The Hedyotis diffusa Willd. (Rubiaceae): A Review on Phytochemistry, Pharmacology, Quality Control and Pharmacokinetics
Abstract
:1. Introduction
2. Phytochemistry
2.1. Iridoids and Triterpenes
2.2. Flavonoids
2.3. Anthraquinones
2.4. Phenolic Acids and Their Derivatives
2.5. Polysaccharides
2.6. Essential Oils
2.7. Cyclotides
2.8. Miscellaneous
3. Pharmacology
3.1. Anti-Cancer Activity
3.1.1. Anti-Colorectal Cancer Activity
3.1.2. Anti-Leukemia Activity
3.1.3. Anti-Liver Cancer Activity
3.1.4. Anti-Lung Cancer Activity
3.1.5. Anti-Breast Cancer Activity
3.1.6. Anti-Cervical Tumor Activity
3.1.7. Anti-Prostate Cancer Activity
3.1.8. Anti-Multiple Myeloma Activity
3.1.9. Other Anti-Cancer Effects
3.2. Immunomodulatory Effect
3.3. Antioxidant Effect
3.4. Anti-Inflammatory Effect
3.5. Others
4. Quality Control
5. Pharmacokinetics
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tao, C.; Taylor, C.M. Rubiaceae. In Flora of China; Wu, Z.Y., Raven, P.H., Hong, D.Y., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2011; Volume 19, pp. 147–174. [Google Scholar]
- Lin, J.M.; Li, Q.Y.; Chen, H.W.; Lin, H.; Lai, Z.J.; Peng, J. Hedyotis diffusa Willd extract suppresses proliferation and induces apoptosis via IL-6-inducible STAT3 pathway inactivation in human colorectal cancer cells. Oncol. Lett. 2015, 9, 1962–1970. [Google Scholar] [PubMed]
- Zhang, P.Y.; Zhang, B.; Gu, J.; Hao, L.; Hu, F.F.; Han, C.H. The study of the effect of Hedyotis diffusa on the proliferation and the apoptosis of the cervical tumor in nude mouse model. Cell Biochem. Biophys. 2015, 72, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, R.F.; Xiao, Q.G.; Li, R.; Shen, X.L.; Zhu, X.G. Hedyotis diffusa Willd extract inhibits the growth of human glioblastoma cells by inducing mitochondrial apoptosis via AKT/ERK pathways. J. Ethnopharmacol. 2014, 158, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.X.; Roubin, H.R.; Hanranhan, R.J. Ethnopharmacological and bioactivity guided investigation of five TCM anticancer herbs. J. Ethnopharmacol. 2013, 148, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.H.; Fu, P.K.; Chang, C.H.; Chang, S.N.; Mao, F.C.; Lin, C.H. Prescription patterns of Chinese herbal products for post-surgery colon cancer patients in Taiwan. J. Ethnopharmacol. 2014, 156, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.C.; Chen, H.Y.; Yang, S.H.; Lin, Y.H.; Chiu, J.H.; Lin, Y.H.; Chen, C.L. Hedyotis diffusa combined with scutellaria barbata Are the core treatment of Chinese herbal medicine used for breast cancer patients: A population-based Study. Evid. Based Complement Alternat. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wong, Y.L.; Jiang, L.L.; Wong, K.L.; Wong, Y.T.; Lau, C.B.; Shaw, P.C. Application of novel loop-mediated isothermal amplification (LAMP) for rapid authentication of the herbal tea ingredient Hedyotis diffusa Willd. Food Chem. 2013, 141, 2522–2525. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Z.; Bau, D.T.; Kuo, C.L.; Tsai, R.Y.; Chen, C.Y.; Chang, Y.H. Clarification of the phenotypic characteristics and anti-tumor activity of Hedyotis diffusa. Am. J. Chin. Med. 2011, 39, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.B.; Cheng, L.; Cheng, B.W.; Yue, G.G.; Wong, E.C.; Lau, C.P.; Leung, P.C.; Fung, K.P. Development of a simple chromatographic method for distinguishing between two easily confused species, Hedyotis diffusa and Hedyotis corymbosa. Nat. Prod. Res. 2012, 26, 1446–1450. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, C.; Mai, H.Z. Analysis of volatile compounds in Hedyotis diffusa and Hedyotis corymbosa. J. Chin. Mater. Med. 2003, 26, 563–564. [Google Scholar]
- Liu, Z.M.; Hao, M.G.; Wang, J.L. Application of allele-specific primer in the identification of Hedyotis diffusa. J. Chin. Mater. Med. 2004, 27, 484–487. [Google Scholar]
