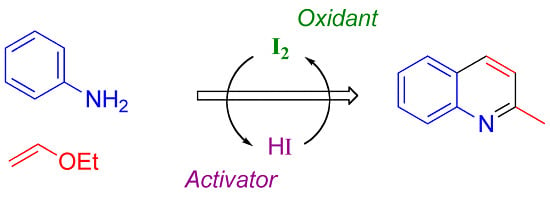
Dual Behavior of Iodine Species in Condensation of Anilines and Vinyl Ethers Affording 2-Methylquinolines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Iodine-Catalyzed Synthesis of 6-Substituted 2-Methylquinolines

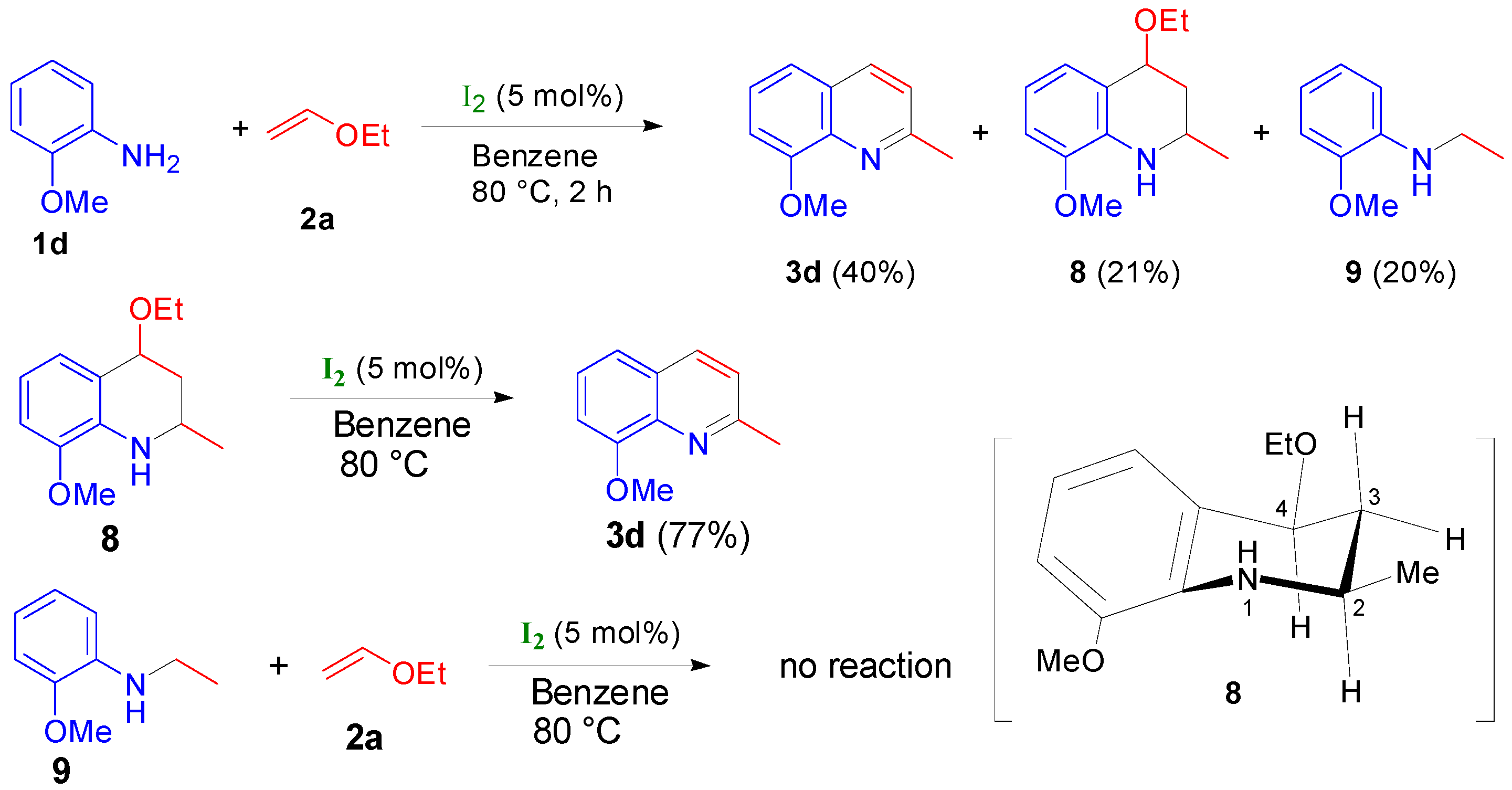
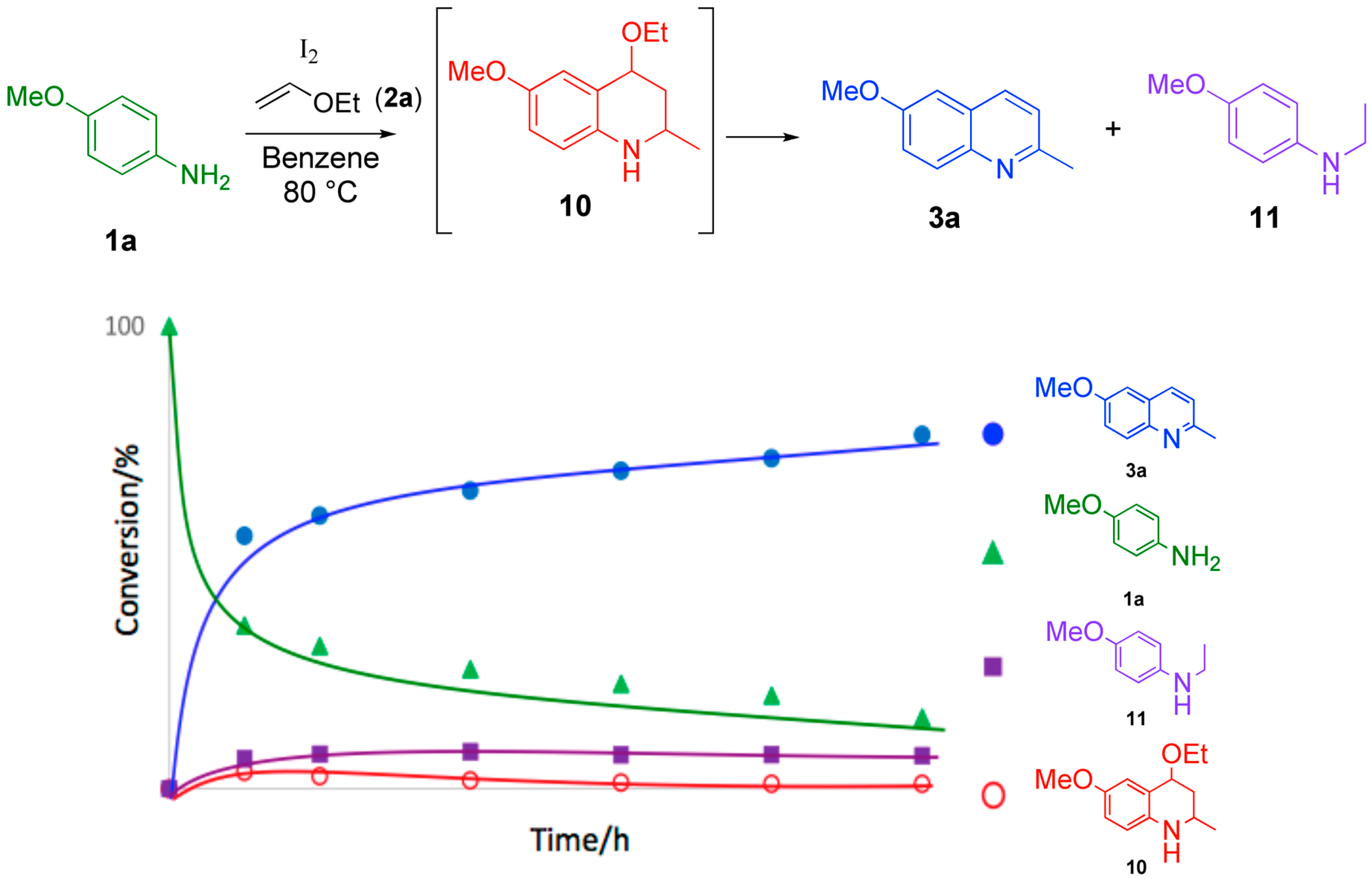
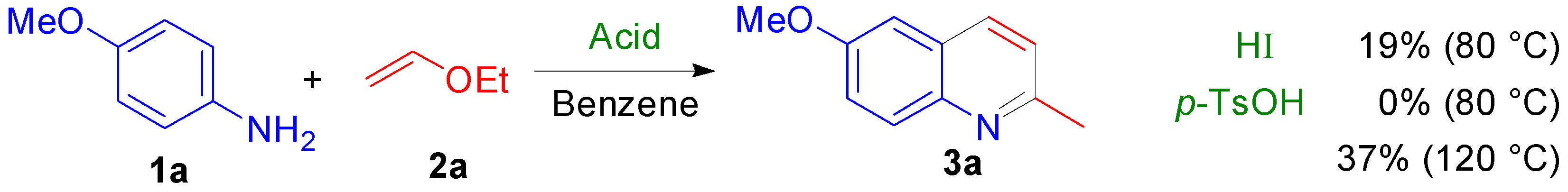
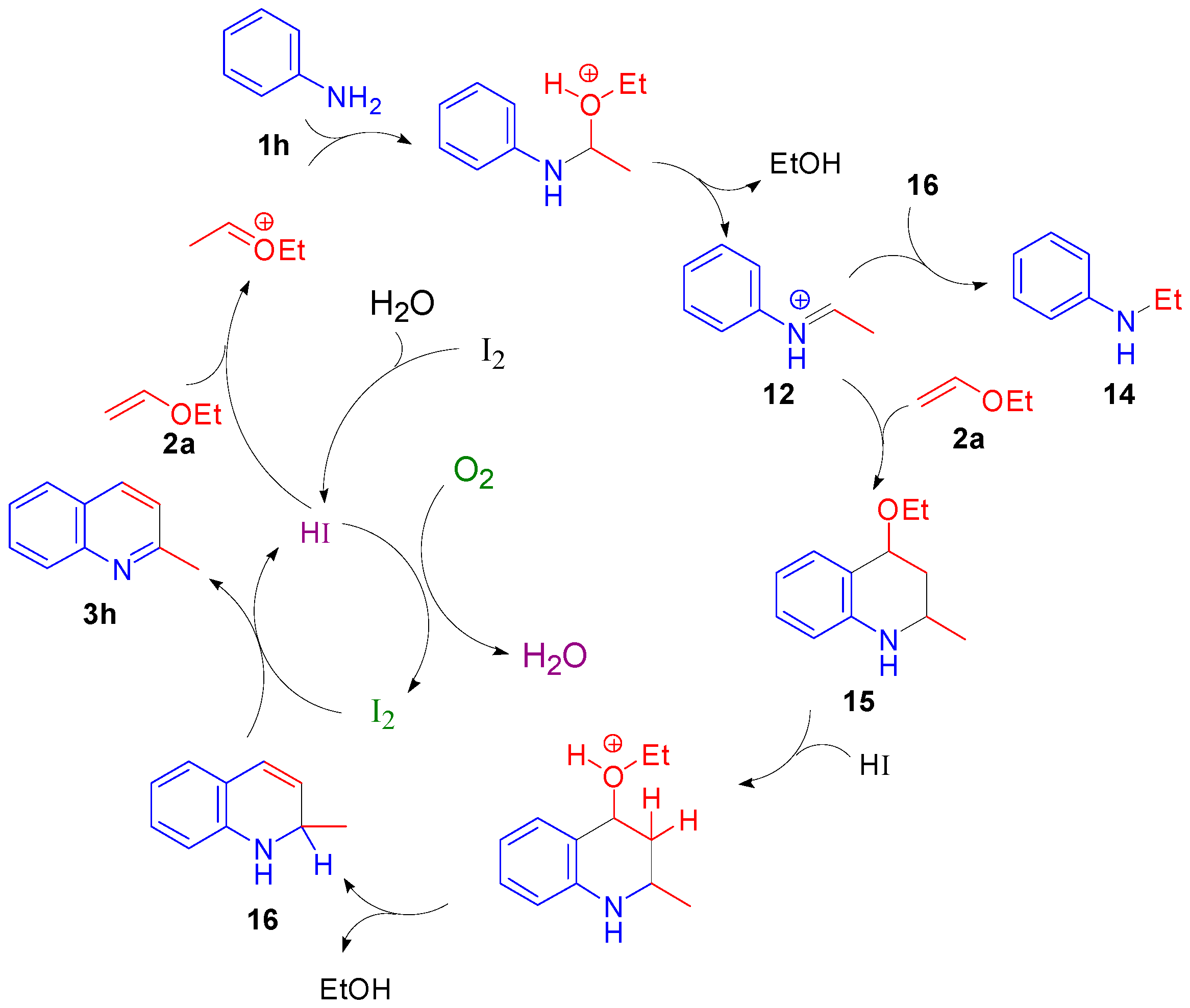
2.2. Study on the Mechanism
3. Materials and Methods
3.1. General Information
3.2. Procedures
Iodine-Mediated Synthesis of 2-Methylquinolines 3
3.3. Compound Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Kaiho, T. Iodine Chemistry and Applications; Kaiho, T., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Zhdankin, V.V. Hypervalent Iodine Chemistry: Preparation, Structure, and Synthetic Application of Polyvalent Iodine Compounds; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 337–380. [Google Scholar] [CrossRef]
- Ko, S.; Sastry, M.N.V.; Lin, C.; Yao, C.F. Molecular iodine-catalyzed one-pot synthesis of 4-substituted-1,4-dihydropyridine derivatives via Hantzsch reaction. Tetrahedron Lett. 2005, 46, 5771–5774. [Google Scholar] [CrossRef]
- Chen, W.Y.; Lu, J. Molecular-Iodine-Catalyzed One-Pot Synthesis of 1,5-Benzodiazepine Derivatives under Solvent-Free Conditions. Synlett 2005. [Google Scholar] [CrossRef]
- Lee, B.S.; Mahajan, S.; Janda, K.D. Molecular Iodine-Catalyzed Imine Activation for Three-Component Nucleophilic Addition Reactions. Synlett 2005. [Google Scholar] [CrossRef]
- Gogoi, P.; Hazarika, P.; Konwar, D. Surfactant/I2/Water: An Efficient System for Deprotection of Oximes and Imines to Carbonyls under Neutral Conditions in Water. J. Org. Chem. 2005, 70, 1934–1936. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Dong, Y.; Cao, L.; Wang, X.; Wang, S.; Hu, Y. Highly Efficient Chemoselective Deprotection of O,O-Acetals and O,O-Ketals Catalyzed by Molecular Iodine in Acetone. J. Org. Chem. 2004, 69, 8932–8934. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, K.V.; Das, B. Iodine Catalyzed One-Pot Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones and Thiones: A Simple and Efficient Procedure for the Biginelli Reaction. Synthesis 2004. [Google Scholar] [CrossRef]
- Take, Y.; Oogose, K.; Kubo, T.; Inouye, Y.; Nakamura, S.; Kitahara, Y.; Kubo, A. Comparative Study on Biological Activities of Heterocyclic Quinones and Streptonigrin. J. Antibiot. 1987, 40, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, I.A.; Johnson, F.; Grollman, A.P. Streptonigrin. 1. Structure-Activity Relationships among Simple Bicyclic Analogs. Rate Dependence of DNA Degradation on Quinone Reduction Potential. J. Med. Chem. 1986, 29, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.K.; Kim, H.J. The Synthesis of 6-(N-Arylamino)-7-chloro-5,8-quinolinedione Derivatives for Evaluation of Antifungal Activities. Arch. Pharm. Res. 1994, 17, 139–144. [Google Scholar] [CrossRef]
- Boger, D.L.; Yasuda, M.; Mitscher, L.A.; Drake, S.D.; Kitos, P.A.; Thompson, S.C. Streptonigrin and Lavendamycin Partial Structures. Probes for the Minimum, Potent Pharmacophore of Streptonigrin, Lavendamycin, and Synthetic Quinoline-5,8-diones. J. Med. Chem. 1987, 30, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.G.; He, H.Q.; Zeng, C.C.; Tan, J.J.; Hu, L.M.; Wang, C.X. Design, Synthesis and Anti-HIV Integrase Evaluation of N-(5-Chloro-8-hydroxy-2-styrylquinolin-7-yl)benzenesulfonamide Derivatives. Molecules 2010, 15, 1903–1917. [Google Scholar] [CrossRef] [PubMed]
- Mouscadet, J.-F.; Desmaële, D. Chemistry and Structure-Activity Relationship of the Styrylquinoline-type HIV Integrase Inhibitors. Molecules 2010, 15, 3048–3078. [Google Scholar] [CrossRef] [PubMed]
