, G.X.; Wang, G.L. Versatile and amplified biosensing through enzymatic cascade reaction by coupling alkaline phosphatase in situ generation of photoresponsive nanozyme. Anal. Chem. 2015, 87, 10429–10436. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Liu, K.L.; Shu, J.X.; Gu, T.T.; Wu, X.M.; Dong, Y.M.; Li, Z.J. A novel photoelectrochemical sensor based on photocathode of PbS quantum dots utilizing catalase mimetics of bio-bar-coded platinum nanoparticles/G-quadruplex/hemin for signal amplification. Biosen. Bioelectron. 2015, 69, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Xu, X.F.; Qiu, L.; Dong, Y.M.; Li, Z.J.; Zhang, C. Dual responsive enzyme mimicking activity of AgX (X = Cl, Br, I) nanoparticles and its application for cancer cell detection. ACS Appl. Mater. Interfaces 2014, 6, 6434–6442. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Xu, B.; Luo, B.; Lin, H.; Chen, S. Preparation, characterization and visible-light photocatalytic activity of AgI/AgCl/TiO2. Appl. Surf. Sci. 2011, 257, 7083–7089. [Google Scholar] [CrossRef]
- An, C.; Wang, J.; Qin, C.; Jiang, W.; Wang, S.; Li, Y.; Zhang, Q. Synthesis of Ag@AgBr/AgCl heterostructured nanocashews with enhanced photocatalytic performance via anion exchange. J. Mater. Chem. 2012, 22, 13153–13158. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, X.; Chen, K. Iron phosphate microflowers as peroxidase mimic and superoxide dismutase mimic for biocatalysis and biosensing. Chem. Commun. 2012, 48, 7289–7291. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Liu, G.; Zhao, J.; Hidaka, H.; Serpone, N. Evidence for H2O2 generation during the TiO2-assisted photodegradation of dyes in aqueous dispersions under visible light illumination. J. Phys. Chem. B 1999, 103, 4862–4867. [Google Scholar] [CrossRef]
- Marquez, L.A.; Dunford, H.B. Mechanism of the oxidation of 3,5,3′,5′-tetramethylbenzidine by myeloperoxidase determined by transient-and steady-state kinetics. Biochemistry 1997, 36, 9349–9355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Zhou, H.; Wu, P.; Zhang, H.R.; Xu, J.J.; Chen, H.Y. In situ activation of CdS electrochemiluminescence film and its application in H2S detection. Anal. Chem. 2014, 86, 8657–8664. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, X.; Pang, S.; Na, W.D.; Yan, X.; Su, X.G. Fluorescence detection of adenosine-5′-triphosphate and alkaline phosphatase based on the generation of CdS quantum dots. Anal. Chim. Acta 2014, 827, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Okuma, H.; Sekimukai, S.; Hoshi, M.; Toyama, K.; Watanabe, E. Biosensor system for continuous flow determination of enzyme activities. I. Determination of glucose oxidase and lactic dehydrogenase activities. Enzyme Microb. Technol. 1989, 11, 824–829. [Google Scholar] [CrossRef]
- Wu, M.; Lin, Z.; Schäferling, M.; Dürkop, A.; Wolfbeis, O.S. Fluorescence imaging of the activity of glucose oxidase using a hydrogen-peroxide-sensitive europium probe. Anal. Biochem. 2005, 340, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, G.-X.; Wu, X.-M.; Dong, Y.-M.; Li, Z.-J.; Wang, G.-L. In Situ Enzymatically Generated Photoswitchable Oxidase Mimetics and Their Application for Colorimetric Detection of Glucose Oxidase. Molecules 2016, 21, 902. https://doi.org/10.3390/molecules21070902
Cao G-X, Wu X-M, Dong Y-M, Li Z-J, Wang G-L. In Situ Enzymatically Generated Photoswitchable Oxidase Mimetics and Their Application for Colorimetric Detection of Glucose Oxidase. Molecules. 2016; 21(7):902. https://doi.org/10.3390/molecules21070902
Chicago/Turabian StyleCao, Gen-Xia, Xiu-Ming Wu, Yu-Ming Dong, Zai-Jun Li, and Guang-Li Wang. 2016. "In Situ Enzymatically Generated Photoswitchable Oxidase Mimetics and Their Application for Colorimetric Detection of Glucose Oxidase" Molecules 21, no. 7: 902. https://doi.org/10.3390/molecules21070902