- Yang, Y.B.; Yang, X.Q.; Ding, Z.T. Chemical constituents from Hedyotis diffusa. Chin. J. Yunnan Univ. (Nat. Sci.) 2007, 29, 187–189. [Google Scholar]
- Liang, Z.T.; He, M.F.; Fong, W.F.; Jiang, Z.H.; Zhao, Z.Z. A comparable, chemical and pharmacological analysis of the traditional Chinese medicinal herbs Oldenlandia diffusa and O. corymbosa and a new valuation of their biological potential. Phytomedicine 2008, 15, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.H.; Zhou, T.; Li, G.B.; Li, J.; Huang, X.N.; Pan, F.; Gao, N. Characterization and identification of iridoid glucosides, flavonoids and anthraquinones in Hedyotis diffusa by high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. J. Sep. Sci. 2012, 35, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Nishihama, Y.; Masuda, K.; Yamaki, M.; Takagi, S.; Sakina, K. Three new iridoid glucosides from Hedyotis diffusa. DlantaMedica 1981, 43, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Luo, J.B. Studies on the chemical constituents in Herb of Hedyotis diffusa. J. Chin. Mater. Med. 2008, 31, 522–524. [Google Scholar]
- Ji, B.Y.; Fan, C.Q.; Fei, L.X.; Ma, Y. Advance on the chemical and pharmacological effects studies of Hedyotis diffusa. Chin. J. Exp. Tradit. Med. Form. 2014, 20, 235–240. [Google Scholar]
- Si, J.Y.; Chen, D.H.; Pan, R.L.; Zhao, X.H. Chemical constituents of Hedyotis Diffusa. Nat. Prod. Res. Dev. 2006, 18, 942–944. [Google Scholar]
- Zhang, Y.Y.; Chen, Y.; Fan, C.L.; Ye, W.C.; Luo, J.B. Two new iridoids from Hedyotis diffusa. Fitoterapia 2010, 81, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Z.; Wang, L. Studies on chemical constituents of Hedyotis diffusa Willd. J. Heibei Med. Univ. 2007, 28, 188–190. [Google Scholar]
- Li, C.M.; Zhao, Y.Y.; Guo, Z.M.; Zhang, X.L.; Xue, X.Y.; Liang, X.M. Effective 2D-RPLC/RPLC enrichment and separation of micro-components from Hedyotis diffusa Willd and characterization by using ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2014, 99, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Gu, D.W.; Zhang, H.; Zhu, Z.Y.; Zhang, G.Q.; Chai, Y.F. HPLC-TOEMS in rapid separation and identification of chemical components in Oldenlanda diffusa and its injection preparations. Acad.J. Second Mil. Med. Univ. 2010, 31, 292–296. [Google Scholar] [CrossRef]
- Li, C.; Xue, X.; Zhou, D.; Zhang, F.; Xu, Q.; Ren, L.; Liang, X. Analysis of iridoid glucosides in Hedyotis diffusa by high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2008, 48, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Li, D.X.; Schmitz, O.J. Comprehensive two-dimensional liquid chromatography tandem diode array detector (DAD) and accurate mass QTOF-MS for the analysis of flavonoids and iridoid glycosides in Hedyotis diffusa. Anal. Bioanal. Chem. 2015, 407, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.H.; Kim, Y.H.; Chi, S.W.; Choo, S.J.; Ryoo, I.J.; Ahn, J.S.; Yoo, I.D. Evaluation of human neutrophil elastase inhibitory effect of iridoid glycosides from Hedyotis diffusa. Bioorg. Med. Chem. Lett. 2010, 20, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.M.; Sun, Z.Y. Study on chemical constituents of Oldenlandia diffusa. Chin. J. Chin. Mater. Med. 2014, 37, 2216–2218. [Google Scholar]
- Ren, R.A. Identification of Chinese Drug; Shanghai Scientific & Technological Publishers: Shanghai, China, 1986; p. 491. [Google Scholar]
- Wu, K.S.; Zhang, K.; Tan, G.S.; Zeng, G.Y.; Zhou, Y.J. Study on constituents of Oldenlandia diffusa. Chin. Pharm. J. 2005, 40, 817–818. [Google Scholar]
- Liang, S.Y.; Chen, F.L.; Tang, Q.F.; Luo, J.B.; Zeng, Y.C. Study of Chemical Constituents from Herba Hedyotis diffusa. Tradit. Chin. Drug Res. Clin. Pharm. 2012, 23, 655–657. [Google Scholar]
- Zhou, Y.J.; Wu, K.S.; Zeng, G.R.; Tang, J.B.; Xu, K.P.; Li, F.S.; Tang, G.S. Study on chemical constituents of Oldenlandia diffusa. Chin. J. Chin. Mater. Med. 2007, 32, 590–593. [Google Scholar]
