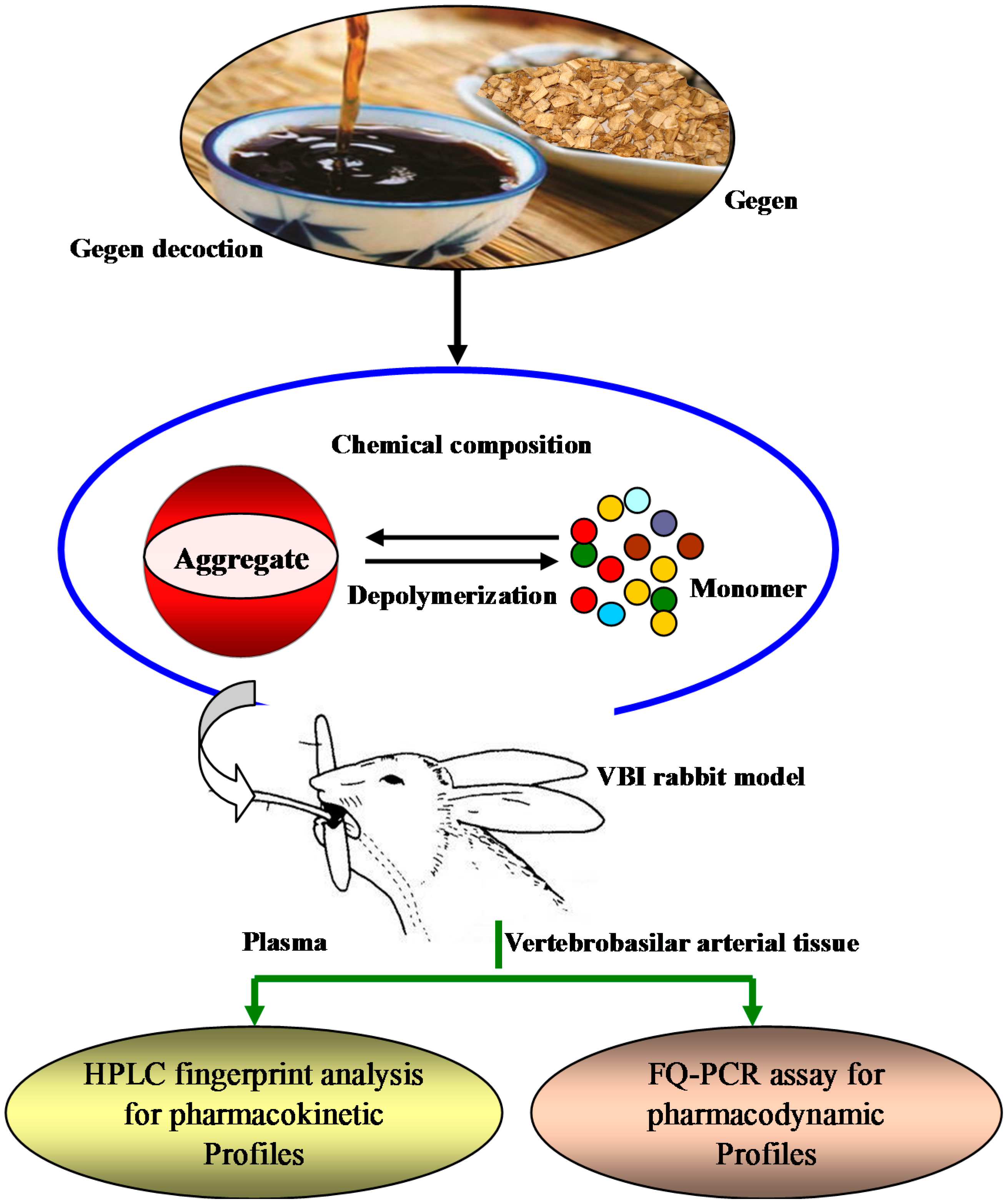
Relevance of the Pharmacokinetic and Pharmacodynamic Profiles of Puerariae lobatae Radix to Aggregation of Multi-Component Molecules in Aqueous Decoctions
Abstract
:1. Introduction
2. Results and Discussion
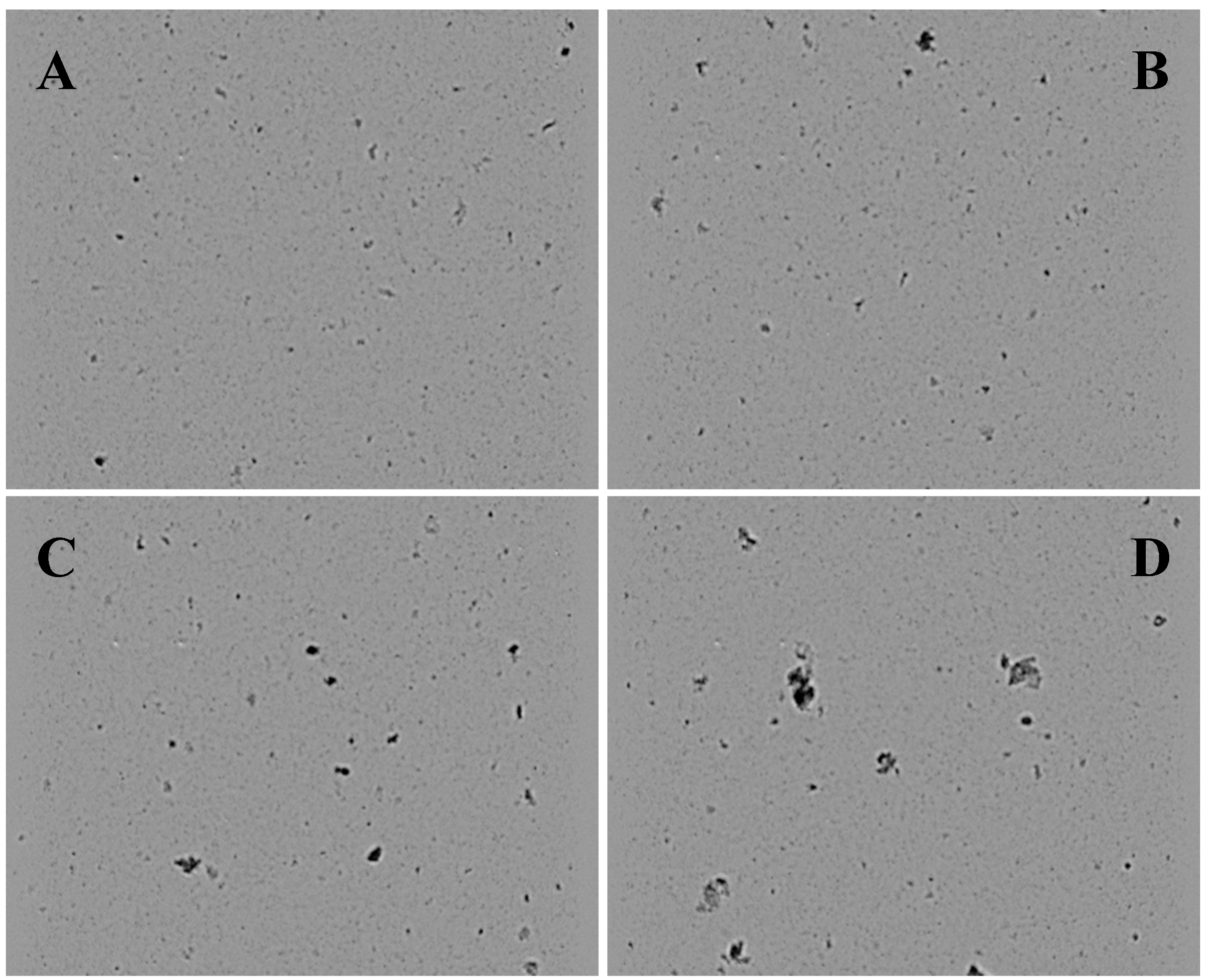
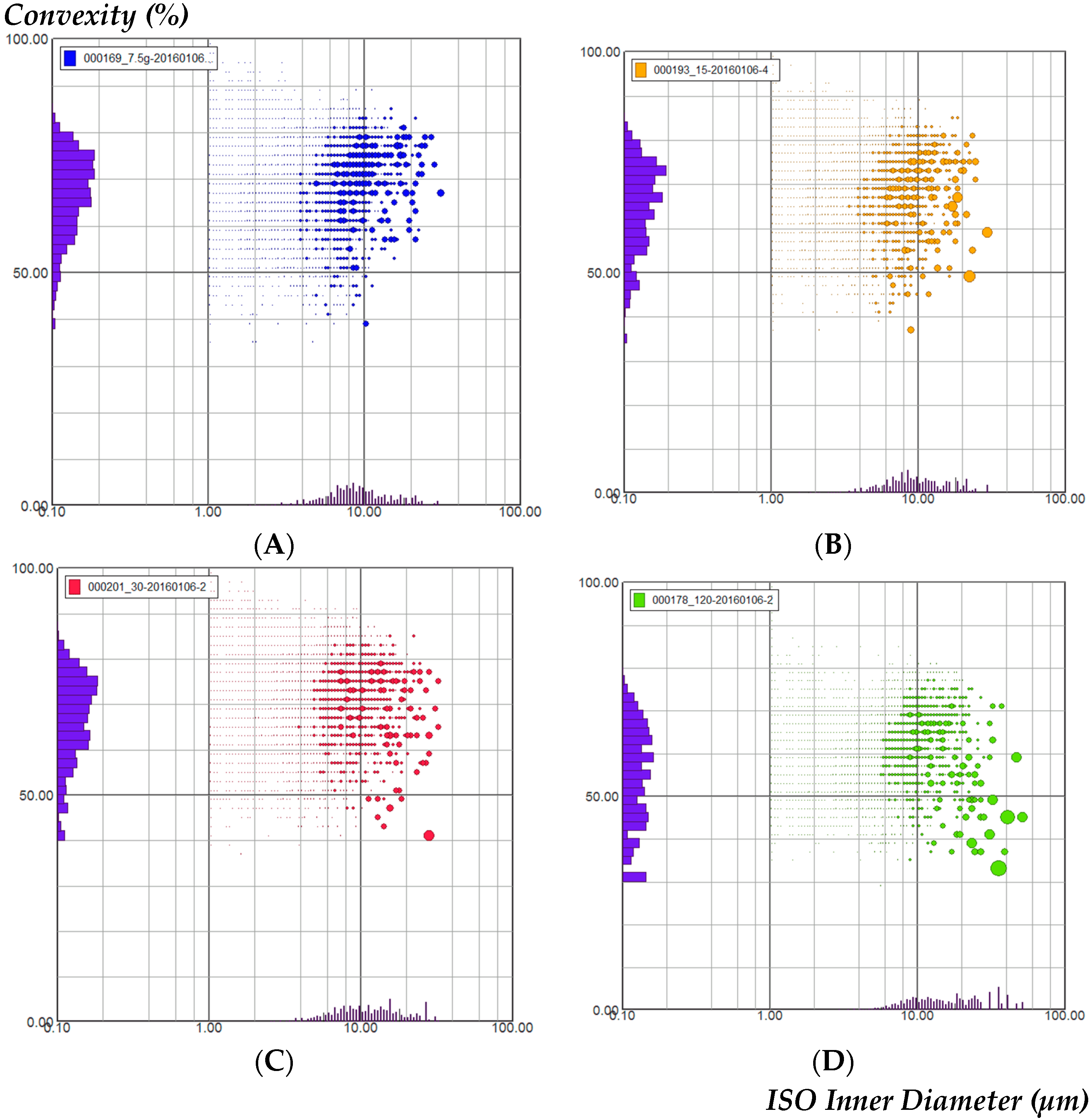
2.1. Particle Size and Shape in the Four GGDs
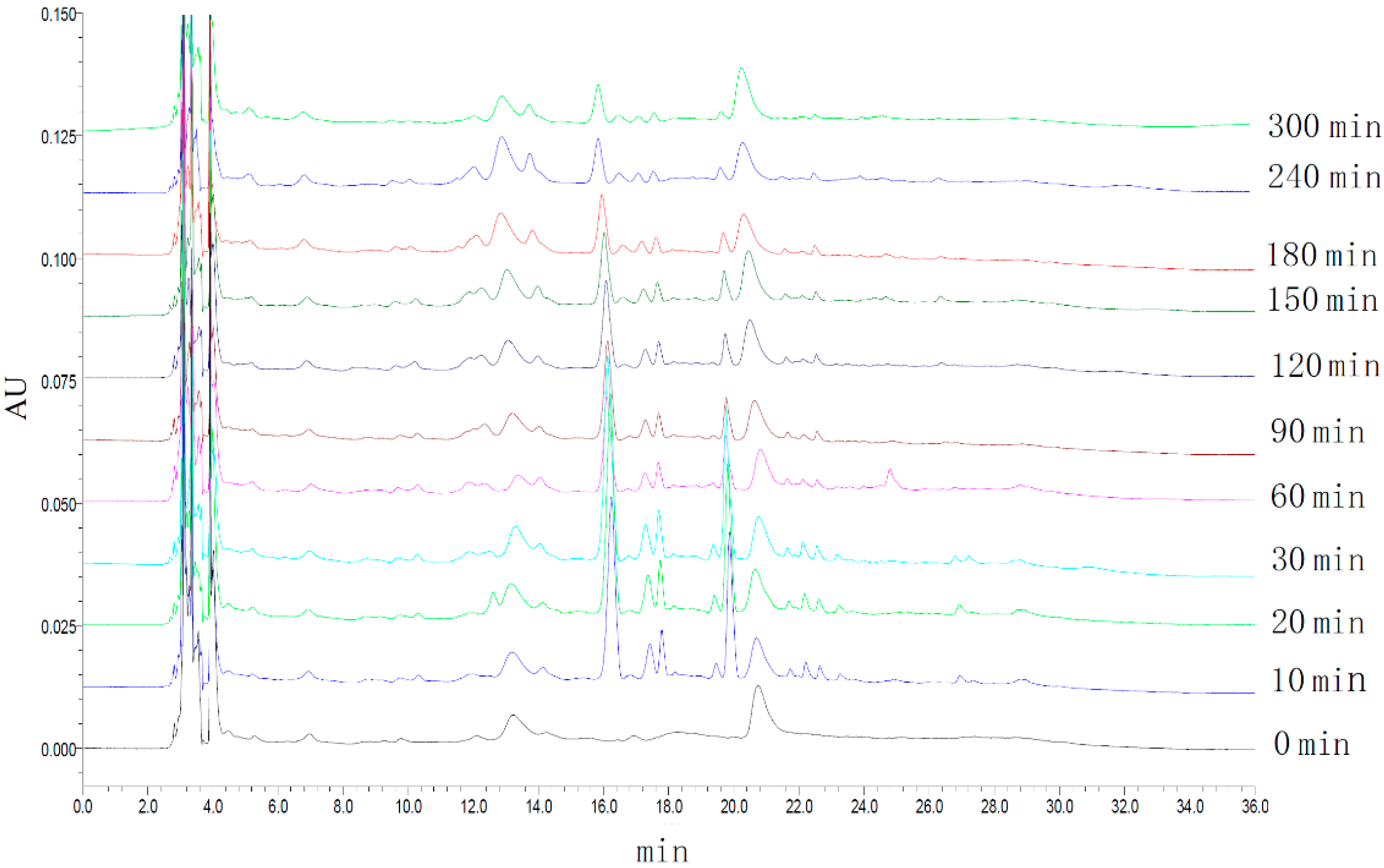
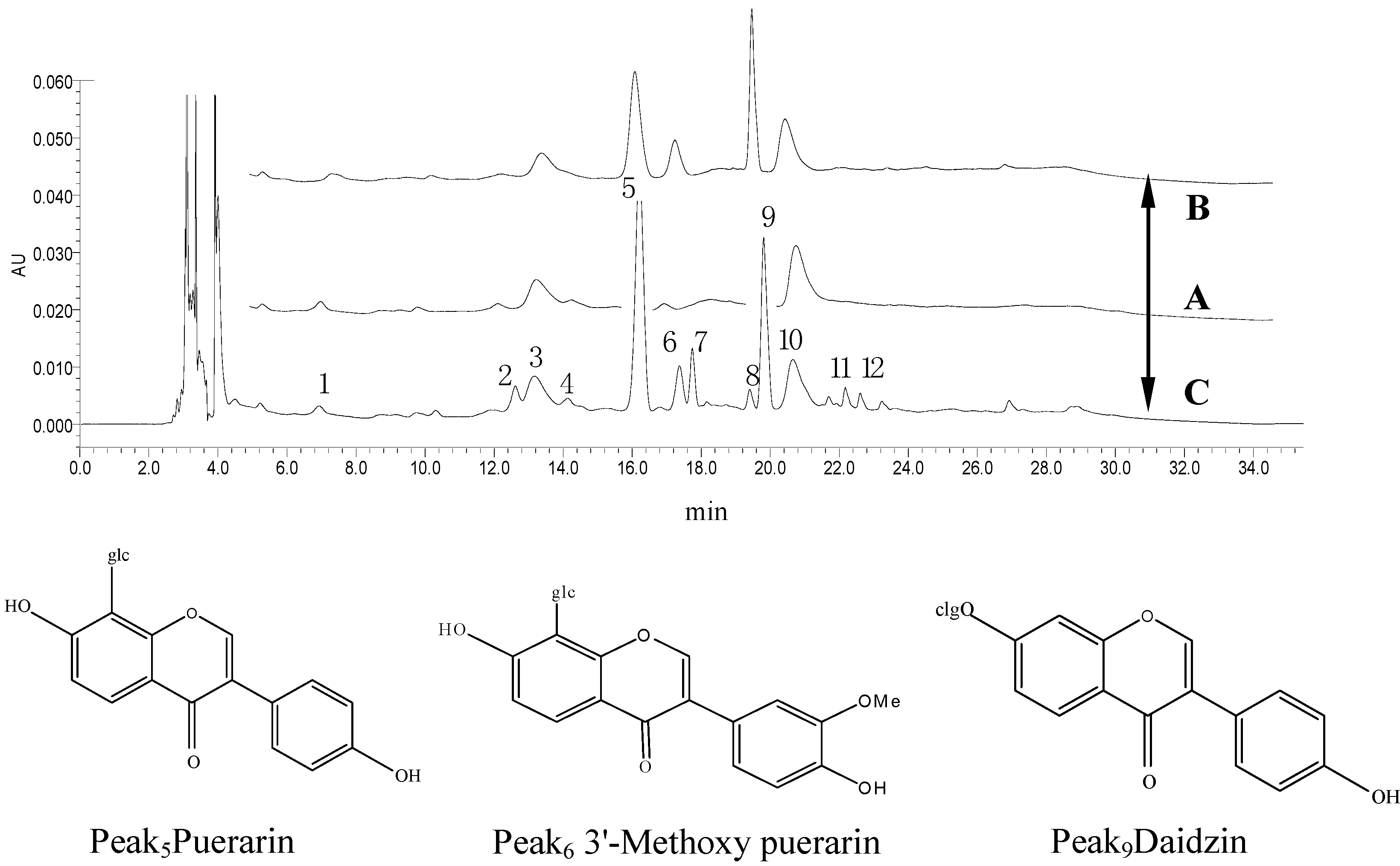
2.2. HPLC Fingerprints of Rabbit Plasma
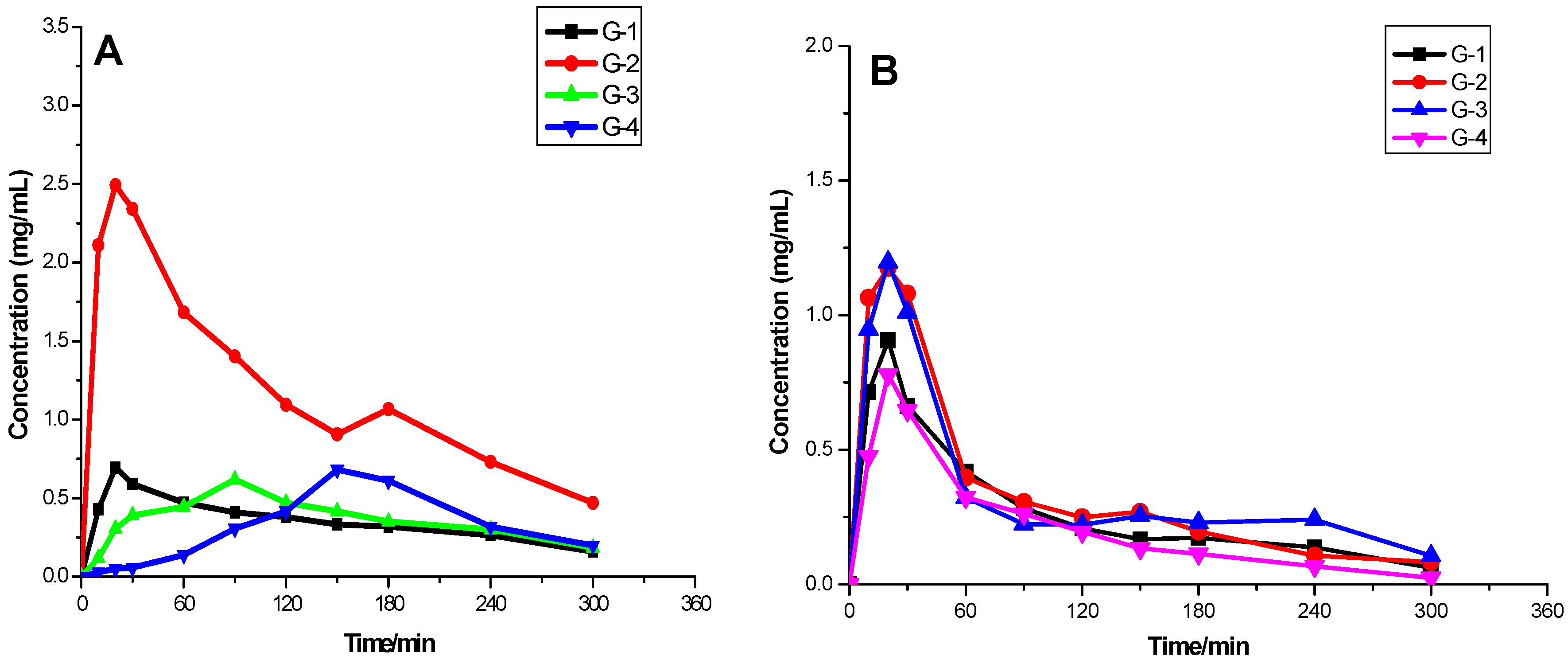
2.3. Rabbit Plasma HPLC Analysis of the Pharmacokinetic Profiles of the Indicative Components Puerarin and Daidzin in GGD
2.3.1. Specificity and Linearity
2.3.2. Accuracy and Precision
2.3.3. Pharmacokinetic Profiles of Puerarin and Daidzin
2.4. Pharmacodynamic Profiles of the Four GGDs in the Anti-Rabbit Vertebrobasilar Artery Ischemia Model
2.4.1. Transcranial Doppler to Evaluate the Vertebrobasilar Artery Ischemia Model of Rabbits
