Alendronate-Loaded Modified Drug Delivery Lipid Particles Intended for Improved Oral and Topical Administration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Alendronate-Loaded Solid Lipid Particles
2.2.2. Characterization of AL-loaded SLPs
SLPs Polydispersity Index and Zeta Potential
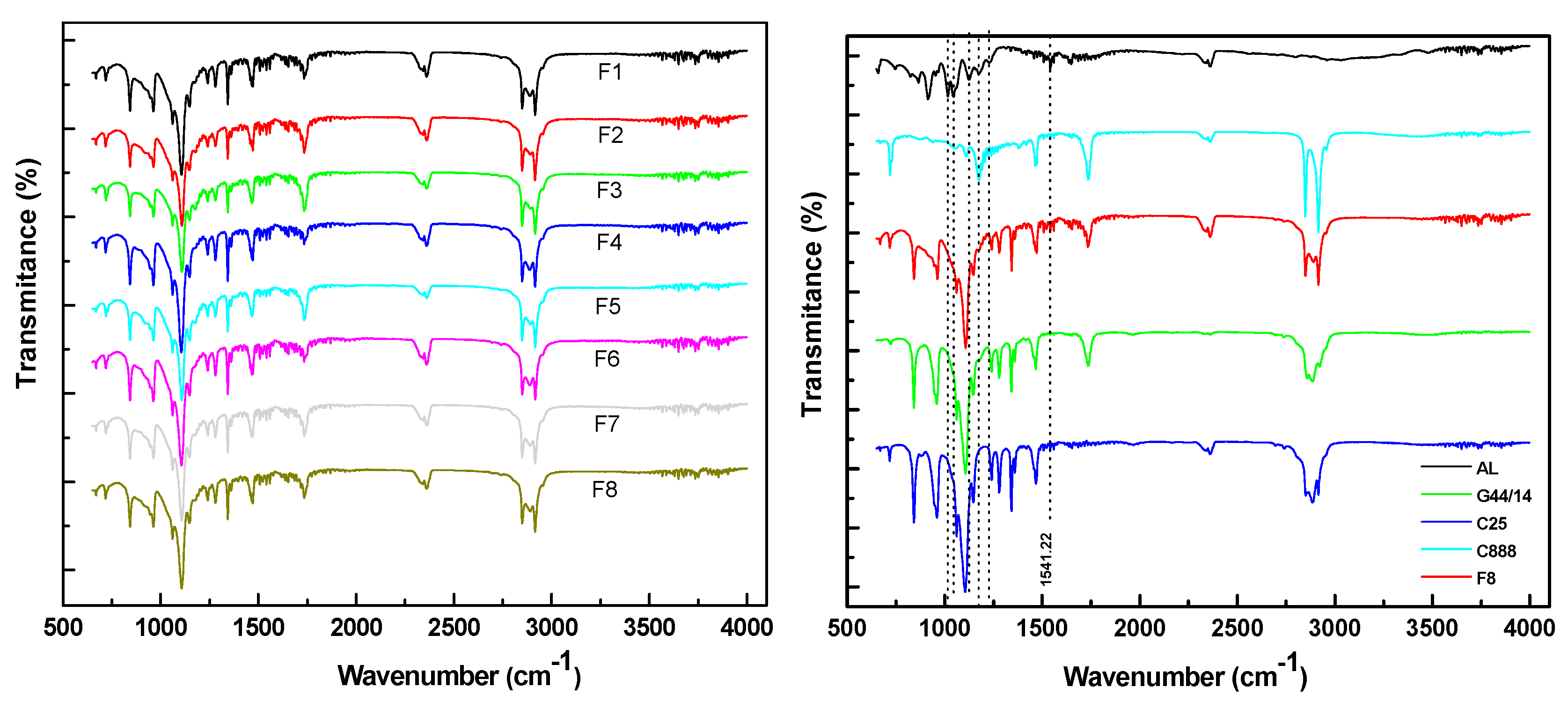
Fourier Transform Infrared Spectroscopy (FTIR)
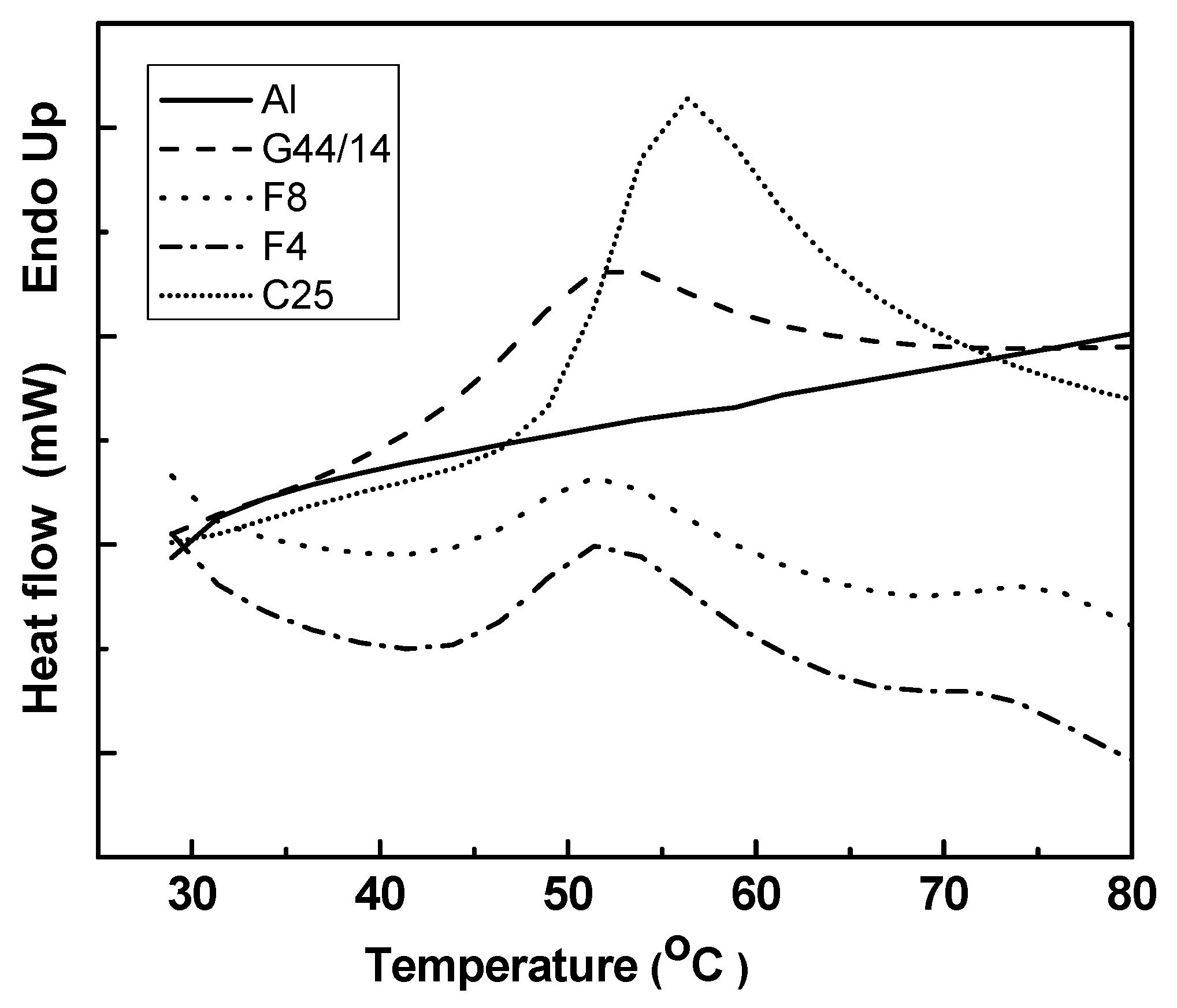
Differential Scanning Calorimetriy (DSC)