- Zhang, H.J.; Chen, Y.G.; Huang, R. Study on flavonoids constituents of Oldenlandia diffusa. Chin. J. Chin. Mater. Med. 2005, 28, 385–387. [Google Scholar]
- Lu, C.M.; Yang, J.J.; Wang, P.Y.; Lin, C.C. A new acylated flavonol glycoside and antioxidant effects of Hedyotis diffusa. Planta Med. 2000, 66, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, E.J.; Kim, J.; Kim, Y.; Kim, S.R.; Kim, Y.Y. Neuroprotective constituents from Hedyotis diffusa. J. Nat. Prod. 2001, 64, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.Z.; Liu, G.S.; Zhang, L.; Niu, G.Y. Study on Chemical constituents of Hedyotis diffusa. Chin. Pharm. J. 2005, 40, 502–504. [Google Scholar]
- Kang, X.D.; Li, X.; Mao, Y.; Zhao, C.C.; Li, N.; Meng, D.L. Chemical constituents of Hedyotis diffusa Willd. J. Shenyang Pharm. Univ. 2007, 24, 479–481. [Google Scholar]
- Liu, Y.Q.; Ying, W.J.; Liu, Y.; Feng, Y.N.; Lv, Q.T. Summarization on the chemical constituents of Oldenlandia Diffusa Willd. Shandong J. Tradit. Chin. Med. 2014, 33, 709–712. [Google Scholar]
- Shi, Y.; Wang, C.H.; Gong, X.G. Apoptosis-inducing effects of two anthraquinones from Hedyotis diffusa Wild. Biol. Pharm. Bull. 2008, 31, 1075–1078. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, J.M.; Jiang, Z.; Guo, X.J. A new anthraquinone from Hedyotis diffusa. Chin. J. Med. Chem. 2008, 18, 298–300. [Google Scholar]
- Tai, D.F.; Lin, Y.M.; Chen, F.C. Component of Hedyotis diffusa willd. Chemistry. 1979, 3, 60–61. [Google Scholar]
- Huang, W.H.; Li, Y.B.; Jiang, J.Q. Chemical constituents from Hedyotis diffusa. Chin. J. Chin. Mater. Med. 2008, 33, 524–526. [Google Scholar]
- Kang, X.D.; Li, X.; Mao, Y. A new anthraquinone from Hedyotis diffusa Willd. Chin. J. Chin. Mater. Med. 2006, 16, 368–370. [Google Scholar]
- Zhou, Y.; Gao, W.Y.; Wang, Y.; Liu, X.J. Studies on constituents of Oldenlandia diffusa. Chin. Tradit. Herb. Drug 2007, 38, 55–57. [Google Scholar]
- Lv, H.C.; He, J. A study on chemical constituents of Oldenlandia diffusa (Willd) Roxb. Nat. Prod. Res. Dev. 1996, 8, 34–37. [Google Scholar]
- Ruehle, P.H.; Browne, C.E.; Vickery, E.H.; Beller, N.R.; Eisenbraun, E.J.; Loghry, R.A.; Van der Helm, D. Synthesis and antifertility activity of 3,9-dihydroxy-5,6,6a alpha,6b beta,11,12,12a beta,12b alpha-octahydrodibenzo[a,g]biphenylene, a structural relative of diethylstilbestrol. J. Med. Chem. 1980, 23, 1410–1414. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Li, Y.; Jiang, J. Chemical constituents from Hedyotis diffusa. Chin. J. Chin. Mater. Med. 2009, 34, 712–714. [Google Scholar]
- Tan, N.H.; Wang, S.M.; Yang, Y.B.; Tian, F. Anticancer activity and principles of Hedyotis diffusa. Nat. Prod. Res. Dev. 2002, 14, 33–36. [Google Scholar]
- Liu, Z.G.; Luo, J.B.; Chen, F.L. The Pilot Study of Volatile Compounds in Hedyotis diffusa from different sources. Tradit. Chin. Drug Res. Clin. Pharm. 2005, 16, 132–134. [Google Scholar]
- Yang, S.; Yang, W.W.; Hu, J.F.; Lv, Q.F.; Rong, R.; Jiang, H.Q.; Gong, L.L. GC-MS combined with Kovats Index analysis for volatile compounds in Hedyoti diffusae. Chin. J. Exp. Tradit. Med. Form. 2012, 18, 93–95. [Google Scholar]
- Ma, H.; Cheng, Y.L.; Zhang, J.J.; Cao, G.S.; Yang, P.M. Effect of preliminary immune activity and structural identification of a polysaccharide extracted from Oldenlandia diffusa. Chin. J. Exp.Tradit. Med. Form. 2014, 20, 37–40. [Google Scholar]
- Hu, E.; Wang, D.G.; Chen, J.Y.; Tao, X.L. Novel cyclotides from Hedyotis diffusa induce apoptosis and inhibit proliferation and migration of prostate cancer cells. Int. J. Clin. Exp. Med. 2015, 8, 4059–4065. [Google Scholar] [PubMed]
- Li, M.; Jiang, R.W.; Hon, P.M.; Cheng, L.; Li, L.L.; Zhou, J.R.; Shaw, P.C.; But, P.P. Authentication of the anti-tumor herb Baihuasheshecao with bioactive marker compounds and molecular sequences. Food Chem. 2010, 119, 1239–1245. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, W.M.; Yao, X.M.; Guo, X.J. Qualitative analysis of the chemical constituents in Hedyotis diffusa by HPLC-TOF-MS. Nat. Prod. Res. 2012, 26, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.Y.; Fan, W.P.; Ju, P.P. Extraction and deputation of polysaccharide from Hedyotis diffusa willd. Acta Universitatis Medicinalis Nanjing 2001, 21, 558. [Google Scholar]