2.4.2. Rabbits Vertebrobasilar Arterial Tissue FQ-PCR Assay for the Pharmacodynamic Profiles of the Four GGDs
3. Experimental Section
3.1. Materials
3.2. Methods
3.2.1. Samples Preparation
3.2.2. Characterization of Molecular Aggregation
3.2.3. Rabbit Plasma HPLC Analysis for Pharmacokinetic Profiles of the Four GGDs
3.2.4. Animal Model Preparation and VBI Rabbit Treatments
3.2.5. Rabbits Vertebrobasilar Arterial Tissue FQ-PCR Assay for Pharmacodynamic Profiles of the Four GGDs
3.3. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pang, W.S.; Lin, S.D.; Dai, Q.W.; Zhang, H.C.; Hu, J. Antitussive activity of Pseudostellaria heterophylla (Miq.) Pax extracts and improvement in lung function via adjustment of multi-cytokine levels. Molecules 2011, 16, 3360–3370. [Google Scholar] [CrossRef] [PubMed]
- Bohets, H.; Annaert, P.; Mannens, G.; van Beijsterveldt, L.; Anciaux, K.; Verboven, P.; Meuldermans, W.; Lavrijsen, K. Strategies for drug screening in drug discovery and development. Curr. Top. Med. Chem. 2001, 1, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Yeh, P.Y.; Ellens, H.; Smith, P.L. Physiological considerations in the design of particulate dosage forms for oral vaccine delivery. Adv. Drug Deliv. Rev. 1998, 34, 123–133. [Google Scholar] [CrossRef]
- Popper, H. Distribution of vitamin A in tissues as visualized by fluorescence microscopy. Physiol. Rev. 1994, 24, 205–224. [Google Scholar]
- Charman, W.N.; Stella, V.J. Lymphatic Transport of Drugs; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Dorland, W.A. Dorland’s Illustrated Medical Dictionary; W.B. Saunders: Philadelphia, PA, USA, 1981; p. 761. [Google Scholar]
- Hu, J.; Wu, Z.S.; Yan, J.J.; Pang, W.S.; Liang, D.H.; Xu, X.J. A promising approach for understanding the mechanism of traditional Chinese medicine by the aggregation morphology. J. Ethnopharmacol. 2009, 123, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhuang, Y.; Qiao, X.B.; Xu, X.J. Predicted model of aggregation of molecules in Chinese herbal drugs by support vector machines. Acta Phys. Chim. Sin. 2007, 23, 1141–1144. [Google Scholar] [CrossRef]
- Bordi, F.; Cametti, C.; Sennato, S.; Diociaiutiy, M. Direct evidence of multicompartment aggregates in polyelectrolyte-charged liposome complexes. Biophys. J. 2006, 91, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Sarah, Z.; Danie, W.; Michael, W.; Gerhard, W.; Wolfgang, F.; Wim, J.; Andrea, H. Flow imaging microscopy for protein particle analysis—A comparative evaluation of four different analytical instruments. AAPS J. 2013, 15, 1200–1211. [Google Scholar]
- Hart, J.; Zhu, Y.; Pirard, E. Particle size and shape characterisation—Current technology and practice. In Advances in the Characterization of Industrial Minerals; Christidis, G., Ed.; The Mineralogical Society of Great Britain and Ireland: Twickenham, UK, 2010. [Google Scholar]
- Wang, G.; Yang, C.M.; Zhang, K.; Hu, J.; Pang, W.S. Molecular clusters size of Puerariae thomsonii Radix aqueous decoction and relevance to oral absorption. Molecules 2015, 20, 12376–12388. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.; Krzeminski, A.; Wille, S.; Weiss, J.; Hinrichs, J. Simultaneous particle size and shape analysis in fermented milk products as influenced by composition and processing. Milchwissenschaft 2012, 67, 6–9. [Google Scholar]
- Zhu, S.M.; Zheng, Z.; Huang, Y.; Hu, X.M.; Yang, S.T.; Zeng, Y.L.; Deng, Y.Z. Animal model of vertebral artery type of cervical spondylosis of vertebral artery type in rabbits by injection curing agent. J. Tradit. Chin. Orthoped. Traumatol. 2000, 12, 11–13. [Google Scholar]
- Ma, Y.X.; Zhang, R.; Min, F.X.; Zhou, H.C.; Wang, Y.L.; Zhou, M.; Jiang, L.M.; Liu, F.C. Effect of cervical pain pill on blood flow velocity of vertebral basilar artery in rabbits with vertebral artery type of cervical spondylosis. Inform. Tradit. Chin. Med. 2012, 29, 50–51. [Google Scholar]
- Sample Availability: Samples of the compounds are not available from the authors.

| Parameter | Unit | G-1 | G-2 | G-3 | G-4 |
|---|---|---|---|---|---|
| t1/2 | min | 69.32 ± 3.58 | 65.28 ± 4.93 | ||