- Sestili, I.; Borioni, A.; Mustazza, C.; Rodomonte, A.; Turchetto, L.; Sbraccia, M.; Riitano, D.; Del Giudice, M.R. A New Synthetic Approach of N-(4-Amino-2-methylquinolin-6-yl)-2-(4-ethylphenoxymethyl) benzamide (JTC-801) and Its Analogues and Their Pharmacological Evaluation as Nociceptin Receptor (NOP) Antagonists. Eur. J. Med. Chem. 2004, 39, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Zouhiri, F.; Mouscadet, J.F.; Mekouar, K.; Desmaële, D.; Savouré, D.; Leh, H.; Subra, F.; le Bret, M.; Auclair, C.; d’Angelo, J. Structure-Activity Relationships and Binding Mode of Styrylquinolines as Potent Inhibitors of HIV-1 Integrase and Replication of HIV-1 in Cell Culture. J. Med. Chem. 2000, 43, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Fakhfakh, M.A.; Fournet, A.; Prina, E.; Mouscadet, J.F.; Franck, X.; Hocquemiller, R.; Figadère, B. Synthesis and Biological Evaluation of Substituted Quinolines: Potential Treatment of Protozoal and Retroviral Co-infections. Bioorg. Med. Chem. 2003, 11, 5013–5023. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Kayakiri, H.; Satoh, S.; Inoue, T.; Sawada, Y.; Inamura, N.; Asano, M.; Aramori, I.; Hatori, C.; Sawai, H.; et al. A Novel Class of Orally Active Non-peptide Bradykinin B2 Receptor Antagonists. 3. Discovering Bioisosteres of the Imidazo[1,2-a] Pyridine Moiety. J. Med. Chem. 1998, 41, 4062–4079. [Google Scholar] [CrossRef] [PubMed]
- Chern, J.; Huang, C.; Chang, P.; Talekar, R.S. Multifunctional Quinoline Derivatives as Anti-neurodegenerative Agents. U.S. Patent WO2014163622, 9 October 2014. [Google Scholar]
- Ariyasu, S.; Sawa, A.; Morita, A.; Hanaya, K.; Hoshi, M.; Takahashi, I.; Wang, B.; Aoki, S. Design and Synthesis of 8-Hydroxyquinoline-Based Radioprotective Agents. Bioorg. Med. Chem. 2014, 22, 3891–3905. [Google Scholar] [CrossRef] [PubMed]
- Fabian, J.; Nakazumi, H.; Matsuoka, M. Near-infrared Absorbing Dyes. Chem. Rev. 1992, 92, 1197–1226. [Google Scholar] [CrossRef]