- Lin, M.H.; Lin, J.M.; Wei, L.H.; Xu, W.; Hong, Z.F.; Cai, Q.Y.; Peng, J.; Zhu, D.Z. Hedyotis diffusa Willd extract inhibits HT-29 cell proliferation via cell cycle arrest. Exp. Ther. Med. 2012, 4, 307–310. [Google Scholar] [PubMed]
- Lin, J.M.; Chen, Y.Q.; Wei, L.H.; Chen, X.Z.; Xu, W.; Hong, Z.F. Hedyotis diffusa Willd extract induces apoptosis via activation of the mitochondrion-dependent pathway in human colon carcinoma cells. Int. J. Oncol. 2010, 37, 1331–1338. [Google Scholar] [PubMed]
- Lin, J.M.; Wei, L.H.; Xu, W.; Hong, Z.F.; Liu, X.X.; Peng, J. Effect of Hedyotis diffusa Willd extract on tumor angiogenesis. Mol. Med. Rep. 2011, 4, 1283–1288. [Google Scholar] [PubMed]
- Lin, J.M.; Wei, L.H.; Shen, A.L.; Cai, Q.Y.; Xu, W.; Li, H. Hedyotis diffusa Willd extract suppresses Sonic hedgehog signaling leading to the inhibition of colorectal cancer angiogenesis. Int. J. Oncol. 2013, 42, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.Y.; Lin, J.M.; Wei, L.H.; Zhang, L.; Wang, L.L.; Zhan, Y.Z. Hedyotis diffusa Willd inhibits colorectal cancer growth In Vivo via inhibition of STAT3 signaling pathway. Int. J. Mol. Sci. 2012, 13, 6117–6128. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.P.; Jin, C.G.; Li, J.; Chen, X.Q.; Yao, Q.; Zhu, Q.S. Inhibition of colon cancer cells by ethanol extract of Oldenlandia diffusa. J. Kunming Med. Univ. 2013, 34, 31–34. [Google Scholar]
- Li, Q.Y.; Wang, X.F.; Shen, A.L.; Zhang, Y.C.; Chen, Y.Q.; Thomas, J.S.; Lin, J.M.; Peng, J. Hedyotis diffusa Willd overcomes 5-fluorouracil resistance in human colorectal cancer HCT-8/5-FU cells by downregulating the expression of P-glycoprotein and ATP-binding casette subfamily G member 2. Exp. Ther. Med. 2015, 10, 1845–1850. [Google Scholar] [PubMed]
- Ganbolda, M.; Barkera, J.; Ma, R.; Jones, L.; Carew, M. Cytotoxicity and bioavailability studies on a decoction of Oldenlandia diffusa and its fractions separated by HPLC. J. Ethnopharmacol. 2010, 131, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.C.; Pan, R.B.; Wang, Q. Research on the mechanisms of inhibiting effects of the aqueous extract of Hedyotis diffusa Willd on CEM cells. Lishizhen Med. Mater. Med. Res. 2014, 25, 827–829. [Google Scholar]
- Lin, C.C.; Kuo, C.L.; Lee, M.H.; Hsu, S.C.; Huang, A.C.; Tang, N.Y.; Lin, J.P.; Yang, J.S.; Lu, C.C.; Chiang, J.H. Extract of Hedyotis diffusa Willd influences murine leukemia WEHI-3 cells in vivo as well as promoting T- and B-cell proliferation in leukemic mice. In Vivo 2011, 25, 633–640. [Google Scholar] [PubMed]
- Chen, X.H.; Gao, R.L.; Qian, X.D.; Wang, X.; Tan, P.L.; Yin, L.M.; Zhou, Y.H. Inhibition effect of Hedyotis diffusa wild injection on HL-60 cells and its mechanism. J. Exp. Hematol. 2008, 16, 1035–1038. [Google Scholar]
- Kuo, Y.J.; Yang, J.S.; Lu, C.C.; Chiang, S.Y.; Lin, J.G.; Chung, J.G. Ethanol extract of Hedyotis diffusa willd upregulates G0/G1 phase arrest and induces apoptosis in human leukemia cells by modulating caspase cascade signaling and altering associated genes expression was assayed by cDNA microarray. Environ. Toxicol. 2015, 30, 1162–1177. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Li, D.Y.; Niu, H.Y.; Zhang, Y.; He, P.; Wang, J.H. 2-Hydroxy-3-methylanthraquinone from Hedyotis diffusa Willd induces apoptosis in human leukemic U937 cells through modulation of MAPK pathways. Arch. Pharm. Res. 2013, 36, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Shu, L.H.; Yang, L.L.; Zhang, M.; Zhang, M.; He, P. 2-Hydroxy-3-methylanthraquinone from Hedyotis diffusa WILLD Induces apoptosis via alteration of Fas/FasL and activation of caspase-8 in human Leukemic THP-1 Cells. Arch. Med. Res. 2011, 42, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, J.; Song, J. Experimental research on effect of Hedyotis diffusa Willd on blood metastasis in H22 mice. Lishizhen Med. Mater. Med. Res. 2012, 23, 2434–2435. [Google Scholar]
