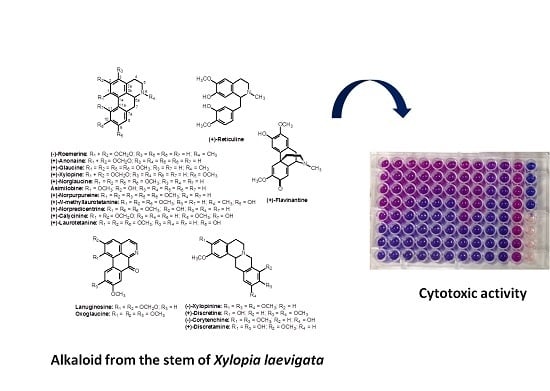
Cytotoxic Alkaloids from the Stem of Xylopia laevigata
Abstract
:1. Introduction
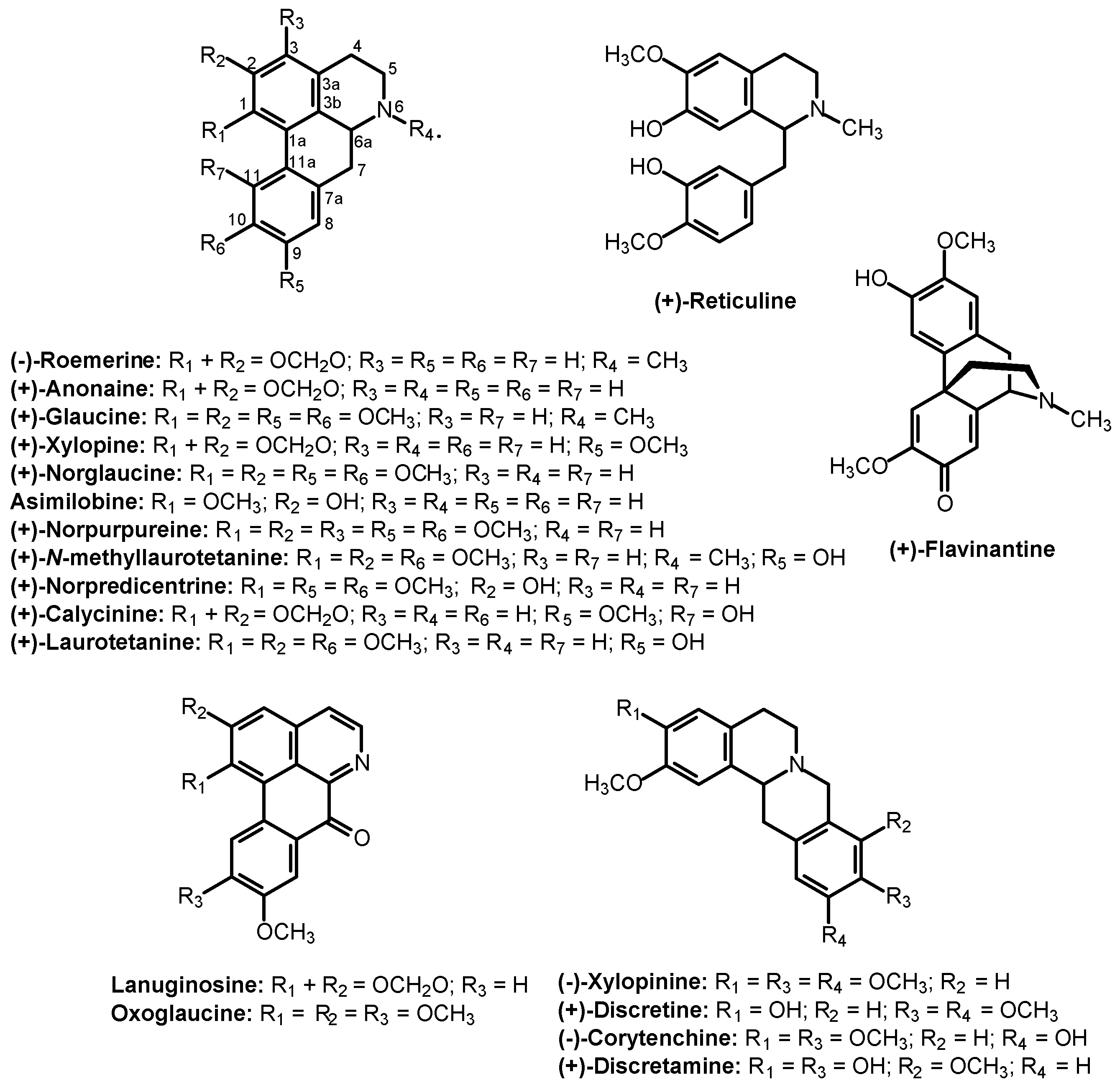
2. Results and Discussion
3. Experimental Section
3.1. Botanical Material
3.2. Phytochemical Analyses
3.2.1. General Procedures
3.2.2. Extraction and Isolation
3.3. Biological Evaluation
3.3.1. Cells
3.3.2. In Vitro Cytotoxic Activity Assay
3.3.3. Morphological Analysis with May-Grünwald-Giemsa Staining
3.3.4. Morphological Analysis with Acridine Orange/Ethidium Bromide Staining
3.3.5. Annexin Assay
3.3.6. Internucleosomal DNA Fragmentation and Cell Cycle Distribution
3.3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Quintans, J.S.S.; Soares, B.M.; Ferraz, R.P.C.; Oliveira, A.C.A.; Silva, T.B.; Menezes, L.R.A.; Sampaio, M.F.C.; Prata, A.P.N.; Moraes, M.O.; Pessoa, C.; et al. Chemical constituents and anticancer effects of the essential oil from leaves of Xylopia laevigata. Planta Med. 2013, 79, 123–130. [Google Scholar]
- Silva, D.M.; Costa, E.V.; Nogueira, P.C.L.; Moraes, V.R.S.; Cavalcanti, S.C.H.; Salvador, M.J.; Ribeiro, L.H.G.; Gadelha, F.R.; Barison, A.; Ferreira, A.G. Ent.-kaurane diterpenoids and other constituents from the stem of Xylopia laevigata (Annonaceae). Quim. Nova 2012, 35, 1570–1576. [Google Scholar] [CrossRef]
- Costa, E.V.; Silva, T.B.; Menezes, L.R.A.; Ribeiro, L.H.G.; Gadelha, F.R.; Carvalho, J.E.; Souza, L.M.B.; Silva, M.A.N.; Siqueira, C.A.T.; Salvador, M.J. Biological activities of the essential oil from the leaves of Xylopia laevigata (Annonaceae). J. Essent. Oil Res. 2013, 25, 179–185. [Google Scholar] [CrossRef]
- Da Silva, T.B.; Menezes, L.R.A.; Sampaio, M.F.C.; Meira, C.S.; Guimaraes, E.T.; Soares, M.B.P.; Prata, A.P.N.; Nogueira, P.C.L.; Costa, E.V. Chemical composition and anti-Trypanosoma cruzi activity of essential oils obtained from leaves of Xylopia frutescens and X. laevigata (Annonaceae). Nat. Prod. Commun. 2013, 8, 403–406. [Google Scholar] [PubMed]
