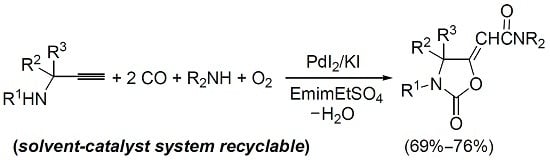
Auto-Tandem Catalysis in Ionic Liquids: Synthesis of 2-Oxazolidinones by Palladium-Catalyzed Oxidative Carbonylation of Propargylic Amines in EmimEtSO4
Abstract
:1. Introduction
2. Result and Discussion
3. Experimental Section
3.1. General Information
3.2. Preparation of Substrates
3.3. Preparation of EmimEtSO4
3.4. General Procedure for the PdI2/KI-Catalyzed Oxidative Carbonylation of Propargylic Amines (1) with CO, O2, and Secondary Amines (2) in EmimEtSO4 and Recycling Experiments
3.5. Characterization of Products
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DME | 1,2-dimethoxyethane |
| EmimEtSO4 | 1-ethyl-3-methylimidazolium ethyl sulfate |
| Et2O | diethyl ether |
| GC | gas chromatograph |
| GC-MS | gas chromatograph/mass spectrometer |
| GLC | gas-liquid chromatography |
| GLC-MS | gas-liquid chromatography/mass spectrometry |
| ILs | Ionic Liquids |
| TLC | thin layer chromatography |
| MS | mass |
| NMR | nuclear magnetic resonance |
| VOCs | Volatile Organic Compounds |
References
- Sun, S.; He, J.; Qu, M.; Li, K. Progress of cooperative catalysis in organic synthesis. Chin. J. Org. Chem. 2015, 35, 1250–1259. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Garcia, H. Cascade reactions catalyzed by metal organic frameworks. ChemSusChem 2014, 7, 2392–2410. [Google Scholar] [CrossRef] [PubMed]
- Filice, M.; Palomo, J.M. Cascade reactions catalyzed by bionanostructures. ACS Catal. 2014, 4, 1588–1598. [Google Scholar] [CrossRef]
- Ball, C.J.; Willis, M.C. Cascade palladium- and copper-catalysed aromatic heterocycle synthesis: The emergence of general precursors. Eur. J. Org. Chem. 2013, 425–441. [Google Scholar] [CrossRef]
- Vlaar, T.; Ruijter, E.; Orru, R.V.A. Recent advances in palladium-catalyzed cascade cyclizations. Adv. Synth. Catal. 2011, 353, 809–841. [Google Scholar] [CrossRef]
- Ambrosini, L.M.; Lambert, T.H. Multicatalysis: Advancing synthetic efficiency and inspiring discovery. ChemCatChem 2010, 2, 1373–1380. [Google Scholar] [CrossRef]
- Grondal, C.; Jeanty, M.; Enders, D. Organocatalytic cascade reactions as a new tool in total synthesis. Nat. Chem. 2010, 2, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J. Recent Advances in Multicatalyst Promoted Asymmetric Tandem Reactions. Chem. Asian J. 2010, 5, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Shindoh, N.; Takemoto, Y.; Takasu, K. Auto-tandem catalysis: A single catalyst activating mechanistically distinct reactions in a single reactor. Chem. Eur. J. 2009, 15, 12168–12179. [Google Scholar] [CrossRef] [PubMed]
- Wender, P.A.; Miller, B.L. Synthesis at the molecular frontier. Nature 2009, 460, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Wheeldon, I.; Minteer, S.D.; Banta, S.; Barton, S.C.; Atanassov, P.; Sigman, M. Substrate channelling as an approach to cascade reactions. Nat. Chem. 2016, 8, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Veltri, L.; Grasso, G.; Rizzi, R.; Mancuso, R.; Gabriele, B. Palladium-catalyzed carbonylative multicomponent synthesis of functionalized benzimidazothiazoles. Asian J. Org. Chem. 2016, 5, 560–567. [Google Scholar] [CrossRef]
- Mancuso, R.; Raut, D.S.; Marino, N.; de Luca, G.; Giordano, C.; Catalano, S.; Barone, I.; Andò, S.; Gabriele, B. A palladium-catalyzed carbonylation approach to eight-membered lactam derivatives with antitumor activity. Chem. Eur. J. 2016, 22, 3053–3064. [Google Scholar] [CrossRef] [PubMed]
- Veltri, L.; Mancuso, R.; Altomare, A.; Gabriele, B. Divergent multicomponent tandem palladium-catalyzed aminocarbonylation-cyclization approaches to functionalized imodazothiazinones and imidazothiazoles. ChemCatChem 2015, 7, 2206–2213. [Google Scholar] [CrossRef]