- Chen, X.Z.; Cao, Z.Y.; Chen, T.S.; Zhang, Y.Q.; Liu, Z.Z.; Su, Y.T. Water extract of Hedyotis Diffusa Willd suppresses proliferation of human HepG2 cells and potentiates the anticancer efficacy of low-dose 5-fluorouracil by inhibiting the CDK2-E2F1 pathway. Oncol. Rep. 2012, 28, 742–748. [Google Scholar] [PubMed]
- Zhang, Y.B.; Zhu, J.; Xiao, J.X.; Guo, Y.H.; Liao, Z.J.; Xu, R. Effect and mechanism of total flavones of Oldenlendia diffusa willd on epithelial-mesenchymal transition of cell line MHCC97-H induced by TGF-β1. J. Xi’an Jiaotong Univ. (Med. Sci.) 2016, 37, 279–282. [Google Scholar]
- Li, Y.L.; Zhang, J.; Min, D.; Hongyan, Z.; Lin, N.; Li, Q.S. Anticancer effects of 1,3-dihydroxy-2-methyl anthraquinone and the ethyl acetate fraction of Hedyotis diffusa willd against HepG2 carcinoma cells mediated via apoptosis. PLoS ONE 2016. [Google Scholar] [CrossRef]
- Dong, Q.; Ling, B.; Gao, B.; Maley, J.; Sammynaiken, R.; Yang, J. Hedyotis diffusa water extract diminished the cytotoxic effects of chemotherapy drugs against human breast cancer MCF7 cells. Nat. Prod. Commun. 2014, 9, 699–700. [Google Scholar] [PubMed]
- Liu, Z.; Liu, M.; Liu, M.; Li, J.C. Methyl anthraquinone from Hedyotis diffusa WILLD induces Ca2+-mediated apoptosis in human breast cancer cells. Toxicol. In Vitro 2010, 24, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Shen, C.Y.; Jiang, J.P.; Wu, L.Q.; Dai, T.Y.; Qian, W.B. Meng HT Apoptosis of multiple myeloid cells induced by polysaccharides extracts from Hedyotis diffusa and its mechanism. Chin. J. Hematol. 2013, 34, 337–340. [Google Scholar]
- Zhang, X.; Ye, B.D.; Lin, S.Y. Effects of Hedyotis diffusa Willd injection on the proliferation of RPMI 8226 cells. Chin. J. Integr. Tradit. West. Med. 2012, 32, 1658–1662. [Google Scholar]
- Zhao, H.R.; Li, R.; Lin, Y.N.; Cheng, N.L. Influence of extraction on process of Hedyotis diffusa on anti-tumor activity. J. Chin. Pharm. Univ. 2002, 33, 510–513. [Google Scholar]
- Huang, Y.L.; Tang, Y.J.; Wang, J.L.; Xie, K.G.; Huang, K. Effect of Hedyotic diffusa willd injection on osteosarcoma MG-63 cells bax gene expression. Chongqing Med. 2014, 43, 4708–4710. [Google Scholar]
- Xie, K.G.; Tang, Y.J.; Huang, Y.L.; Huang, K.; Lu, L.; Lin, J.J.; Lu, X.Z. Effect of different concentration and action time spreading Hedyotis herb injection induced MG-63 cells apoptosis. Med. Innov. China 2016, 13, 255–263. [Google Scholar]
- Pu, F.; Chen, F.; Lin, S.; Chen, S.; Zhang, Z.; Wang, B.; Shao, Z. The synergistic anticancer effect of cisplatin combined with Oldenlandia diffusa in osteosarcoma MG-63 cell line in vitro. OncoTargets Ther. 2016, 9, 255–263. [Google Scholar]
- Yue, G.G.L.; Lee, J.K.M.; Kwok, H.F.; Cheng, L.; Wong, E.C.W.; Jiang, L.; Yu, H.; Leung, H.W.; Wong, Y.L.; Leung, P.C.; et al. Novel PI3K/AKT targeting anti-angiogenic activities of 4-vinylphenol, a new therapeutic potential of a well-known styrene metabolite. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.J.; Lin, J.P.; Hsiao, Y.T.; Chou, G.L.; Tsai, Y.H.; Chiang, S.Y.; Lin, J.G.; Chuang, J.G. Ethanol extract of Hedyotis diffusa Willd affects immune responses in vivo. In Vivo 2015, 29, 453–460. [Google Scholar] [PubMed]
- Wang, Y.L.; Zhang, Y.; Fang, M.; Li, Q.J.; Jiang, Q.; Ming, L. Immunomdulatory effects of total flavones of Oldenlandia diffusa willd. Chin. Pharmacol. Bull. 2005, 21, 444–447. [Google Scholar]
- Yang, X.Z.; Hao, Z.Y.; Zhu, Y.C.; Dong, Y. Effects of different solvents and extraction methods on antioxidant activity of Hedyotis diffusa Extract. Guizhou Agric. Sci. 2014, 42, 43–45. [Google Scholar]