- Mishra, A.; Behera, R.K.; Behera, P.K.; Mishra, B.K.; Behera, G.B. Cyanines during the 1990s: A Review. Chem. Rev. 2000, 100, 1973–2012. [Google Scholar] [CrossRef] [PubMed]
- Denmark, S.E.; Venkatraman, S. On the Mechanism of the Skraup-Doebner-Von Miller Quinoline Synthesis. J. Org. Chem. 2006, 71, 1668–1676. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Comprehensive Organic Name Reactions and Reagents; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Steck, E.A.; Hallock, L.L.; Holland, A.J.; Fletcher, L.T. Quinolines. V. Some Polysubstituted 4-(4’-Diethylamino-1’-methylbutylamino)-quinolines. J. Am. Chem. Soc. 1948, 70, 1012–1015. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.S.; Reddy, B.V.; Premalatha, K. Bi(OTf)3-Catalyzed Friedländer Hetero-Annulation: A Rapid Synthesis of 2,3,4-Trisubstituted Quinolines. Synlett 2004. [Google Scholar] [CrossRef]
- Henze, H.R.; Carroll, D.W. Utilization of n-Alkyl Methyl Ketones in the Pfitzinger Reaction. J. Am. Chem. Soc. 1954, 76, 4580–4584. [Google Scholar] [CrossRef]
- Matsubara, Y.; Hirakawa, S.; Yamaguchi, Y.; Yoshida, Z. Assembly of Substituted 2-Alkylquinolines by a Sequential Palladium-Catalyzed C-N and C-C Bond Formation. Angew. Chem. Int. Ed. Engl. 2011, 50, 7670–7673. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Xi, C. Copper-Promoted Tandem Reaction of Azobenzenes with Allyl Bromides via N=N Bond Cleavage for the Regioselective Synthesis of Quinolines. Org. Lett. 2015, 17, 5836–5839. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Verma, D.; Jain, S.L. Magnetically Separable Palladium–Graphene Nanocomposite as Heterogeneous Catalyst for the Synthesis of 2-Alkylquinolines via One Pot Reaction of Anilines with Alkenyl Ethers. Tetrahedron Lett. 2014, 55, 2406–2409. [Google Scholar] [CrossRef]