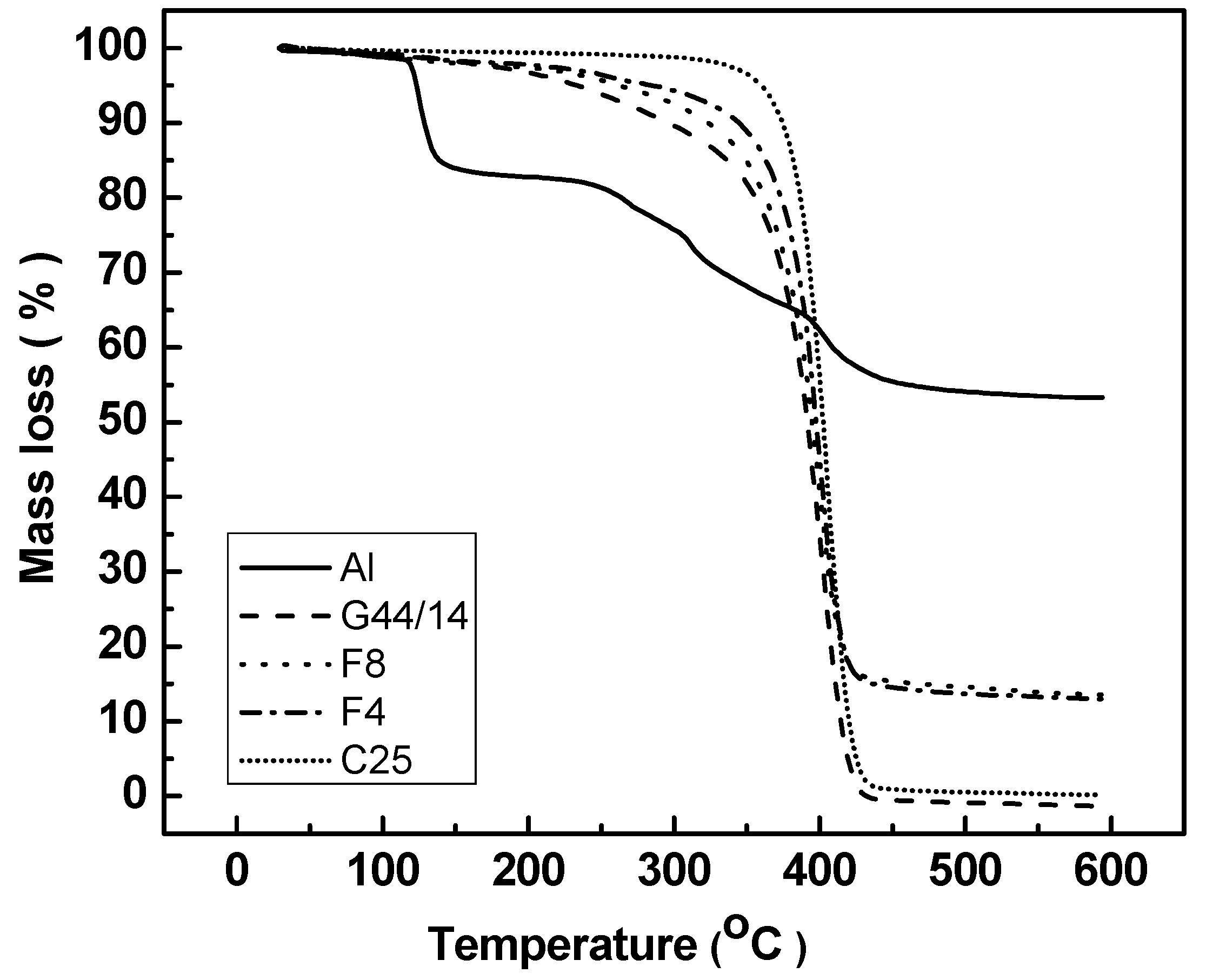
Thermogravimetric Analysis (TGA)
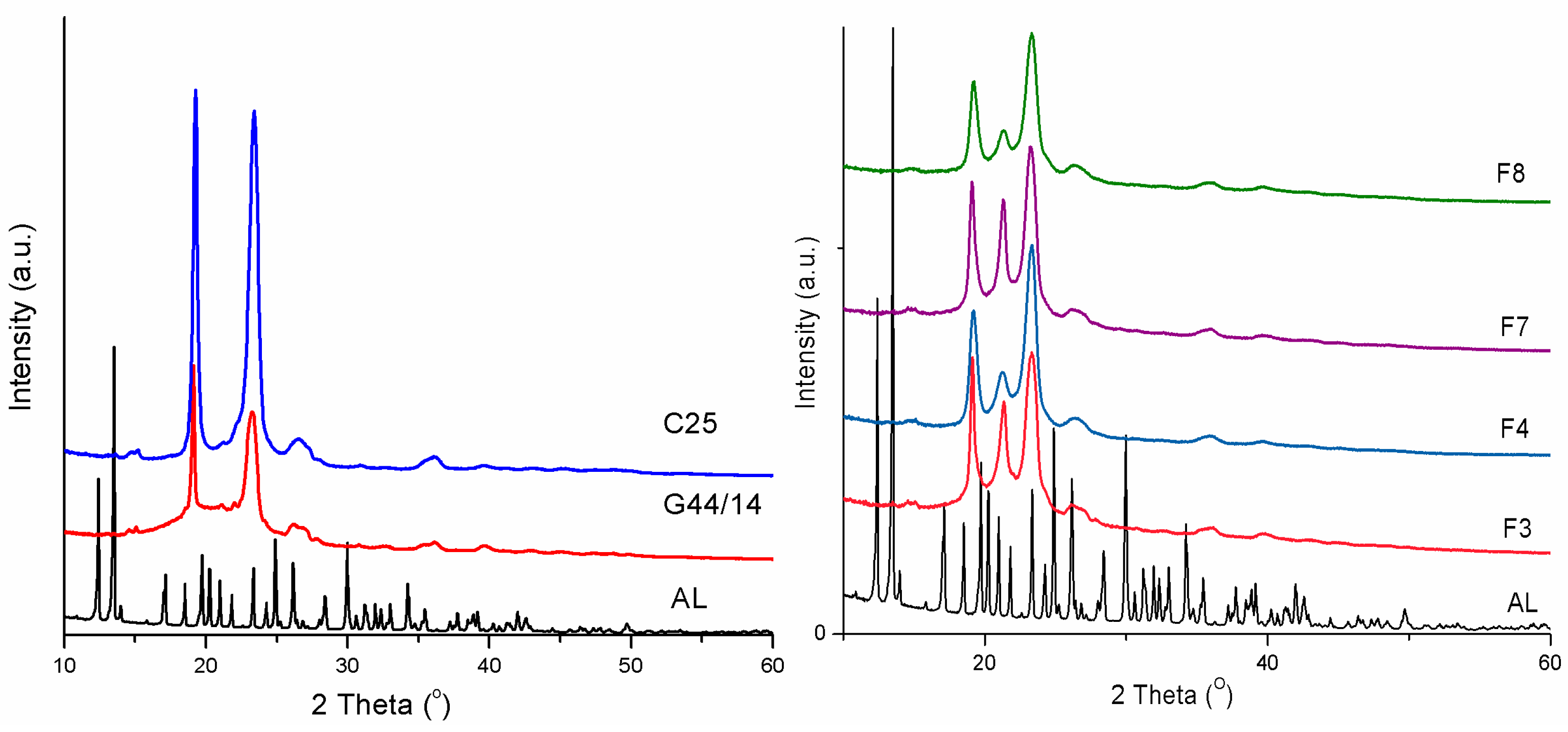
Powder X-ray Diffraction (PXRD) Analysis
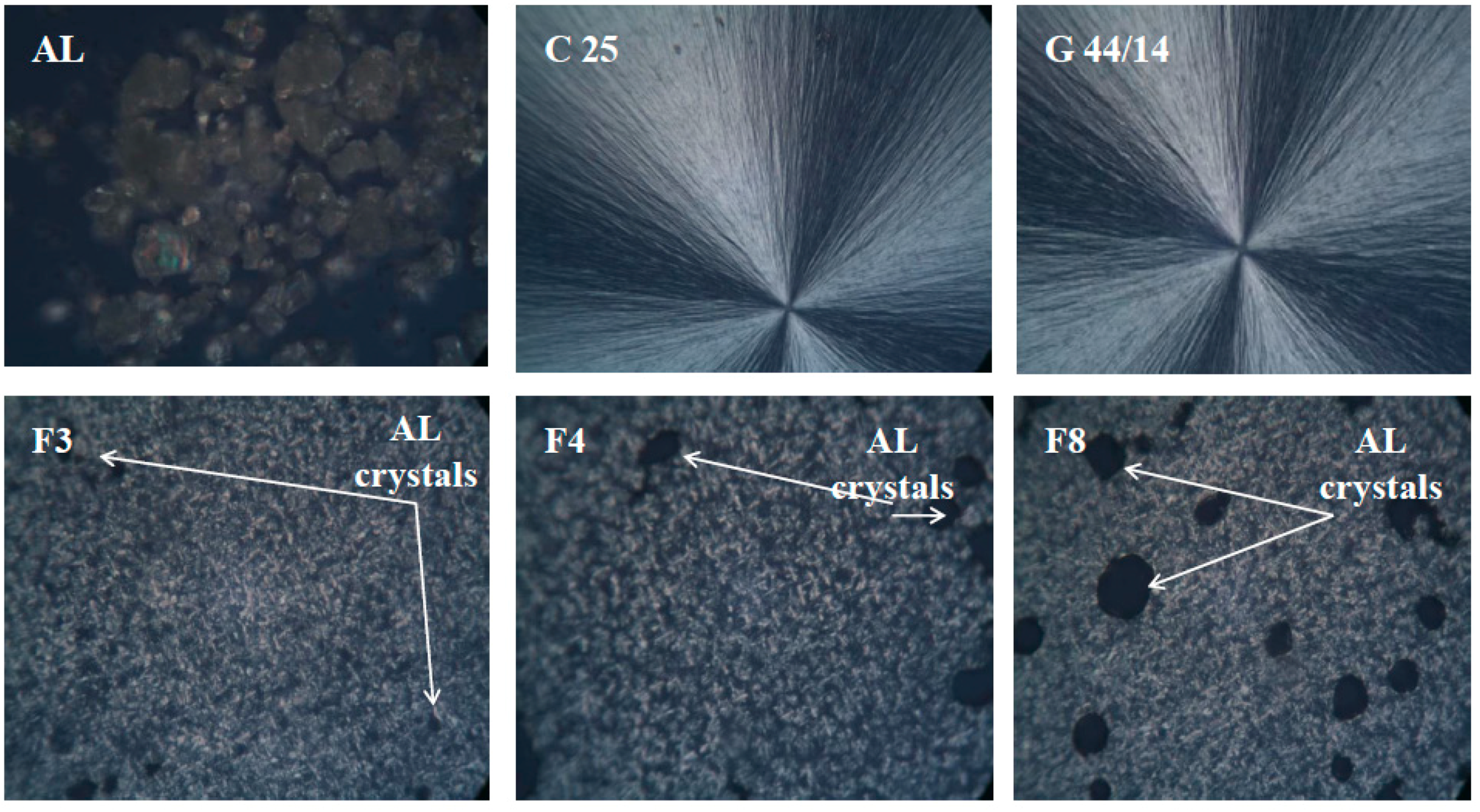
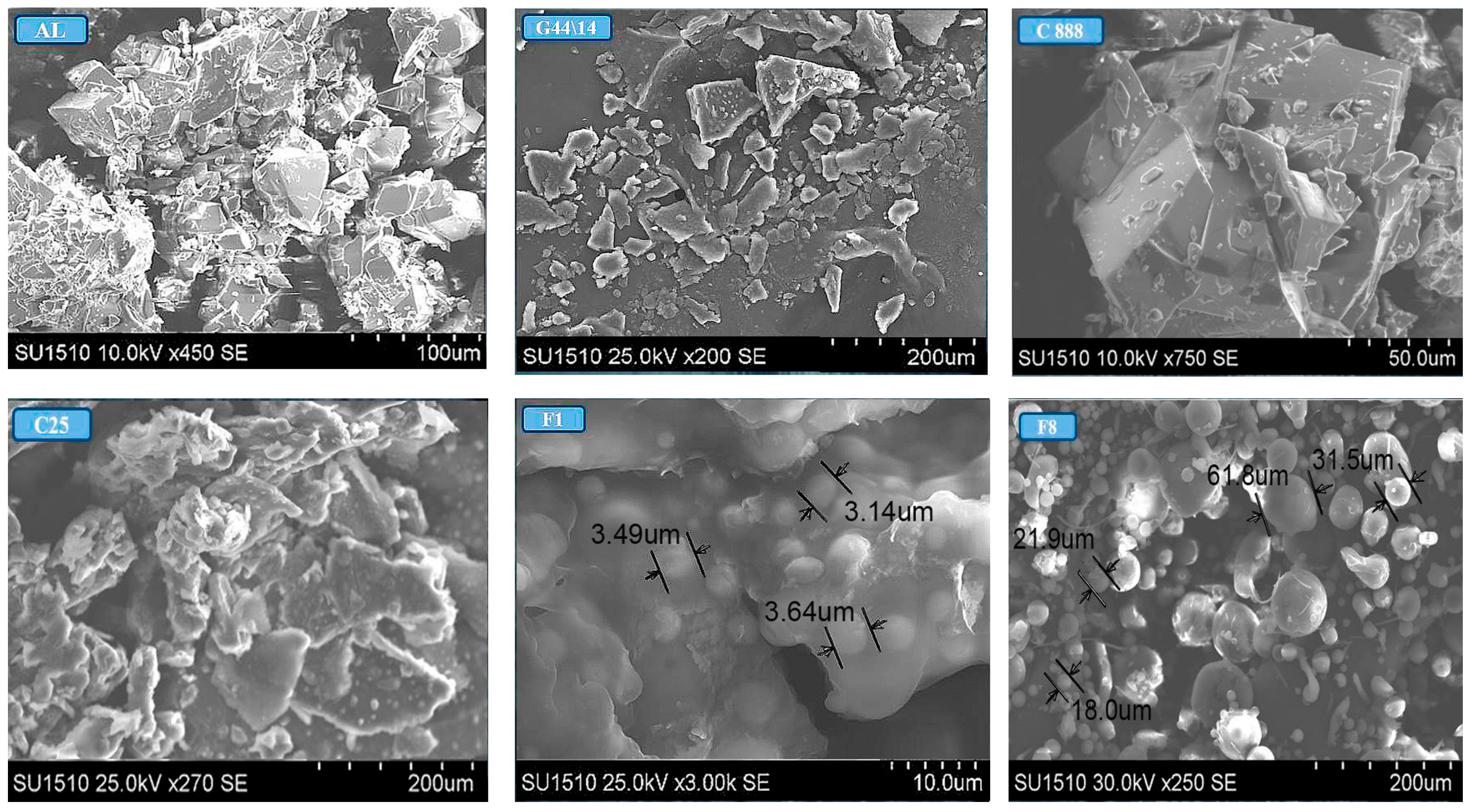
Optical and Scanning Electron Microscopy
2.2.3. HPLC Analysis and Method Validation
2.2.4. Assessment of Entrapment Efficacy
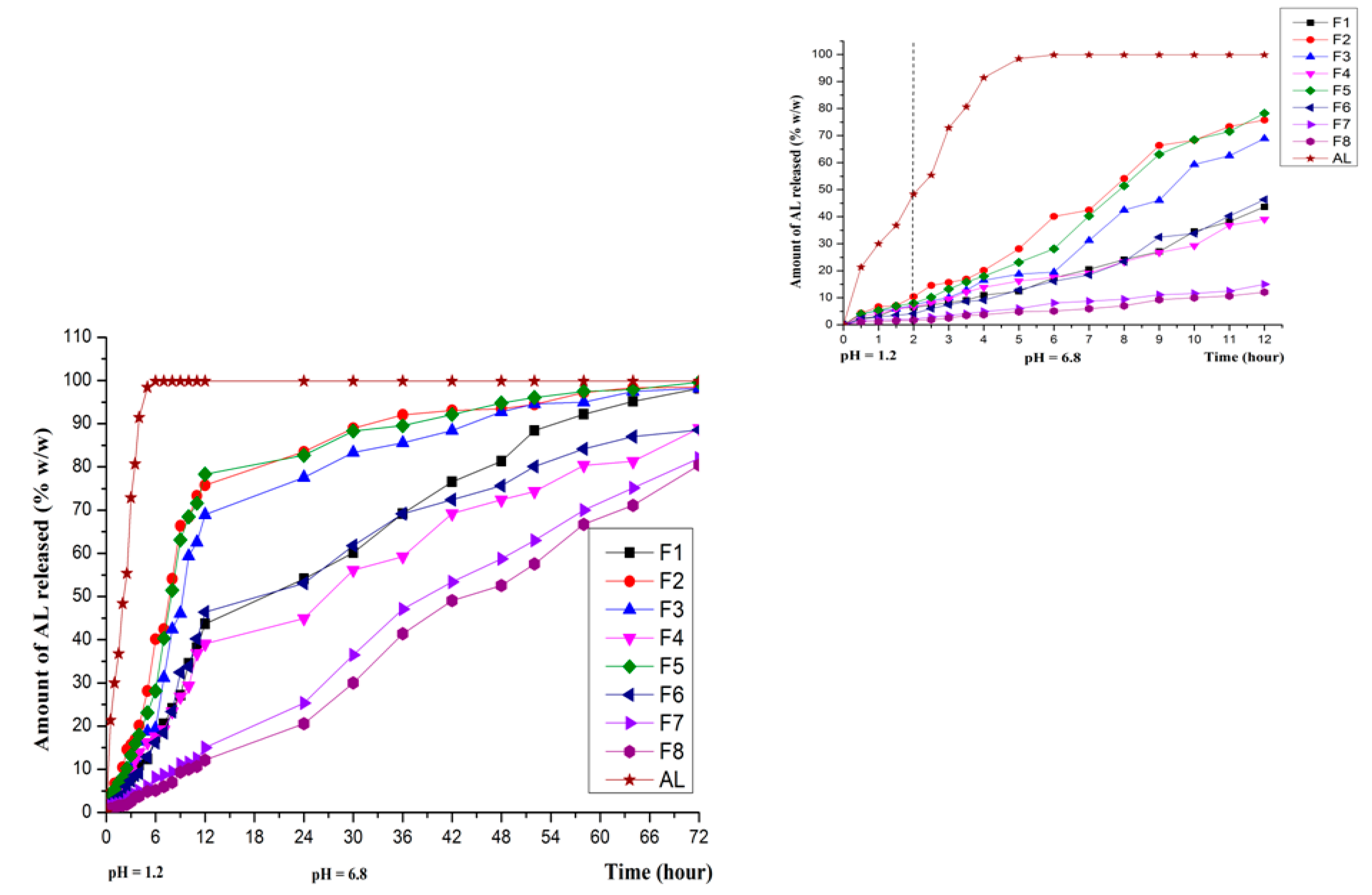
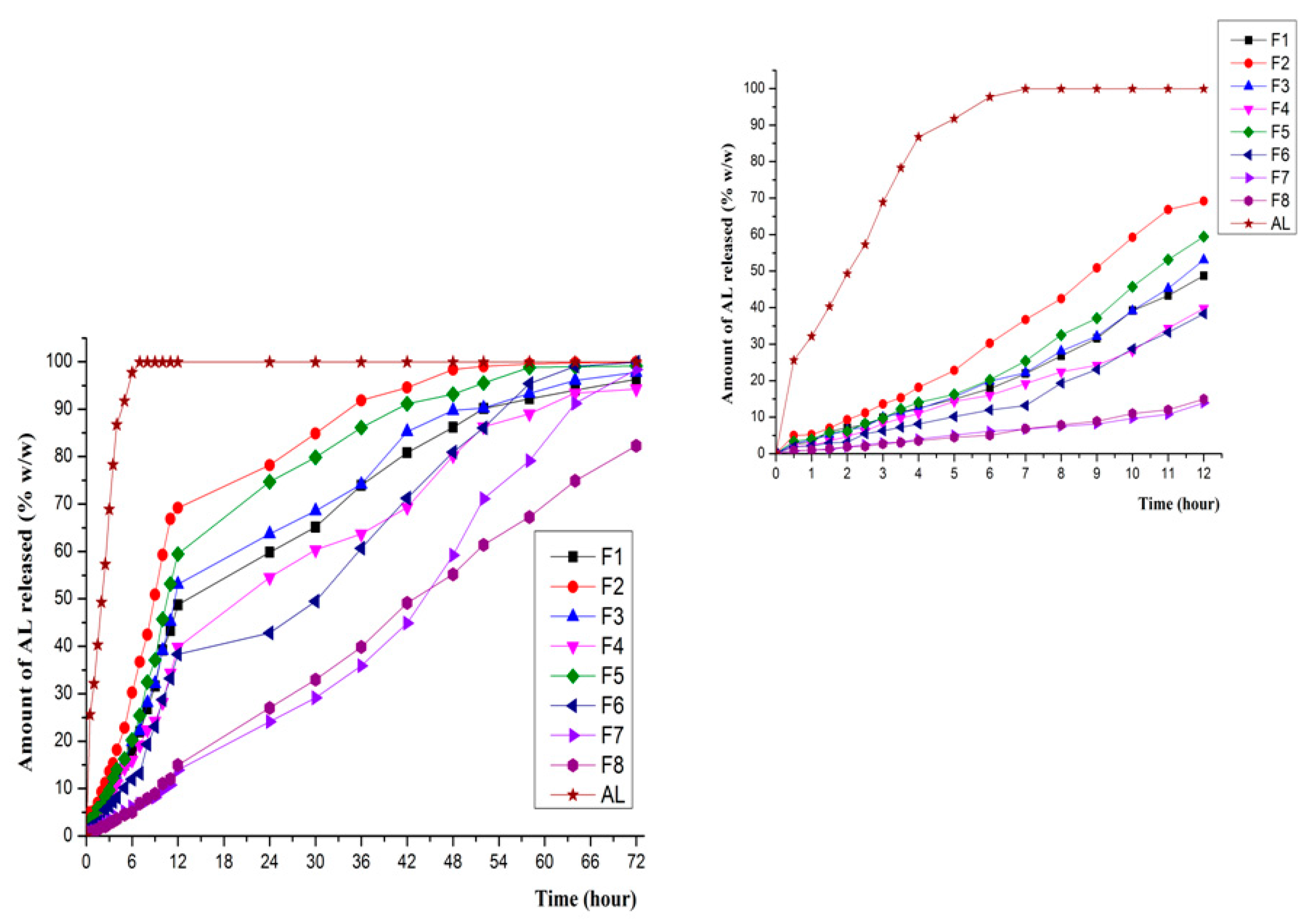