- Queiroz, J.C.C.; Antoniolli, A.R.; Quintans-Júnior, L.J.; Brito, R.G.; Barreto, R.S.S.; Costa, E.V.; da Silva, T.B.; Prata, A.N.P.; de Lucca Junior, W.; Almeida, J.R.G.S.; et al. Evaluation of the anti-Inflammatory and antinociceptive effects of the essential oil from leaves of Xylopia laevigata in experimental models. Sci. World J. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.V.; da Silva, T.B.; Costa, C.O.; Soares, M.B.; Bezerra, D.P. Chemical composition of the essential oil from the fresh fruits of Xylopia laevigata and its cytotoxic evaluation. Nat. Prod. Commun. 2016, 11, 417–418. [Google Scholar] [PubMed]
- Costa, E.V.; Dutra, L.M.; Nepel, A.; Barison, A. Isoquinoline alkaloids from the leaves of Xylopia laevigata (Annonaceae). Biochem. Syst. Ecol. 2013, 51, 331–334. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, M.; Wang, D.; Duan, W.; Wang, X.; Zheng, C. Preparative separation of alkaloids from Nelumbo nucifera leaves by conventional and pH-zone-refining counter-current chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.V.; Sampaio, M.F.C.; Salvador, M.J.S.; Nepel, A.; Barison, A. Chemical constituents from the stem bark of Annona pickelii (Annonaceae). Quim. Nova 2015, 38, 769–776. [Google Scholar]
- Da Silva, F.M.; De Souza, A.D.; Koolen, H.H.; Barison, A.; Vendramin, M.E.; Costa, E.V.; Ferreira, A.G.; Pinheiro, M.L. Phytochemical study of the alkaloidal fractions of Unonopsis duckei R.E. Fr. guided by electrospray ionisation ion-trap tandem mass spectrometry. Phytochem. Anal. 2014, 25, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.S.; Tavares, J.F.; Queiroga, K.F.; Agra, M.F.; Barbosa-Filho, J.M.; Almeida, J.R.G.S.; da Silva, S.A.S. Alcaloides e outros constituintes de Xylopia langsdorffiana (Annonaceae). Quim. Nova 2009, 3, 1566–1570. [Google Scholar] [CrossRef]
- Chiou, C.M.; Lin, C.T.; Huang, W.J.; Chang, Y.M.; Ho, Y.J.; Su, M.J.; Lee, S.S. Semisynthesis and myocardial activity of thaliporphine N-homologues. J. Nat. Prod. 2013, 76, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Stermitz, F.R.; Castro, O. Pentasubstituted aporphine alkaloids from Phoebe molicella. J. Nat. Prod. 1983, 46, 913–916. [Google Scholar] [CrossRef]
- Zanin, S.M.W.; Lordello, A.L.L. Alcalóides aporfinóides do gênero Ocotea (Lauraceae). Quim. Nova 2007, 30, 92–98. [Google Scholar] [CrossRef]