- Hu, Y.-Z.; Zhang, G.; Thummel, R.P. Friedländer Approach for the Incorporation of 6-Bromoquinoline into Novel Chelating Ligands. Org. Lett. 2003, 5, 2251–2253. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, B.R.; Miller, B.L. A Mild and Efficient One-step Synthesis of Quinolines. Org. Lett. 2003, 5, 4257–4259. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.S.; Reddy, B.V.; Sreedhar, P.; Rao, R.S.; Nagaiah, K. Silver Phosphotungstate: A Novel and Recyclable Heteropoly Acid for Friedländer Quinoline Synthesis. Synthesis 2004. [Google Scholar] [CrossRef]
- Mogilaiah, K.; Reddy, C.S. An Efficient Friedlander Condensation Using Sodium Fluoride as Catalyst in the Solid State. Synth. Commun. 2003, 33, 3131–3134. [Google Scholar] [CrossRef]
- Arumugam, P.; Karthikeyan, G.; Atchudan, R.; Muralidharan, D.; Perumal, P.T. A Simple, Efficient and Solvent-Free Protocol for the Friedländer Synthesis of Quinolines by Using SnCl2·2H2O. Chem. Lett. 2005, 34, 314–315. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, L.; Diao, T.-N. An Expeditious Approach to Quinolines via Friedländer Synthesis Catalyzed by FeCl3 or Mg(ClO4)2. Synlett 2005. [Google Scholar] [CrossRef]
- Lin, X.F.; Cui, S.L.; Wang, Y.G. Molecular Iodine-Catalyzed One-pot Synthesis of Substituted Quinolines from Imines and Aldehydes. Tetrahedron Lett. 2006, 47, 3127–3130. [Google Scholar] [CrossRef]
- Wang, X.S.; Li, Q.; Yao, C.S.; Tu, S.J. An Efficient Method for the Synthesis of Benzo[f]quinoline and Benzo[a]phenanthridine Derivatives Catalyzed by Iodine by a Three-Component Reaction of Arenecarbaldehyde, Naphthalen-2-amine, and Cyclic Ketone. Eur. J. Org. Chem. 2008. [Google Scholar] [CrossRef]
- Wang, X.S.; Li, Q.; Wu, J.-R.; Li, Y.L.; Yao, C.S.; Tu, S.J. An Efficient and Highly Selective Method for the Synthesis of 3-Arylbenzoquinoline Derivatives Catalyzed by Iodine via Three-Component Reactions. Synthesis 2008. [Google Scholar] [CrossRef]