| t1/2α | min | 5.15 ± 2.01 ▼▼ | 17.79 ± 9.82 | ||
| t1/2β | min | 69.32 ± 1.82 | 69.32 ± 2.88 | ||
| V1/F | L/kg | 446.1 ± 8.53 **,▼▼ | 192.9 ± 9.22 ** | 527.4 ± 7.23 **,▼▼ | 975.1 ± 10.28 **,▼▼ |
| CL/F | L/min/kg | 8.38 ± 6.38 **,▼▼ | 1.74 ± 0.19 ** | 5.27 ± 0.74 **,▼▼ | 10.46 ± 1.96 **,▼▼ |
| Tmax | min | 21.32 ± 1.35 **,▼▼ | 26.45 ± 5.77 ** | 91.12 ± 3.80 **,▼▼ | 157.5 ± 1.67 **,▼▼ |
| Cmax | mg/L | 0.69 ± 0.16 **,▼▼ | 2.62 ± 0.61 ** | 0.62 ± 0.05 **,▼▼ | 0.038 ± 0.063 **,▼▼ |
| AUC0–t | mg/L·min | 106.9 ± 5.96 **,▼▼ | 357.9 ± 5.25 ** | 110.5 ± 6.34 **,▼▼ | 60.00 ± 10.88 **,▼▼ |
| AUC0–∞ | mg/L·min | 147.5 ± 9.85 **,▼▼ | 474.6 ± 7.58 ** | 158.1 ± 2.65 **,▼▼ | 80.34 ± 1.11 **,▼▼ |
| Parameter | Unit | G-1 | G-2 | G-3 | G-4 |
|---|---|---|---|---|---|
| t1/2α | min | 56.29 ± 2.55 ** | 25.29 ± 4.44 ** | 10.56 ± 2.44 ** | 8.02 ± 0.80 ** |
| t1/2β | min | 69.31 ± 2.35 | 68.62 ± 1.20 | 69.31 ± 1.82 | 69.31 ± 3.12 |
| V1/F | L/kg | 676.4 ± 15.63 **,▼▼ | 220.8 ± 7.98 ** | 238.5 ± 8.71 ** | 369.3 ± 6.22 **,▼▼ |