2.2.5. Study of the in Vitro Dissolution Release
2.2.6. Analysis of in Vitro Drug Release Kinetics
3. Results and Discussion
3.1. Preparation of AL-Loaded SLPs
3.2. Characterization of AL-loaded SLPs
3.2.1. SLPs Polydispersity Index and Zeta Potential
3.2.2. Fourier Transform Infrared Spectroscopy
3.2.3 Differential Scanning Calorimetry
3.2.4. Thermogravimetric Analysis
3.2.5. Powder X-ray Diffraction
3.2.6. Optical and Scanning Electron Microscopy
3.3. Entrapment Efficacy
3.4. In Vitro Release in Various Dissolution Media
3.5. In Vitro Drug Release Kinetics
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Whitaker, M.; Guo, J.; Kihoe, T.; Benson, G. Bisphosphonates for osteoporosis e where do we go from here? N. Engl. J. Med. 2012, 366, 2048–2051. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Bauer, D.C.; Schwartz, A.V.; Cummings, S.R.; Rosen, C.J. Continuing bisphosphonate treatment for osteoporosis for whom and for how long? N. Engl. J. Med. 2012, 366, 2051–2053. [Google Scholar] [CrossRef] [PubMed]
- Roelofs, A.J.; Ebetino, H.F.; Reszka, A.A. Bisphosphonates: Mecanism of actions. In Principles of Bone Biology, 3rd ed.; Bilezikian, P.J., Raiz Martin, T.J., Eds.; Academic Press Inc.: New York, NY, USA, 2008; pp. 333–368. [Google Scholar]
- Medication Guide Fosama Plus® Reference ID: 3403221. Available online: http://www.fda.gov/downloads/Drugs/DrugSafety (accessed on 13 October 2014).