- Wang, X.-S.; Zhou, J.; Yin, M.-Y.; Yang, K.; Tu, S.-J. Efficient and Highly Selective Method for the Synthesis of Benzo(naphtho)quinoline Derivatives Catalyzed by Iodine. J. Comb. Chem. 2010, 12, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Li, Q.; Wu, J.R.; Tu, S.J. Efficient Method for the Synthesis of Pyranoquinoline, Thiopyranoquinoline, Thienoquinoline, and Naphtho[2,7]naphthyridine Derivatives Catalyzed by Iodine. J. Comb. Chem. 2009, 11, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xia, H.G.; Gao, K. Molecular Iodine: A Highly Efficient Catalyst in the Synthesis of Quinolines via Friedländer Annulation. Org. Biomol. Chem. 2006, 4, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.F.; Cui, S.L.; Wang, Y.-G. A Highly Efficient Synthesis of 1,2,3,4-Tetrahydroquinolines by Molecular Iodine-Catalyzed Domino Reaction of Anilines with Cyclic Enol Ethers. Tetrahedron Lett. 2006, 47, 4509–4512. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, S.; Liu, W. Discovery and efficient synthesis of a biologically active alkaloid inspired by thiostrepton biosynthesis. Tetrahedron 2014, 70, 7686–7690. [Google Scholar] [CrossRef]
- Selvam, K.; Swaminathan, M. Cost effective one-pot photocatalytic synthesis of quinaldines from nitroarenes by silver loaded TiO2. J. Mol. Catal. A Chem. 2011, 351, 52–61. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Wang, B.; Li, W.; Huang, L.; Li, X. Design, Synthesis, and Evaluation of Orally Available Clioquinol-Moracin M Hybrids as Multitarget-Directed Ligands for Cognitive Improvement in a Rat Model of Neurodegeneration in Alzheimer’s Disease. J. Med. Chem. 2015, 58, 8616–8637. [Google Scholar] [CrossRef] [PubMed]
- Nizar, P.N.H.; Chauhan, S.M.S. Use of 5,8-dimethyoxyquinolines in the synthesis of novel 5,12-dihydroxy-6,11-dioxo-azanapthacenes. Org. Prep. Proced. Int. 1989, 21, 243–245. [Google Scholar] [CrossRef]