| CL/F | L/min/kg | 7.81 ± 1.55 | 6.93 ± 1.60 | 5.72 ± 2.99 | 11.32 ± 0.54 ▼ |
| Tmax | min | 20.00 ± 4.08 | 22.50 ± 2.30 | 22.50 ± 3.22 | 20.00 ± 2.06 |
| Cmax | min | 0.91 ± 0.10 | 1.24 ± 0.38 | 1.12 ± 0.24 | 0.78 ± 0.14 ▼ |
| AUC0–t | mg/L·min | 82.79 ± 7.33 ▼ | 104.8 ± 3.05 | 102.9 ± 2.66 | 65.54 ± 3.71 ▼ |
| AUC0–∞ | mg/L·min | 108.1 ± 3.99 ▼ | 122.7 ± 8.40 | 115.82 ± 7.58 | 72.54 ± 3.45 ▼▼ |
| Groups | Vm (dm/s) | BA (dm/s) | 2−△△ct(AT1R) | 2−△△ct(AT2R) |
|---|---|---|---|---|
| Blank control | 21.84 ± 0.036 | 16.02 ± 1.47 | 1.02 ± 0.070 | 1.02 ± 0.033 |
| Model control | 5.60 ± 0.24 ** | 6.99 ± 0.081 ** | 2.81 ± 0.20 ** | 0.29 ± 0.061 ** |
| G-1 | 6.92 ± 1.33 | 7.73 ± 0.32 | 2.42 ± 0.24 | 0.49 ± 0.12 * |
| G-2 | 18.38 ± 0.79 ▲▲ | 14.16 ± 1.47 ▲▲ | 1.29 ± 0.33 ▲▲ | 1.06 ± 0.17 ▲▲ |
| G-3 | 17.29 ± 1.36 ▲▲ | 15.63 ± 0.95 ▲▲ | 1.51 ± 0.26 ▲▲ | 0.99 ± 0.14 ▲▲ |
| G-4 | 10.14 ± 1.28 ▲ | 10.42 ± 1.56 ▲ | 2.21 ± 0.34 | 0.31 ± 0.022 |
| t/min | Solvent/% | |
|---|---|---|
| Acetonitrile | 0.2% Formic Acid | |