- Binkley, N.; Ringe, J.D.; Reed, J.I. Alendronate/vitamin D3 70/2800 with and without additional 2800 IU vitamin D3 for osteoporosis: Results from the 24-week extension of a 15-week randomized, controlled trial. Bone 2009, 44, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Fleish, H. Bisphosphonates in Bone Diseases: From the Laboratory to the Patient; Academic Press: Cambridge, MA, USA, 2003; pp. 2–3. [Google Scholar]
- Bartl, R.; Frisch, B. Bisphosphonates in Medical Practice; Springer: New York City, NY, USA, 2005; pp. 7–27. [Google Scholar]
- Cremers, S.; Papapoulos, S. Pharmacology of bisphosphonates. Bone 2011, 49, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Kusamori, K.; Katsumi, H.; Abe, M.; Ueda, A.; Sakai, R.; Hayashi, R.; Hirai, Y.; Quan, Y.S.; Kamiyama, F.; Sakane, T.; et al. Development of a novel transdermal patch of alendronate, a nitrogen-containing bisphosphonate, for the treatment of osteoporosis. J. Bone Min. Res. 2010, 25, 2582–2591. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.; Assumpção, E.; Andrade, S.F.; Conrado, D.J.; Guterres, S.S.; Pohlmann, A.R. Microencapsulation of sodium alendronate reduces drug mucosal damages in rats. Drug Deliv. 2010, 17, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Asikoğlu, M.; Ozguney, I.; Ozcan, I.; Orumlu, O.; Guneri, T.; Koseoğlu, K.; Ozkilic, H. The absorption of (99m)Tc-alendronate given by rectal route in rabbits. Pharm. Dev. Technol. 2008, 13, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Miladi, K.; Sfar, S.; Fessi, H.; Elaissari, A. Drug carriers in osteoporosis: Perparation, drug encapsulation and applications. Int. J. Pharm. 2013, 445, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Barrat, G. Colloidal drug carriers: Achievements and perspectives. Cell. Mol. Life Sci. 2003, 60, 21–37. [Google Scholar] [CrossRef]
- Gagliardi, M.; Bardi, G.; Bifone, A. Polymeric nanocarriers for controlled and enhanced delivery of therapeutic agents to the CNS. Ther. Deliv. 2012, 3, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Katsumi, H.; Nakatani, M.; Sano, J.I.; Abe, M.; Kusamori, K.; Kurihara, M.; Shiota, R.; Takashima, M.; Fujita, T.; Sakan, T.; et al. Absorption and safety af alendronate, a nitrogen-containing bisphosphonate, after intrapulmonary administration in rats. Int. J. Pharm. 2010, 400, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Bock, O.; Felsenberg, D. Bisphosphonates in the management of postmenopausal osteoporosis–optimizing efficacy in clinical practice. Clin. Interv. Aging 2010, 3, 279–297. [Google Scholar]
- Naahidi, S.; Jafari, M.; Edalat, F.; Raymond, K.; Khademhosseini, A.; Chen, P. Biocompatibility of engineered nanoparticles for drug delivery. J. Control. Release 2013, 166, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine 2012, 8, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.B.; Banerjee, A.; Onyuksel, H. Improvement of drug safety by the use of lipid-based nanocarriers. J. Control. Release 2012, 163, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Negi, J.S.; Chattopadhyay, P.; Sharma, A.K.; Ram, V. Development of solid lipid nanoparticles (SLNs) of lopinavir using hot delf nano-emulsification (SNE) technique. Eur. J. Pharm. Sci. 2013, 48, 231–245. [Google Scholar]
- Bose, S.; Du, Y.; Takhistov, P.; Michniak-Kohn, B. Formulation optimization and topical delivery of quercetin from solid lipid based nanosystems. Int. J. Pharm. 2013, 56, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Dolatabadi, J.E.N.; Hamishehkar, H.; Eskandani, M.; Valizadeh, H. Formulation, characterization and cytotoxicity studies of alendronate sodium-loaded solid lipid nanoparticles. Coll. Surf. B Biointerfaces 2014, 117, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Merisko-Liversidge, E.; Liversidge, G.G. Drug nanoparticles: Formulating poorly water-soluble compounds. Toxicol. Pathol. 2008, 36, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.H.; Shegokar, R.; Keck, C.M. 20 years of lipid nanoparticles (SLN and NLC): Present state of development and industrial applications. Curr. Drug Discov. Technol. 2011, 8, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, W.; Mader, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- U.S. Pharmacopeial Medicines Compendium. Available online: https://mc.usp.org.monographs/alendronate (accessed on 3 March 2014).