- Yu, X.; Du, Z.J.; Chen, Y.J.; Huang, T.Q. Study on antioxidant effect from Oldenlandia diffusa Willd. Food Ferment. Ind. 2002, 28, 10–13. [Google Scholar]
- Gao, X.; Li, C.; Tang, Y.L.; Zhang, H.; Chan, S.W. Effect of Hedyotis diffusa water extract on protecting human hepatocyte cells (LO2) from H2O2-induced cytotoxicity. Pharm. Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.H.; Liu, M.H.; Zhang, X.L.; He, J.Y. Chemical profiles and protective effect of Hedyotis diffusa Willd in lipopolysaccharide-induced renal inflammation mice. Int. J. Mol. Sci. 2015, 16, 27252–27269. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lin, Y.; Li, Y.; Li, C. Total flavonoids of Hedyotis diffusa willd inhibit inflammatory responses in LPS-activated macrophages via suppression of the NF-κB and MAPK signaling pathways. Exp. Ther. Med. 2016, 11, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.K.; Tai, W.C.S.; Liang, Z.T.; Zhao, Z.Z.; Hsiao, W.L.W. Oleanolic acid isolated from Oldenlandia diffusa exhibits a unique growth inhibitory effect against ras-transformed fibroblasts. Life Sci. 2009, 85, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.S.; Liu, M.H.; He, J.Y. Hypericum japonicum Thunb. ex murray: Phytochemistry, pharmacology, quality Ccontrol and pharmacokinetics of an important herbal medicine. Molecules 2014, 19, 10733–10754. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Yang, Y.H.; Yang, J.Y.; Chen, B.M.; Chen, Y.X.; Yu, S.Y.; Duan, J.P.; Ouyang, H.T.; Cheng, J.P. Finger print of Hedyotis diffusa Willd by LC-MS. Chin. Med. Herb. 2007, 4, 21–23. [Google Scholar]
- Liang, Z.T.; Jiang, Z.H.; Ho, H.; Zhao, Z.Z. Comparative analysis of Oldenlandia diffusa and its substitues by high performance liquid chromatographic fingerprint and mass spectrometric analysis. Planta Med. 2007, 73, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.Q.; Li, R.R.; Jin, Y.; Li, H.X.; Feng, X.F. Quality standard of Hedyotis diffusae. Chin. J. Exp. Tradit. Med. Form. 2014, 20, 98–101. [Google Scholar]
- Lu, W.B. Quantitative determination of oleanolic acid in Oldenlandia diffusa (Willd) Roxb. by TLC-scaning. Lishizhen Med. Mater. Med. Res. 2001, 12, 961–962. [Google Scholar]
- Cao, G.S.; Yang, P.M.; Wang, X.F.; Li, H.; Gao, P. Determination of isoseutellarein in different parts of Hedyotis diffusa in different harvest time by HPLC. Chin. J. Exp. Tradit. Med. Form. 2014, 20, 49–51. [Google Scholar]
- Zhang, C.H.; Guo, X.J.; Xue, X.F.; Yang, L.; Ran, G.M.; Li, F.M. Determination of p-comnaric acid in Baihua Sheshecao injection by HPLC. Chin. Pharm. J. 2004, 39, 854–855. [Google Scholar]
- Zhang, C.H.; Guo, X.J.; Bao, L.D.; Qin, F.; Li, M. Determination of p-coumaric acid in Hedyotis diffusae Willd. From different source by reversed-phase high-performance liquid chromatography. Chin. J. Chromatogr. 2005, 23, 180–182. [Google Scholar]
- Ma, L.; Li, J.M.; Chen, Y.Q.; Li, Y.; Guo, X.J. Determination of 3,4-dihydroxy Methyl Benzoate in Hedyotis diffusa Willd by HPLC. Lishizhen Med. Mater. Med. Res. 2009, 20, 528–529. [Google Scholar]
- Ling, Y.Z. Separation and content determination of polysassharides in Hedyotis diffusa Willd. Biotechnology 2005, 15, 48–50. [Google Scholar]
- Yang, Y.C.; Wei, M.C.; Chiu, H.F.; Huang, T.C. Development and validation of a modified ultrasound-assisted extraction method and a HPLC method for the quantitative determination of two triterpenic acids in Hedyotis diffusa. Nat. Prod. Commun. 2013, 8, 1683–1686. [Google Scholar] [PubMed]
- Zhang, Y.; Tan, X.H.; Cui, X.B.; Jiang, G.B.; Zhu, Y.L. Quantitative determination of ursolic acid and oleanolic acid in Baihuasheshecao (Herba Hedyotis diffusae) produced from different places by HPLC. J. Beijing Univ. Tradit. Chin. Med. 2010, 33, 274–276. [Google Scholar]
- Zhou, S.Q.; Leng, G.H. Mensunation of content of oleanolic acid and maloic acid of Hedyotis diffuse Wild by HPLC. J. Anhui Agric. Sci. 2006, 34, 1785–1787. [Google Scholar]