- Mancuso, R.; Raut, D.S.; Della Ca’, N.; Fini, F.; Carfagna, C.; Gabriele, B. Catalytic oxidative carbonylation of amino moieties to ureas, oxamides, 2-oxazolidinones, and benzoxazolones. ChemSusChem 2015, 8, 2204–2211. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, B.; Veltri, L.; Mancuso, R.; Carfagna, C. Cascade reactions: a multicomponent approach to functionalized indane derivatives by a tandem palladium-catalyzed carbamoylation/carbocyclization process. Adv. Synth. Catal. 2014, 356, 2547–2558. [Google Scholar] [CrossRef]
- Mancuso, R.; Ziccarelli, I.; Armentano, D.; Marino, N.; Giofrè, S.V.; Gabriele, B. Divergent palladium iodide catalyzed multicomponent carbonylative approaches to functionalized isoindolinone and isobenzofuranimine derivatives. J. Org. Chem. 2014, 79, 3506–3518. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, B.; Mancuso, R.; Salerno, G. Oxidative carbonylation as a powerful tool for the direct synthesis of carbonylated heterocycles. Eur. J. Org. Chem. 2012, 6825–6839. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, B.; Backvall, J.-E. Highly selective cascade C–C bond formation via palladium-catalyzed oxidative carbonylation–carbocyclization–carbonylation–alkynylation of enallenes. J. Am. Chem. Soc. 2015, 137, 11868–11871. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, B.; Plastina, P.; Salerno, G.; Mancuso, R.; Costa, M. An unprecedented Pd-catalyzed, water-promoted sequential oxidative aminocarbonylation-cyclocarbonylation process leading to 2-oxazolidinones. Org. Lett. 2007, 9, 3319–3322. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, B.; Salerno, G.; Veltri, L.; Costa, M. Synthesis of 2-ynamides by direct palladium-catalyzed oxidative aminocarbonylation of alk-1-ynes. J. Organomet. Chem. 2001, 622, 84–88. [Google Scholar] [CrossRef]
- Cattaneo, D.; Alffenaar, J.-W.; Neely, M. Drug monitoring and individual dose optimization of antimicrobial drugs: oxazolidinones. Expert Opin. Drug Metab. Toxicol. 2016, 12, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Phillips, O.A.; Sharaf, L.H. Oxazolidinone antimicrobials: A patent review (2012–2015). Expert Opin. Ther. Patents 2016, 26, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Douros, A.; Grabowski, K.; Stahlmann, R. Drug-drug interactions and safety of linezolid, tedizolid, and other oxazolidinones. Expert Opin. Drug Metab. Toxicol. 2015, 11, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Renslo, A.R. Antibacterial oxazolidinones: emerging structure-toxicity relationships. Expert Rev. Anti-Infect. Ther. 2010, 8, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, X. Imidazolium Ionic Liquids, Imidazolylidene Heterocyclic Carbenes, and Zeolitic Imidazolate Frameworks for CO2 Capture and Photochemical Reduction. Angew. Chem. Int. Ed. 2016, 55, 2308–2320. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.A.; Marvey, B.B. Room Temperature Ionic Liquids as Green Solvent Alternatives in the Metathesis of Oleochemical Feedstocks. Molecules 2016, 21, 184. [Google Scholar] [CrossRef] [PubMed]
- Laali, K.K. Ionic liquids as novel media for electrophilic/onium ion chemistry and metal-mediated reactions: A progress summary. Arkivoc 2016. [Google Scholar] [CrossRef]
- Kuchenbuch, A.; Giernoth, R. Ionic Liquids beyond Simple Solvents: Glimpses at the State of the Art in Organic Chemistry. ChemistryOpen 2015, 4, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, A.R.; Rafiee, F. Recent Progress in Ionic Liquids and their Applications in Organic Synthesis. Org. Prep. Proced. Int. 2015, 47, 1–60. [Google Scholar] [CrossRef]
- Holbrey, J.D.; Reichert, W.M.; Swatloski, R.P.; Broker, G.A.; Pitner, W.R.; Seddon, K.R.; Rogers, R.D. Efficient, halide free synthesis of new, low cost ionic liquids: 1,3-Dialkylimidazolium salts containing methyl- and ethyl-sulfate anions. Green Chem. 2002, 4, 407–413. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 3 are available from the authors.