- Dong, X.; Heo, C.H.; Chen, S.; Kim, H.M.; Liu, Z. Quinoline-based two-photon fluorescent probe for nitric oxide in live cells and tissues. Anal. Chem. 2014, 86, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Petit, M.; Tran, C.; Roger, T.; Gallavardin, T.; Dhimane, H.; Palma-Cerda, F.; Blanchard-Desce, M.; Acher, F.C.; Ogden, D.; Dalko, P.I. Substitution effect on the one- and two-photon sensitivity of DMAQ “caging” groups. Org. Lett. 2012, 14, 6366–6369. [Google Scholar] [CrossRef] [PubMed]
- Krogul, A.; Litwinienko, G. One pot synthesis of ureas and carbamates via oxidative carbonylation of aniline-type substrates by CO/O2 mixture catalyzed by Pd-complexes. J. Mol. Catal. A Chem. 2015, 407, 204–211. [Google Scholar] [CrossRef]
- Chen, S.; Lu, G.; Cai, C. Synthesis of Quinolines from Allylic Alcohols via Iridium-Catalyzed Tandem Isomerization/Cyclization Combined with Potassium Hydroxide. Synthesis 2015, 47, 976–984. [Google Scholar] [CrossRef]
- Khusnutdinov, R.I.; Bayguzina, A.R.; Aminov, R.I. Synthesis of Substituted Quinolines by the Reaction of Anilines with Alcohols and CCl4 in the Presence of Fe Containing Catalysts. Russ. Chem. Bull. 2013, 62, 133–137. [Google Scholar] [CrossRef]
- Sridharan, V.; Avendaño, C.; Carlos Menéndez, J. CAN-catalyzed three-component reaction between anilines and alkyl vinyl ethers: Stereoselective synthesis of 2-methyl-1,2,3,4-tetrahydroquinolines and studies on their aromatization. Tetrahedron 2007, 63, 673–681. [Google Scholar] [CrossRef]
- Plas, A.; Martin, C.; Joubert, N.; Viaud-Massuard, M.-C. Palladium-Catalyzed Amination of N-Free 2-Chloro-7-azaindole. Org. Lett. 2015, 17, 4710–4713. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, A.; Spanedda, M.V.; Ourévitch, M.; Crousse, B.; Bonnet-Delpon, D. Uncatalysed Domino Reaction in Hexafluoroisopropanol: A Simple Protocol for the Synthesis of Tetrahydroquinoline Derivatives. Synthesis 2003. [Google Scholar] [CrossRef]