- Yang, Y.H.; Chen, Y.X. Determination of oleanolic acid and ursolic acid in Oldenlandia diffusa (Willd) Roxb. by LC/MS. Herb. Med. 2008, 27, 589–591. [Google Scholar]
- Liu, Y.Q.; Ying, W.J.; Zuo, L.; Man, Q.Q.; Lv, H.T.; Jiang, H.Q.; Gong, L.L. Determination of two anthraquinones in Hedyotis diffusa by HPLC. Chin. J. Exp. Tradit. Med. Form. 2014, 20, 42–44. [Google Scholar]
- Liang, Z.T.; Jiang, Z.H.; Leung, K.S.Y.; Zhao, Z.Z. Determination of iridoid glucosides for quality assessment of herba oldenlandiae by high-performance liquid chromatography. Chem. Phann. Bull. 2006, 54, 1131–1137. [Google Scholar] [CrossRef]
- Zhang, H.F.; Zhang, H.Y.; Li, Y.; Guo, X.J. Simultaneous determination of four components in Hedyotis diffusa oral solution by reversed-phase HPLC. Chin. J. Chin. Mater. Med. 2008, 33, 2329–2331. [Google Scholar]
- Cheung, H.Y.; Cheung, S.H.; Law, M.L.; Lai, W.P. Simultaneous determination of key bioactive components in Hedyotis diffusa by capillary electrophoresis. J. Chromatogr. B 2006, 834, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.M.; Cao, G.S.; Li, F.; Gao, P. Contents of five iridoids in Ooldenlandia diffusa (Wind) Roxb. based on HPLC-DAD. Chin. Hosp. Pharm. J. 2015, 35, 9–12. [Google Scholar]
- Cao, G.S.; Yang, P.M.; Li, F.; Li, J. Determination of six active flavonoids in Oldenlandia diffusa based on HPLC-DAD. Chin. J. Exp. Tradit. Med. Form. 2014, 20, 52–55. [Google Scholar]
- Zhai, X.; Lv, Y. Simultaneous determination of 7 active components in Hedyotis diffuse by HPLC. Chin. Pharm. 2016, 19, 70–72. [Google Scholar]
- Liu, K.; Yan, L.Q.; Yao, G.C.; Guo, X.J. Estimation of p-coumaric acid as metabolite of E-6-O-p-coumaroyl scandoside methyl ester in rat plasma by HPLC and its application to a pharmacokinetic study. J. Chromatogr. B 2006, 831, 303–306. [Google Scholar] [CrossRef] [PubMed]
- An, L.Z.; Wang, Y.F.; Yu, Q.R. HPLC-TOF-MS analysis of metabolites of Oldenlandia diffusa effective extracts in rats. Chin. J. Chin. Mater. Med. 2011, 36, 1301–1304. [Google Scholar]
| Compounds | R1 | R2 |
|---|---|---|
| 45. Quercetin | OH | H |
| 46. Rutin | OH | rutinose |
| 47. Quercetin-3-O-β-d-glucopyranside | OH | β-d-Glc |
| 48. Quercetin-3-O-β-d-galactopyranoside | OH | β-d-Gal |
| 49. Quercetin-3-O-(2-O-glucopyranosyl)-β-d-glucopyranside | OH | β-d-Glc-(1→2)-d-Glc |
| 50. Quercetin-3-O-(2-O-glucopyranosyl)-β-d-galactopyranoside | OH | β-d-Glc-(1→2)-d-Gal |
| 51. Quercetin-3-O-sambubioside | OH | β-d-Xyl-(1→2)-d-Glc |
| 52. Quercetin-3-O-[2-O-(6-O-E-ferloyl)-β-d-glucopyranosyl]-β-d-galactopyranoside | OH | 6′-O-E-feruloyl-β-d-Glc-(1→2)-d-Gal |
| 53. Quercetin-3-O-[2-O-(6-O-E-feruloyl)-β-d-glucopyranosyl]-β-d-glucopyanoside | OH | 6′-O-E-feruloyl-β-d-Glc-(1→2)-d-Glc |
| 54. Quercetin-3-O-[2-O-(6-O-E-sinapoyl)-β-d-glucopyranosyl]-β-d-glucopyanoside | OH | 6′-O-E-sinapoyl-β-d-Glc-(1→2)-d-Glc |
| 55. Quercetin-3-O-[2-O-(6-O-E-sinapoyl)-β-d-glucopyranosyl]-β-d-galactopyranoside | OH | 6′-O-E-sinapoyl-β-d-Glc-(1→2)-d-Gal |
| 56. Kaempferol | H | H |
| 57. Kaempferol-3-O-β-d-glucopyranside | H | β-d-Glc |
| 58. Kaempferol-3-O-β-d-galactopyranoside | H | β-d-Gal |
| 59. Kaempferol-3-O-(2-O-β-d-glucopyranosyl)-β-d-galactopyranoside | H | β-d-Glc-(1→2)-d-Gal |
| 60. Kaempferol-3-O-(6-O-α-l-rhamnosyl)-β-d-glucopyranside | H | α-l-Rha-(1→6)-β-d-Glc |
| 61. Kaempferol-3-O-[2-O-(6-O-E-ferloyl)-β-d-glucopyranosyl]-β-d-glucopyranside | H | 6′-O-E-feruloyl-β-d-Glc-(1→2)-d-Glc |
| 62. Kaempferol-3-O-[2-O-(6-O-E-feruloyl)-β-d-glucopyranosyl]-β-d-galactopyranoside | H | 6′-O-E-feruloyl-β-d-Glc-(1→2)-d-Gal |
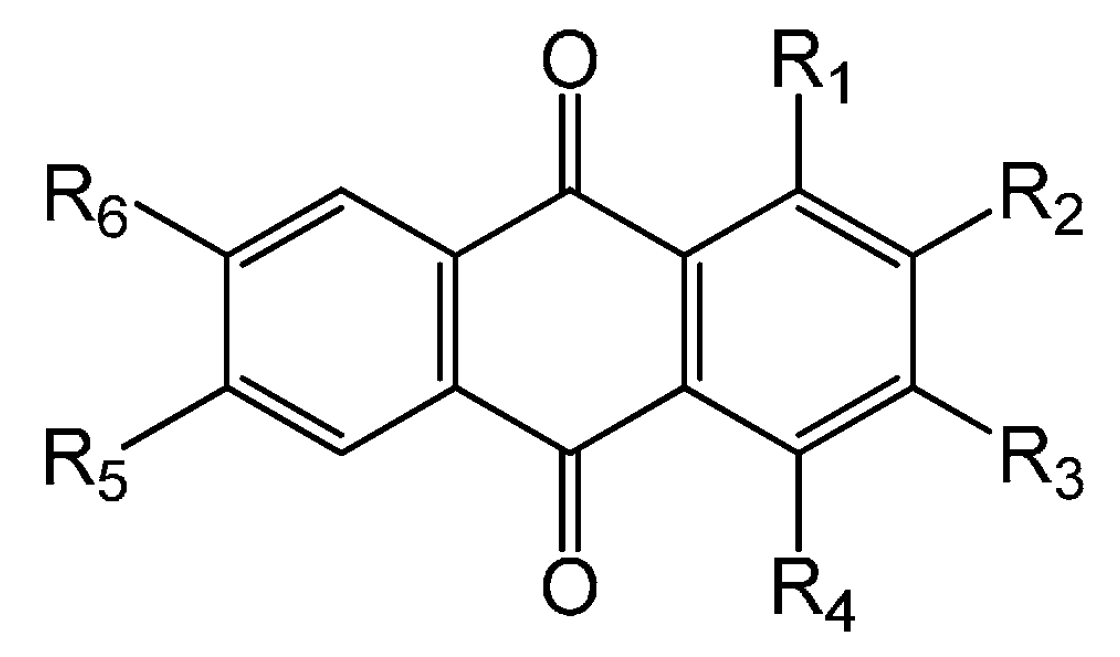
| Compounds | R1 | R2 | R3 | R4 | R5 | R6 |
|---|---|---|---|---|---|---|
| 63. 2-Methyl-3-methoxy anthraquinone | H | CH3 | OCH3 | H | H | H |
| 64. 2-Hydroxy-1,3-dimethoxy anthraquinone | OCH3 | OH | OCH3 | H | H | H |
| 65. 2-Hydroxy-3-methyl-1-methoxy anthraquinone | OCH3 | OH | CH3 | H | H | H |