| Entry | 1 | 2 | 3 | Yield of 3 (%) b (Z/E ratio) c | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Run 1 | Run 2 | Run 3 | Run 4 | Run 5 | Run 6 | Run 7 d | ||||
| 1 |  |  |  | 70 (3.1) | 71 (3.2) | 74 (2.9) | 74 (3.4) | 71 (3.2) | 70 (3.4) | 70 (3.2) |
| 2 | 1a |  |  | 74 (3.1) | 75 (3.4) | 73 (3.2) | 75 (3.0) | 74 (3.3) | 73 (3.2) | 74 (3.3) |
| 3 | 1a |  |  | 75 (4.0) | 75 (3.9) | 74 (3.9) | 76 (4.0) | 76 (3.9) | 75 (3.7) | 75 (3.8) |
| 4 | 1a |  |  | 70 (3.1) | 71 (2.9) | 71 (3.1) | 70 (3.4) | 70 (3.4) | 69 (3.0) | 71 (3.1) |
| 5 |  | 2a |  | 75 (2.9) | 75 (3.4) | 74 (3.3) | 75 (3.0) | 76 (3.1) | 74 (3.1) | 74 (3.2) |
| 6 |  | 2a |  | 71 (6.1) | 74 (7.2) | 72 (6.9) | 74 (6.4) | 73 (7.1) | 75 (7.3) | 74 (7.3) |
| 7 |  | 2a |  | 69 (5.3) | 70 (5.4) | 70 (6.0) | 71 (5.5) | 72 (5.0) | 69 (5.9) | 72 (5.6) |
| 8 | 1d | 2d |  | 74 (6.4) e | 76 (5.9) e | 76 (6.0) e | 75 (6.3) e | 74 (6.2) e | 75 (6.1) e | 74 (6.0) e |
| 9 |  | 2a |  | 69 (4.3) | 72 (4.5) | 71 (4.5) | 71 (4.9) | 72 (4.5) | 72 (4.5) | 70 (4.8) |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancuso, R.; Maner, A.; Ziccarelli, I.; Pomelli, C.; Chiappe, C.; Della Ca’, N.; Veltri, L.; Gabriele, B. Auto-Tandem Catalysis in Ionic Liquids: Synthesis of 2-Oxazolidinones by Palladium-Catalyzed Oxidative Carbonylation of Propargylic Amines in EmimEtSO4. Molecules 2016, 21, 897. https://doi.org/10.3390/molecules21070897
Mancuso R, Maner A, Ziccarelli I, Pomelli C, Chiappe C, Della Ca’ N, Veltri L, Gabriele B. Auto-Tandem Catalysis in Ionic Liquids: Synthesis of 2-Oxazolidinones by Palladium-Catalyzed Oxidative Carbonylation of Propargylic Amines in EmimEtSO4. Molecules. 2016; 21(7):897. https://doi.org/10.3390/molecules21070897
Chicago/Turabian StyleMancuso, Raffaella, Asif Maner, Ida Ziccarelli, Christian Pomelli, Cinzia Chiappe, Nicola Della Ca’, Lucia Veltri, and Bartolo Gabriele. 2016. "Auto-Tandem Catalysis in Ionic Liquids: Synthesis of 2-Oxazolidinones by Palladium-Catalyzed Oxidative Carbonylation of Propargylic Amines in EmimEtSO4" Molecules 21, no. 7: 897. https://doi.org/10.3390/molecules21070897