- Rasoanaivo, P.; Ratsimamanga-Urverg, S.; Rafatro, H.; Ramanitrahasimbola, D.; Palazzino, G.; Galeffi, C.; Nicoletti, M. Alkaloids of Hernandia voyronni: Chloroquine-potentiating activity and structure elucidation of Herveline D. Planta Med. 1998, 64, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Navarro, R.V.; Sette, I.M.F.; Da-Cunha, E.V.L.; Silva, M.S.; Barbosa-Filho, J.M.; Maia, J.G.S. Alcaloides de Duguettia flagellaris Huber (Annonaceae). Rev. Bras. Plantas Med. 2001, 3, 23–29. [Google Scholar]
- De Siqueira, J.M.; Ziminiani, M.G.; Resende, U.M.; Boaventura, M.A.D. Estudo fitoquímico das cascas do caule de Duguetia glabriuscula–Annonaceae, biomonitorado pelo ensaio de toxicidade frente a Artemia salina Leach. Quim. Nova 2001, 24, 185–187. [Google Scholar] [CrossRef]
- Chen, Z.F.; Shi, Y.F.; Liu, Y.C.; Hong, X.; Geng, B.; Peng, Y.; Liang, H. TCM active ingredient oxoglaucine metal complexes: Crystal structure, cytotoxicity, and interaction with DNA. Inorg. Chem. 2012, 51, 1998–2009. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz, P.E.O.; Costa, E.V.; Moraes, V.R.S.; Nogueira, P.C.L.; Vendramin, M.E.; Barison, A.; Ferreira, A.G.; Prata, A.P.N. Chemical constituents from the leaves of Annona pickelii (Annonaceae). Biochem. Syst. Ecol. 2012, 41, 115–118. [Google Scholar]
- Gozler, B.; Ozic, P.; Freyer, A.J.; Shamma, M. Morphinandienone alkaloids from Roemeria refracta. J. Nat. Prod. 1990, 53, 986–988. [Google Scholar] [CrossRef]
- Suffness, M.; Pezzuto, J.M. Assays related to cancer drug discovery. In Methods in Plant Biochemistry: Assays for Bioactivity; Hostettmann, K., Ed.; Academic Press: London, UK, 1990; Volume 6, pp. 71–133. [Google Scholar]
- Chiu, C.C.; Chou, H.L.; Wu, P.F.; Chen, H.L.; Wang, H.M.; Chen, C.Y. Bio-functional constituents from the stems of Liriodendron tulipifera. Molecules 2012, 17, 4357–4372. [Google Scholar] [PubMed]
- Ahmed, S.A.; Gogal, R.M.; Walsh, J.E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H] thymidine incorporation assay. J. Immunol. Methods 1994, 170, 211–224. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the isolated alkaloids are unavailable.