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Ahuja, N.; Om, P.K.; Singh, B. Studies on dissolution enhancement and mathematical modelling of drug release of a poorly watersoluble drug using water-soluble carriers. Eur. J. Pharm. Biopharm. 2007, 65, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Karalis, V.; Magklara, E.; Shah, V.P.; Macheras, P. From drug delivery systems to drug release, dissolution, IVIVC, BCS, BDDCS, bioequivalence and biowaivers. Pharm. Res. 2010, 27, 2018–2029. [Google Scholar] [CrossRef] [PubMed]
- Gohel, M.C.; Sarvaiya, K.G.; Shah, A.R.; Brahmbhatt, B.K. Mathematical approach for the assessment of similarity factor using a new scheme for calculating weight. Indian J. Pharm. Sci. 2009, 71, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Fangueiro, J.H.; Smith, J. Predictive modeling of insulin release profile from cross-linked chitosan microspheres. Eur. J. Med. Chem. 2013, 60, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Briejesh, T.; Singh, N. Pulse Chemistry and Technology; The Royal Society and Chemistry Publisher: London, UK, 2012; pp. 134–138. [Google Scholar]
- Ibrahim, S.; Khalivulla, S.I.; Akowuah, G.A. Determination of Alendronate Sodium in Tablets by Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy. Curr. Bioact. Compd. 2016, 12. [Google Scholar]
- Martin, A.; Swarbrick, J.; Cammarata, A. Physical pharmacy. In States of Matter and Phase Equilibria Philadelphia; Lea & Febiger: Philadelphia, PA, USA, 1983; pp. 62–92. [Google Scholar]
- Libster, D.; Aserin, A.; Wachtel, E. Surfactant science series. In Crystallization and Polymorphism of Fats and Fatty Acids; Garti, N., Sato, K., Eds.; Marcel Dekker: New York, NY, USA, 1988; pp. 3–87. [Google Scholar]
- Ochiuz, L.; Grigoras, A.; Grigoras, C.; Profire, L.; Peptu, C.; Popa, G. Preparation and thermal stability evaluation on solid lipid particles loaded with alendronate. In Proceedings of the 14th SGEM GeoConference on Nano, Bio and Green—Technologies for A Sustainable Future, Albena, Bulgaria, 19–25 June 2014; Volume 1, pp. 151–158.
- Tandon, P.; Forster, G.; Neubert, R. Phase transition in oleic acid as studied by X-ray diffraction and FT-Raman spectroscopy. J. Mol. Struct. 2005, 524, 201–215. [Google Scholar] [CrossRef]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Influence of process and formulation parameters on the formation of submicron particles by solvent displacement and emulsification-diffusion methods: Critical comparison. Adv. Colloid Interface Sci. 2011, 163, 90–122. [Google Scholar] [CrossRef] [PubMed]
- Ochiuz, L.; Peris, J.E. Preparation and Characterisation of Alendronate-Loaded Chitosan Microparticles Obtained through the Spray Drying Technique. Med. Chem. 2009, 5, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Fassihi, R.; Pillay, V. Evaluation and comparison of dissolution data derived from different modified release dosage forms: An alternative method. J. Control. Release 1998, 55, 45–55. [Google Scholar]
- Ju, H.L.; Liaw, S.J. On the assessment of similarity of drug dissolution profiles—A simulation study. Drug Inf. J. 1997, 31, 1273–1289. [Google Scholar] [CrossRef]
- Moore, J.W.; Flanner, H.H. Mathematical comparison of dissolution profiles. Pharm. Tech. 1996, 20, 64–74. [Google Scholar]
- Kim, H.; Fassihi, R. Application of binary polymer system in drug release rate modulation. Influence of formulation variables and hydrodynamic conditions on release kinetics. J. Pharm. Sci. 1997, 86, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.M.; Buri, P.; Peppas, N.A. Mechanism of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Kabanov, A.V. Pluronic block copolymers: Evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J. Control. Release 2008, 130, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Rosca, I.D.; Watari, F.; Uo, M. Microparticle formation and its mechanism in single and double emulsion solvent evaporation. J. Control. Release 2004, 99, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Kallakunta, V.R.; Eedara, B.B.; Jukanti, R.; Ajmeera, R.K.; Bandari, S. A Gelucire 44/14 and labrasol based solid self emulsifying drug delivery system: Formulation and evaluation. J. Pharm. Investig. 2013, 43, 185–196. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Parveen, S.; Penda, J.J. The present and future of nanotechnology in human health care. Nanomedicine 2000, 73, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Silva, A.H.; Filippin-Monteiro, F.B.; Mattei, B.; Zanetti-Ramos, B.G.; Creczynski-Pasa, T.B. In vitro biocompatibility of solid lipid nanoparticles. Sci. Total Environ. 2012, 432, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Doktorovova, S.; Souto, E.B.; Silva, A.M. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers—A systematic review of in vitro data. Eur. J. Pharm. Biopharm. 2014, 87, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.