| 0 | 8 | 92 |
| 13 | 15 | 85 |
| 14 | 20 | 80 |
| 16 | 20 | 80 |
| 18 | 25 | 75 |
| 25 | 30 | 70 |
| 30 | 15 | 85 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, B.; Kan, Y.; Xie, J.; Hu, J.; Pang, W. Relevance of the Pharmacokinetic and Pharmacodynamic Profiles of Puerariae lobatae Radix to Aggregation of Multi-Component Molecules in Aqueous Decoctions. Molecules 2016, 21, 845. https://doi.org/10.3390/molecules21070845
Su B, Kan Y, Xie J, Hu J, Pang W. Relevance of the Pharmacokinetic and Pharmacodynamic Profiles of Puerariae lobatae Radix to Aggregation of Multi-Component Molecules in Aqueous Decoctions. Molecules. 2016; 21(7):845. https://doi.org/10.3390/molecules21070845
Chicago/Turabian StyleSu, Bili, Yongjun Kan, Jianwei Xie, Juan Hu, and Wensheng Pang. 2016. "Relevance of the Pharmacokinetic and Pharmacodynamic Profiles of Puerariae lobatae Radix to Aggregation of Multi-Component Molecules in Aqueous Decoctions" Molecules 21, no. 7: 845. https://doi.org/10.3390/molecules21070845