| Alkaloids | IC50 in μg/mL (µM) | ||||
|---|---|---|---|---|---|
| B16-F10 | HepG2 | HL60 | K562 | PBMC | |
| (−)-Roemerine | NA | NA | NA | NA | NA |
| (+)-Anonaine | 18.80 (70.66) | 14.04 (52.77) | 10.09 (37.92) | 10.62 (39.91) | NA |
| 15.53–22.76 | 12.19–16.16 | 7.94–12.82 | 8.96–12.58 | ||
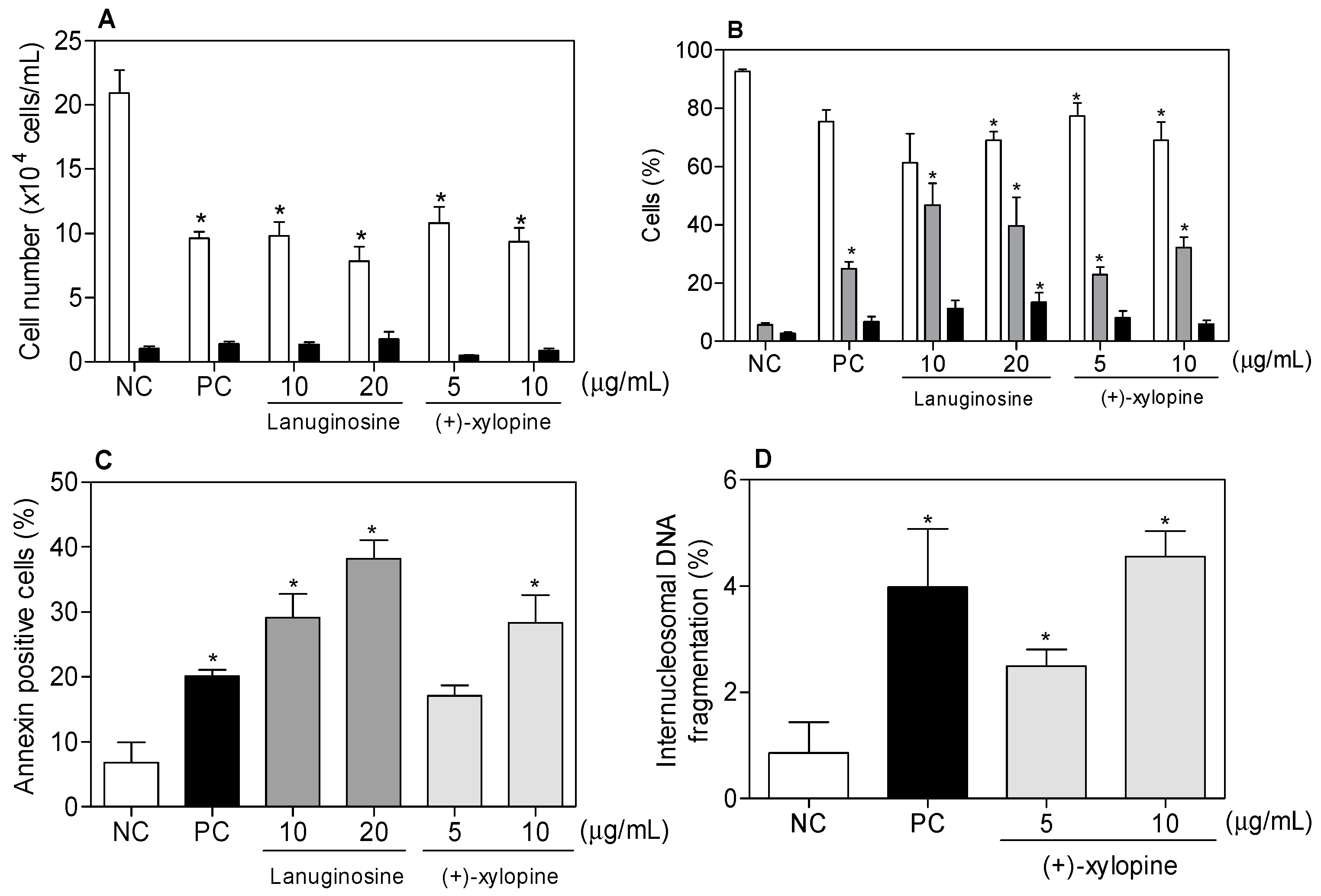
| Lanuginosine | 8.46 (27.63) | 3.89 (12.70) | 7.81 (25.51) | 6.61 (21.59) | 24.53 (80.11) |
| 7.40–9.68 | 3.23–4.69 | 7.32–8.33 | 5.69–7.68 | 16.98–35.43 | |
| (+)-Glaucine | NA | NA | NA | NA | NA |
| (+)-Xylopine | 3.77 (12.73) | 1.87 (6.31) | 1.87 (6.31) | 3.12 (10.53) | 4.08 (13.77) |
| 3.39–4.19 | 1.56–2.23 | 1.67–2.10 | 2.85–3.41 | 2.17–7.66 | |
| Oxoglaucine | 19.14 (54.36) | NA | 5.90 (16.76) | 12.48 (35.45) | 10.25 (29.11) |
| 15.19–24.11 | 4.09–8.52 | 8.84–17.61 | 7.96–13.20 | ||
| (+)-Norglaucine | 8.48 (24.77) | 3.78 (11.04) | 6.84 (19.98) | 7.84 (22.90) | 6.70 (19.57) |
| 7.62–9.44 | 3.11–4.61 | 6.25–7.48 | 6.78–9.06 | 3.74–12.00 | |
| (−)-Xylopinine | NA | NA | NA | NA | NA |
| (+)-Norpurpureine | 21.08 (56.61) | NA | 10.11 (27.15) | 16.72 (44.90) | 17.94 (48.18) |
| 16.74–26.53 | 5.75–17.81 | 11.73–23.84 | 13.23–24.33 | ||
| (+)-N-Methyllaurotetanine | NA | NA | NA | NA | NA |
| (+)-Norpredicentrine | NA | NA | NA | NA | NA |
| (+)-Discretine | 16.15 (47.20) | 7.89 (23.06) | 12.97 (37.91) | 14.85 (43.40) | NA |
| 14.35–18.17 | 5.83–10.68 | 10.75–15.66 | 11.89–18.54 | ||
| (+)-Calycinine | 22.17 (71.03) | NA | 18.59 (59.56) | NA | NA |
| 12.67–38.81 | 13.45–25.69 | ||||
| (+)-Laurotetanine | NA | NA | NA | NA | NA |
| (+)-Reticuline | NA | 15.35 (46.47) | 23.81 (72.09) | NA | NA |
| 12.45–18.92 | 21.44–26.45 | ||||