- Satpute, S.R.; Park, J.K.; Revankar, S.T. Effects of Excess Iodine and Water on Bunsen Reaction for Over-Azeotropic Limit. Adv. Chem. Eng. Res. 2015, 4, 1–14. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
| Entry | I2/mol % | Solv. | Yield/% | Recovery of 1a/% |
|---|---|---|---|---|
| 1 | 0 | CH2Cl2 | 0 | 88 |
| 2 | 5 | CH2Cl2 | 44 | 22 |
| 3 | 5 | MeCN | 43 | 24 |
| 4 | 5 | PhH | 64 | 3 |
| 5 | 10 | PhH | 55 | 11 |
| 6 | 1 | PhH | 11 | 71 |
| Entry | Aniline | Product | Yield of 3/% | Recovery of 1/% |
|---|---|---|---|---|
| 1 |  |  | 64 | 3 |
| 2 |  |  | 30 | 30 |
| 3 |  |  | 36 | 0 |
| 4 |  |  | 55 | 12 |
| 5 |  |  | 0 | 0 |
| 6 |  |  | 83 | 0 |
| 7 |  |  | 53 | 18 |
| 8 |  |  | 37 | 7 |
| Entry | Temp./°C | Time/h | Vinyl Ether | Product | Yield/% |
|---|---|---|---|---|---|
| 1 | 80 | 2 |  | 3a | 64 1 |
| 2 | 80 | 2 |  | 3a | 54 1 |
| 3 | 120 | 14 |  | 4 | 64 |
| 4 | 120 | 2 |  | 5 | 45 |
| 5 | 120 | 2 |  | 6 | 0 |
| 6 | 120 | 14 |  | 7 | 0 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, S.T.; Yasuoka, C.; Asahara, H.; Nishiwaki, N. Dual Behavior of Iodine Species in Condensation of Anilines and Vinyl Ethers Affording 2-Methylquinolines. Molecules 2016, 21, 827. https://doi.org/10.3390/molecules21070827
Le ST, Yasuoka C, Asahara H, Nishiwaki N. Dual Behavior of Iodine Species in Condensation of Anilines and Vinyl Ethers Affording 2-Methylquinolines. Molecules. 2016; 21(7):827. https://doi.org/10.3390/molecules21070827
Chicago/Turabian StyleLe, Song Thi, Chisa Yasuoka, Haruyasu Asahara, and Nagatoshi Nishiwaki. 2016. "Dual Behavior of Iodine Species in Condensation of Anilines and Vinyl Ethers Affording 2-Methylquinolines" Molecules 21, no. 7: 827. https://doi.org/10.3390/molecules21070827
APA StyleLe, S. T., Yasuoka, C., Asahara, H., & Nishiwaki, N. (2016). Dual Behavior of Iodine Species in Condensation of Anilines and Vinyl Ethers Affording 2-Methylquinolines. Molecules, 21(7), 827. https://doi.org/10.3390/molecules21070827