| Formulation | Raw Materials (Ratio w/w) | |||
|---|---|---|---|---|
| AL | C 888 | G 44/14 | C 25 | |
| F1 | 1 | 1 | 0.7 | 1.7 |
| F2 | 1 | 1 | 1.7 | 0.7 |
| F3 | 1 | 1 | 2.4 | 0 |
| F4 | 1 | 1 | 0 | 2.4 |
| F5 | 1 | 1 | 2 | 0.4 |
| F6 | 1 | 1 | 0.4 | 2 |
| F7 | 1 | 1 | 1.4 | 1 |
| F8 | 1 | 1 | 1 | 1 |
| Formulation | DLS Results | Drug Content | |||
|---|---|---|---|---|---|
| Particle Size (µm) | PdI | Zeta Potential (mV) | Actual Drug Content (%) Mean ± SD | Entrapment Efficacy (%) Mean ± SD | |
| F1 | 1.26 | 1.000 | −7.95 | 13.24 ± 1.25 | 57.2 ± 2.30 |
| F2 | 2.52 | 0.231 | −10.90 | 10.22 ± 0.73 | 50.1 ± 0.99 |
| F3 | 1.13 | 0.040 | −13.10 | 10.96 ± 1.83 | 49.2 ± 1.27 |
| F4 | 1.65 | 1.000 | −7.43 | 14.38 ± 2.52 | 62.3 ± 1.31 |
| F5 | 3.87 | 0.364 | −11.60 | 11.39 ± 1.95 | 45.6 ± 2.78 |
| F6 | 2.40 | 0.044 | −6.43 | 12.08 ± 3.72 | 54.9 ± 2.68 |
| F7 | 1.24 | 0.507 | −10.30 | 14.83 ± 1.19 | 65.2 ± 0.97 |
| F8 | 1.04 | 0.030 | −13.30 | 14.56 ± 1.74 | 60.7 ± 1.16 |
| Kinetic Model | Model Coefficients | Formula | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | ||
| Zero-order | K0 | 1.6623 | 1.9536 | 1.8738 | 1.4653 | 1.9490 | 1.5535 | 1.1950 | 1.1075 |
| R2 | 0.8955 | 0.3726 | 0.6165 | 0.8632 | 0.4447 | 0.8425 | 0.9964 | 0.9950 | |
| AIC | 120.1776 | 168.7918 | 156.6207 | 118.6883 | 66.5988 | 126.9713 | 25.0111 | 30.9225 | |
| First-order | K1 | 0.0437 | 0.0627 | 0.0559 | 0.0279 | 0.0670 | 0.0313 | 0.0202 | 0.0180 |
| R2 | 0.9719 | 0.9161 | 0.9597 | 0.9807 | 0.9409 | 0.9790 | 0.9652 | 0.9469 | |
| AIC | 87.3210 | 118.4923 | 100.3231 | 69.7062 | 110.6022 | 76.5813 | 81.8319 | 90.0754 | |
| Higuchi | KH | 11.2320 | 14.1181 | 12.2087 | 9.9892 | 13.9926 | 10.5979 | 7.6690 | 7.0183 |
| R2 | 0.9483 | 0.8433 | 0.9031 | 0.9666 | 0.8566 | 0.9420 | 0.8518 | 0.8183 | |
| AIC | 102.587 | 134.1020 | 122.2216 | 83.4667 | 132.7607 | 102.0109 | 118.0376 | 120.8344 | |
| Korsmeyer-Peppas | n | 0.65 | 0.46 | 0.49 | 0.58 | 0.45 | 0.62 | 0.91 | 0.97 |
| KP | 6.5280 | 16.3025 | 13.6880 | 7.5003 | 16.7236 | 6.6184 | 1.7005 | 1.2456 | |
| R2 | 0.9823 | 0.8564 | 0.9029 | 0.9852 | 0.8649 | 0.9629 | 0.9955 | 0.9933 | |
| AIC | 77.8468 | 133.9255 | 124.2792 | 65.1419 | 133.2584 | 92.8413 | 32.4754 | 40.3637 | |
| Kinetic Model | Model Coefficients | Formula | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | ||
| Zero-order | K0 | 2.1352 | 2.6844 | 2.2234 | 1.8829 | 2.4625 | 1.7802 | 1.0844 | 1.1316 |
| R2 | 0.8601 | 0.6682 | 0.8580 | 0.9076 | 0.8209 | 0.9365 | 0.9834 | 0.9966 | |
| AIC | 98.5480 | 124.7318 | 100.8783 | 85.3670 | 110.2489 | 76.7587 | 31.7981 | 0.0578 | |
| First-order | K1 | 0.0395 | 0.0743 | 0.0439 | 0.0311 | 0.0561 | 0.0294 | 0.0144 | 0.0150 |
| R2 | 0.9803 | 0.9692 | 0.9782 | 0.9874 | 0.9738 | 0.9655 | 0.9509 | 0.9754 | |
| AIC | 57.4289 | 74.8169 | 61.5543 | 43.5773 | 69.8784 | 63.9492 | 54.5410 | 41.4065 | |
| Higuchi | KH | 11.4848 | 14.9391 | 11.9499 | 10.0080 | 13.3191 | 9.2991 | 5.3645 | 5.6246 |
| R2 | 0.9167 | 0.9079 | 0.9101 | 0.9088 | 0.9018 | 0.8676 | 0.7579 | 0.7810 | |
| AIC | 87.6629 | 97.8056 | 91.2823 | 85.0790 | 97.6354 | 92.1918 | 88.0630 | 87.3350 | |
| Korsmeyer-Peppas | n | 0.65 | 0.46 | 0.49 | 0.58 | 0.45 | 0.62 | 0.91 | 0.97 |
| KP | 7.2105 | 16.8176 | 12.3038 | 7.8719 | 15.3785 | 6.4837 | 1.4813 | 1.2569 | |
| R2 | 0.9642 | 0.8923 | 0.9036 | 0.9527 | 0.8673 | 0.9379 | 0.9715 | 0.9947 | |
| AIC | 71.9129 | 103.0962 | 94.7373 | 73.2816 | 105.9575 | 78.3024 | 45.1629 | 11.3721 | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochiuz, L.; Grigoras, C.; Popa, M.; Stoleriu, I.; Munteanu, C.; Timofte, D.; Profire, L.; Grigoras, A.G. Alendronate-Loaded Modified Drug Delivery Lipid Particles Intended for Improved Oral and Topical Administration. Molecules 2016, 21, 858. https://doi.org/10.3390/molecules21070858
Ochiuz L, Grigoras C, Popa M, Stoleriu I, Munteanu C, Timofte D, Profire L, Grigoras AG. Alendronate-Loaded Modified Drug Delivery Lipid Particles Intended for Improved Oral and Topical Administration. Molecules. 2016; 21(7):858. https://doi.org/10.3390/molecules21070858
Chicago/Turabian StyleOchiuz, Lacramioara, Cristian Grigoras, Marcel Popa, Iulian Stoleriu, Corneliu Munteanu, Daniel Timofte, Lenuta Profire, and Anca Giorgiana Grigoras. 2016. "Alendronate-Loaded Modified Drug Delivery Lipid Particles Intended for Improved Oral and Topical Administration" Molecules 21, no. 7: 858. https://doi.org/10.3390/molecules21070858