| (−)-Corytenchine | NA | NA | NA | NA | NA |
| (+)-Discretamine | 18.80 (57.29) | 14.04 (42.79) | 10.09 (30.75) | 10.62 (32.36) | NA |
| 15.53–22.76 | 12.19–16.16 | 7.94–12.82 | 8.96–12.58 | ||
| (+)-Flavinantine | NA | NA | NA | NA | NA |
| Doxorubicin | 0.08 (0.15) | 0.08 (0.15) | 0.09 (0.17) | 0.15 (0.28) | 2.47 (4.54) |
| 0.05–0.14 | 0.06–0.10 | 0.06–0.12 | 0.08–0.31 | 1.80–3.39 | |
| Drug | Concentration (µg/mL) | Cell Cycle Phases (%) | ||
|---|---|---|---|---|
| G1 | S | G2/M | ||
| NC | - | 51.50 ± 4.02 | 16.11 ± 1.89 | 19.93 ± 6.12 |
| PC | 1 | 21.44 ± 4.07 * | 12.00 ± 1.43 * | 61.94 ± 5.52 * |
| (+)-xylopine | 5 | 28.05 ± 3.15 * | 10.80 ± 1.50 * | 64.16 ± 2.56 * |
| 10 | 20.43 ± 3.06 * | 11.56 ± 1,78 * | 39.96 ± 9.59 * | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menezes, L.R.A.; Costa, C.O.D.; Rodrigues, A.C.B.d.C.; Santo, F.R.d.E.; Nepel, A.; Dutra, L.M.; Silva, F.M.A.; Soares, M.B.P.; Barison, A.; Costa, E.V.; et al. Cytotoxic Alkaloids from the Stem of Xylopia laevigata. Molecules 2016, 21, 890. https://doi.org/10.3390/molecules21070890
Menezes LRA, Costa COD, Rodrigues ACBdC, Santo FRdE, Nepel A, Dutra LM, Silva FMA, Soares MBP, Barison A, Costa EV, et al. Cytotoxic Alkaloids from the Stem of Xylopia laevigata. Molecules. 2016; 21(7):890. https://doi.org/10.3390/molecules21070890
Chicago/Turabian StyleMenezes, Leociley R. A., Cinara O. D´Sousa Costa, Ana Carolina B. da C. Rodrigues, Felipe R. do E. Santo, Angelita Nepel, Lívia M. Dutra, Felipe M. A. Silva, Milena B. P. Soares, Andersson Barison, Emmanoel V. Costa, and et al. 2016. "Cytotoxic Alkaloids from the Stem of Xylopia laevigata" Molecules 21, no. 7: 890. https://doi.org/10.3390/molecules21070890