| 66. 2-Hydroxy-3-methyl-4-methoxy anthraquinone | H | OH | CH3 | OCH3 | H | H |
| 67. 2-Hydroxy-7-methyl-3-methoxy anthraquinone | H | OH | OCH3 | H | H | CH3 |
| 68. 2-Hydroxy-1-methoxy-3-methyl anthraquinone | OCH3 | OH | CH | H | H | H |
| 69. 2-Hydroxy-3-methyl anthraquinone | H | OH | CH3 | H | H | H |
| 70. 2-Hydroxy-1-methoxy anthraquinone | OCH3 | OH | H | H | H | H |
| 71. 2-Hydroxy-4-methoxy anthraquinone | H | OH | H | OCH3 | H | H |
| 72. 2-Hydroxy-3-methoxy-7-methyl anthraquinone | H | OH | OCH3 | H | H | CH3 |
| 73. 2-Hydroxy-6-methyl anthraquinone | H | OH | H | H | CH3 | H |
| 74. 2-Hydroxy-3-methoxy-6-methyl anthraquinone | H | OH | OCH3 | H | CH3 | H |
| 75. 2,7-Dihydroxy-3-methyl anthraquinone | H | OH | CH3 | H | H | OH |
| 76. 3-Hydroxy-2-methyl anthraquinone | H | CH3 | OH | H | H | H |
| 77. 3-Hydroxy-2-methyl-4-methoxy anthraquinone | H | CH3 | OH | OCH3 | H | H |
| 78. 2,3-Dimethoxy-6-methyl anthraquinone | H | OCH3 | OCH3 | H | CH3 | H |
| 79. 1,3-Dihydroxy-2-methyl anthraquinone | OH | CH3 | OH | H | H | H |
| 80. 1,7- Dihydroxy-6-methoxy-2-methyl anthraquinone | OH | CH3 | H | H | OCH3 | OH |
| 81. 3-Hydroxy-2-methyl-4-methoxy anthraquinone | H | CH3 | OH | OCH3 | H | H |
| 82. 2,6-Dihydroxy-3-methyl-4-methoxy anthraquinone | H | OH | CH3 | OCH3 | OH | H |
| 83. 2,6-Dihydroxy-1-methoxy-3-methyl anthraquinone | OCH3 | OH | CH3 | H | OH | H |
| 84. 1-Hydroxy-4-methoxy anthraquinone | OH | H | H | OCH3 | H | H |
| 85. 2-hydroxymethyl-1-hydroxy anthraquinone | OH | CH2OH | H | H | H | H |
| 86. 2-hydroxymethyl anthraquinone | H | CH2OH | H | H | H | H |
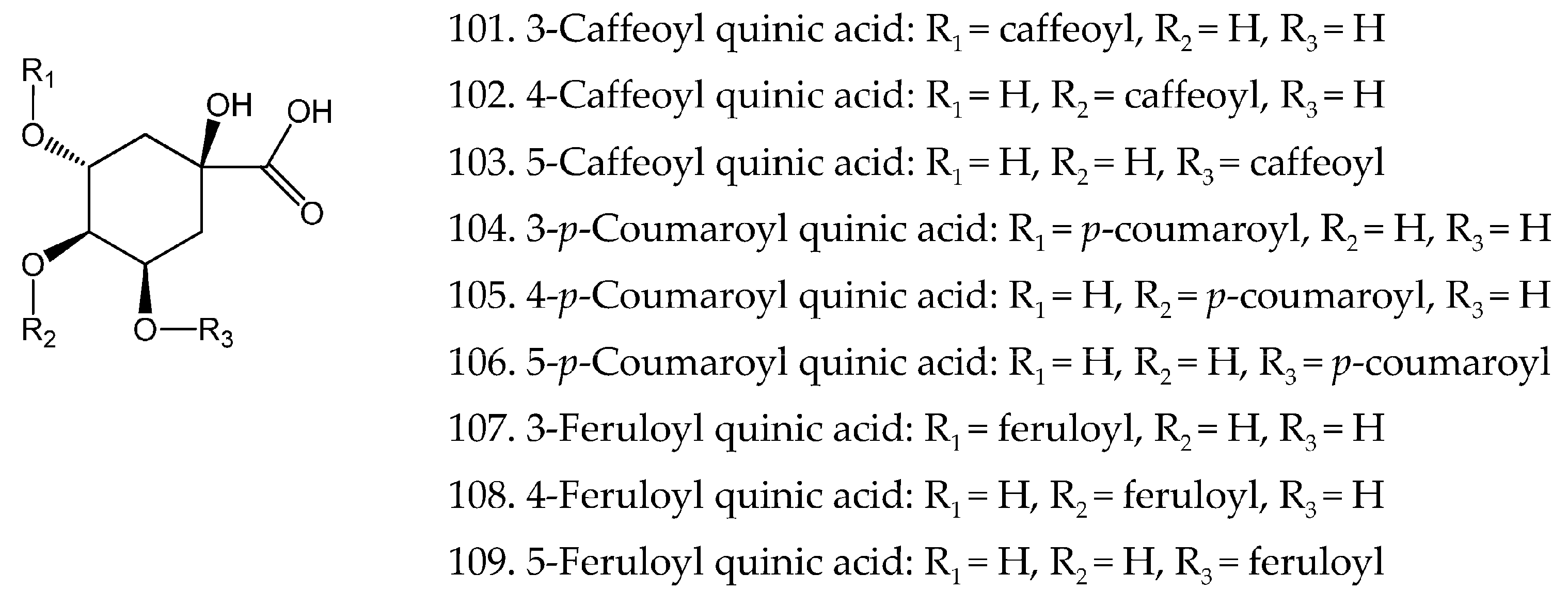
| NO. | Compound Name | Molecular Formula | Reference |
|---|---|---|---|
| Iridoids | |||
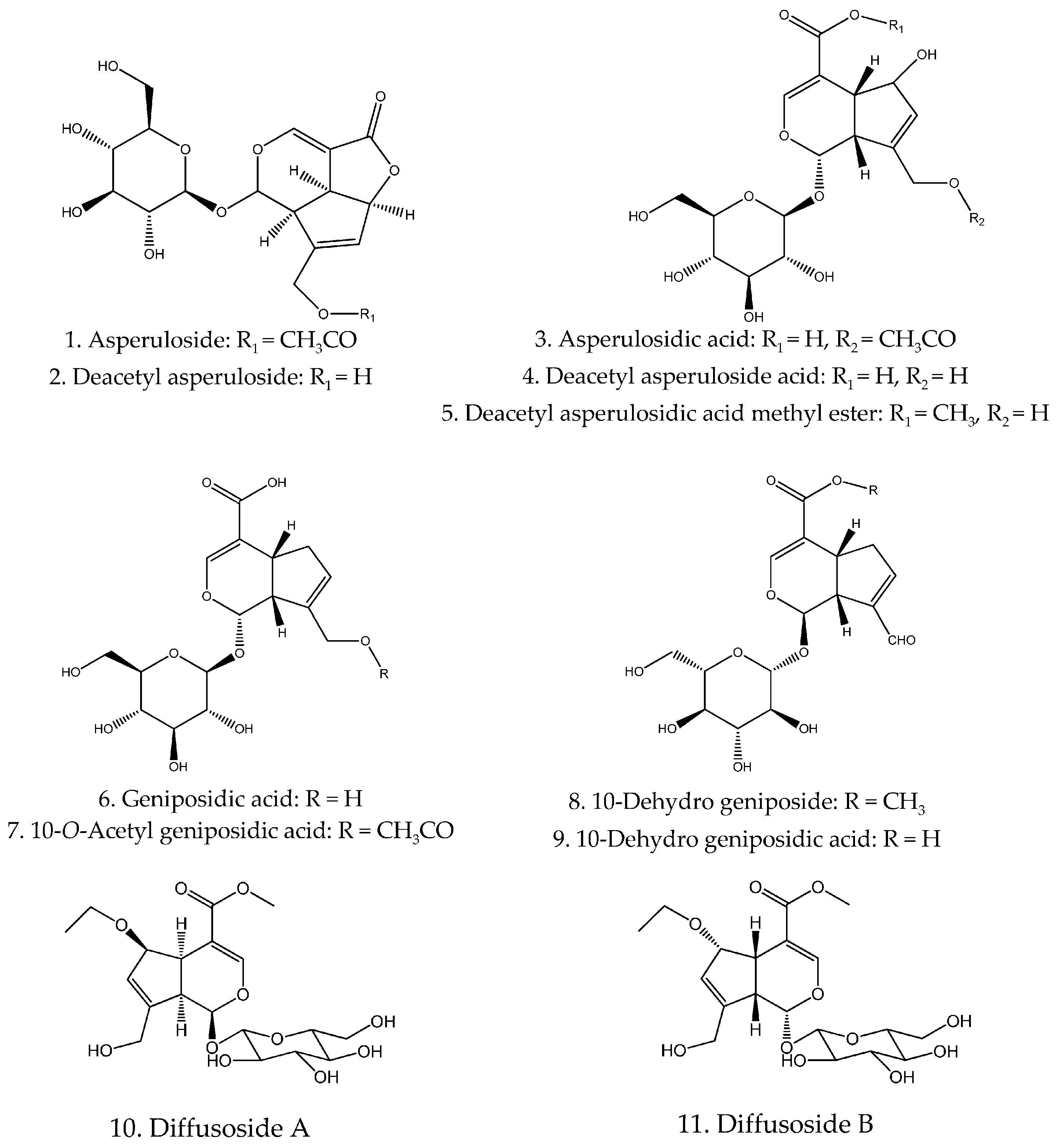
| 1 | Asperuloside | C18H22O11 | [13,14] |
| 2 | Deacetyl asperuloside | C16H20O10 | [15] |
| 3 | Asperuloside acid | C18H24O12 | [16] |
| 4 | Deacetyl asperulosidic acid | C16H22O11 | [15] |
| 5 | Deacetyl asperulosidic acid methyl ester | C17H24O11 | [15,17] |
| 6 | Geniposidic acid | C16H22O10 | [18] |
| 7 | 10-O-Acetyl geniposidic acid | C18H24O11 | [15] |
| 8 | 10-Dehydro geniposide | C17H22O10 | [17] |
| 9 | 10-Dehydro geniposidic acid | C16H20O10 | [19] |
| 10 | Diffusoside A | C19H28O11 | [20] |
| 11 | Diffusoside B | C19H28O11 | [20] |
| 12 | Lupenylacetate | C32H52O2 | [21] |
| 13 | Alpigenoside | C18H28O12 | [15] |
| 14 | Oldenlandoside III | C34H44O20 | [22] |
| 15 | 5-O-Feruloyl scandoside methyl ester | C27H32O14 | [23] |
| 16 | Hehycoryside C | C23H26O11 | [22] |
| 17 | 6-α-Hydro scandoside | C16H22O11 | [24] |
| 18 | 6-β-Hydro scandoside | C16H22O11 | [24] |
| 19 | 6-Dehydro scandoside | C16H22O10 | [19] |
| 20 | 6-α-Hydro scandoside methyl ester | C17H24O11 | [24] |
| 21 | 6-β-Hydro scandoside methyl ester | C17H24O11 | [24] |
| 22 | 6-α-Hydro-10-acetyl asperuloside acid | C18H24O12 | [24] |
| 23 | 6-β-Hydro-10-acetyl asperuloside acid | C18H24O12 | [24] |
| 24 | 6-O-Methoxyl cinnamoyl scandoside | C27H32O13 | [23] |
| 25 | 6-O-p-Hydro cinnamoyl scandoside | C26H30O13 | [23] |
| 26 | (E)-6-O-p-Coumaroyl-10-O-formoxyl scandoside methyl ester | C27H32O13 | [14] |
| 27 | (E)-6-O-p-Coumaroyl scandoside methyl ester | C26H30O13 | [14,15,25] |
| 28 | (Z)-6-O-p-Coumaroyl scandoside methyl ester | C26H30O13 | [18] |
| 29 | (E)-6-O-p-Methoxy cinnamoyl scandoside methyl ester | C27H32O13 | [15,25,26] |
| 30 | (Z)-6-O-p-Methoxy cinnamoyl scandoside methyl ester | C27H32O13 | [26] |
| 31 | (E)-6-O-Feruloyl scandoside methyl ester | C27H32O14 | [15,25,26] |
| 32 | (Z)-6-O-Feruloyl scandoside methyl ester | C27H32O14 | [27] |
| Triterpenes | |||
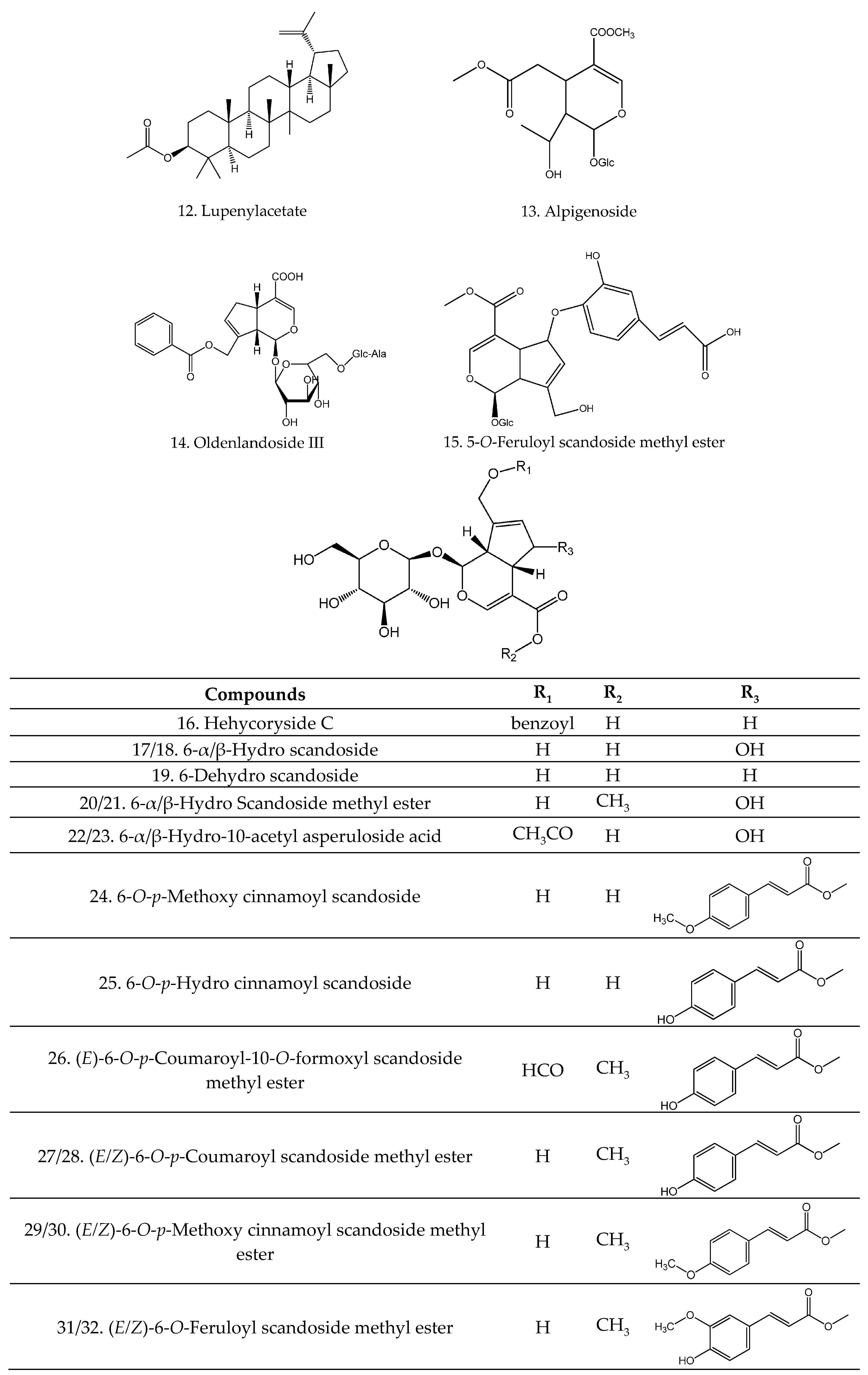
| 33 | Arborinone | C30H48O | [28] |
| 34 | Isoarborinol | C30H50O | [28] |
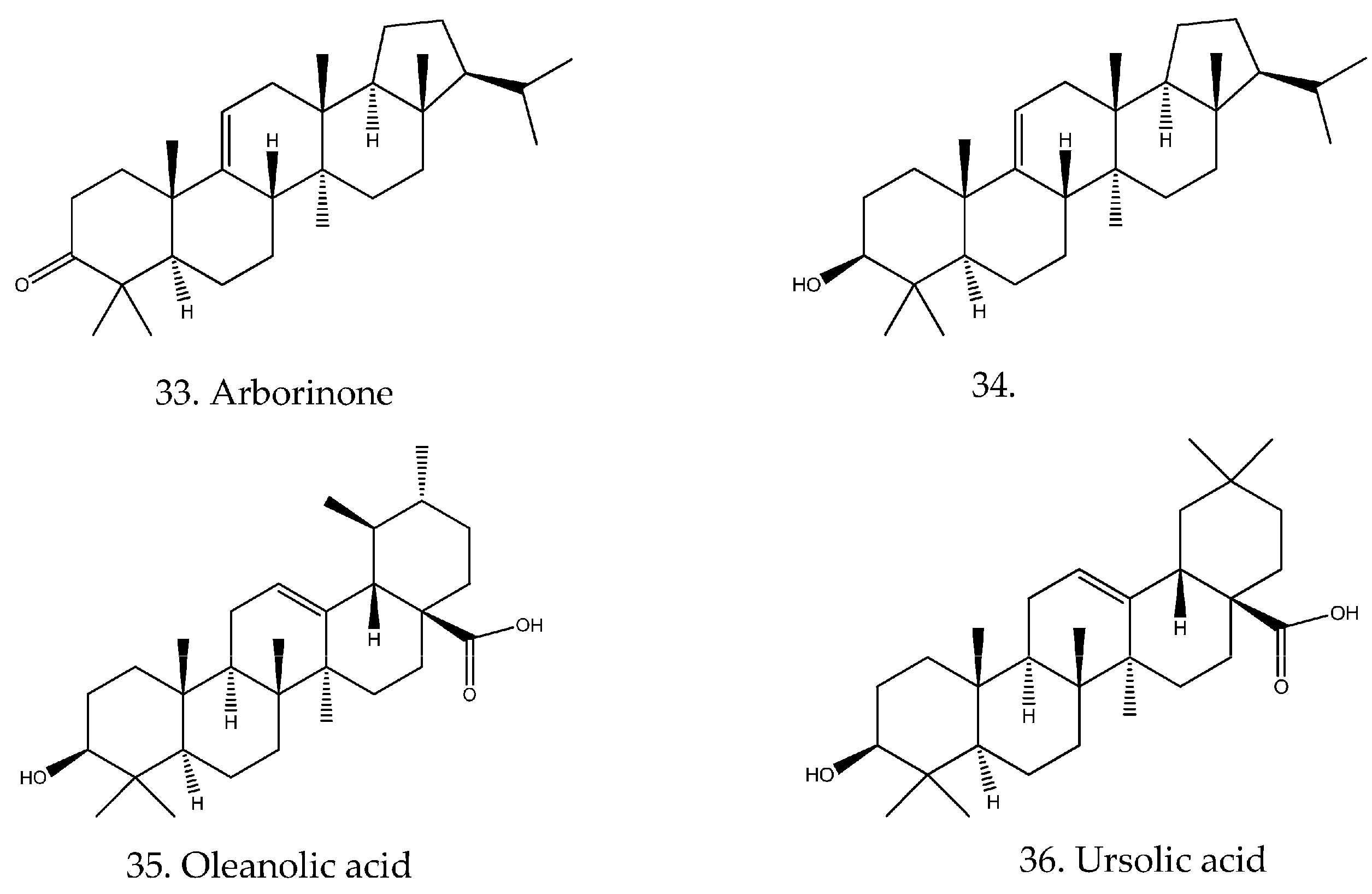
| 35 | Oleanolic acid | C30H48O3 | [19] |
| 36 | Ursolic acid | C30H48O3 | [19] |
| Flavonoids | |||
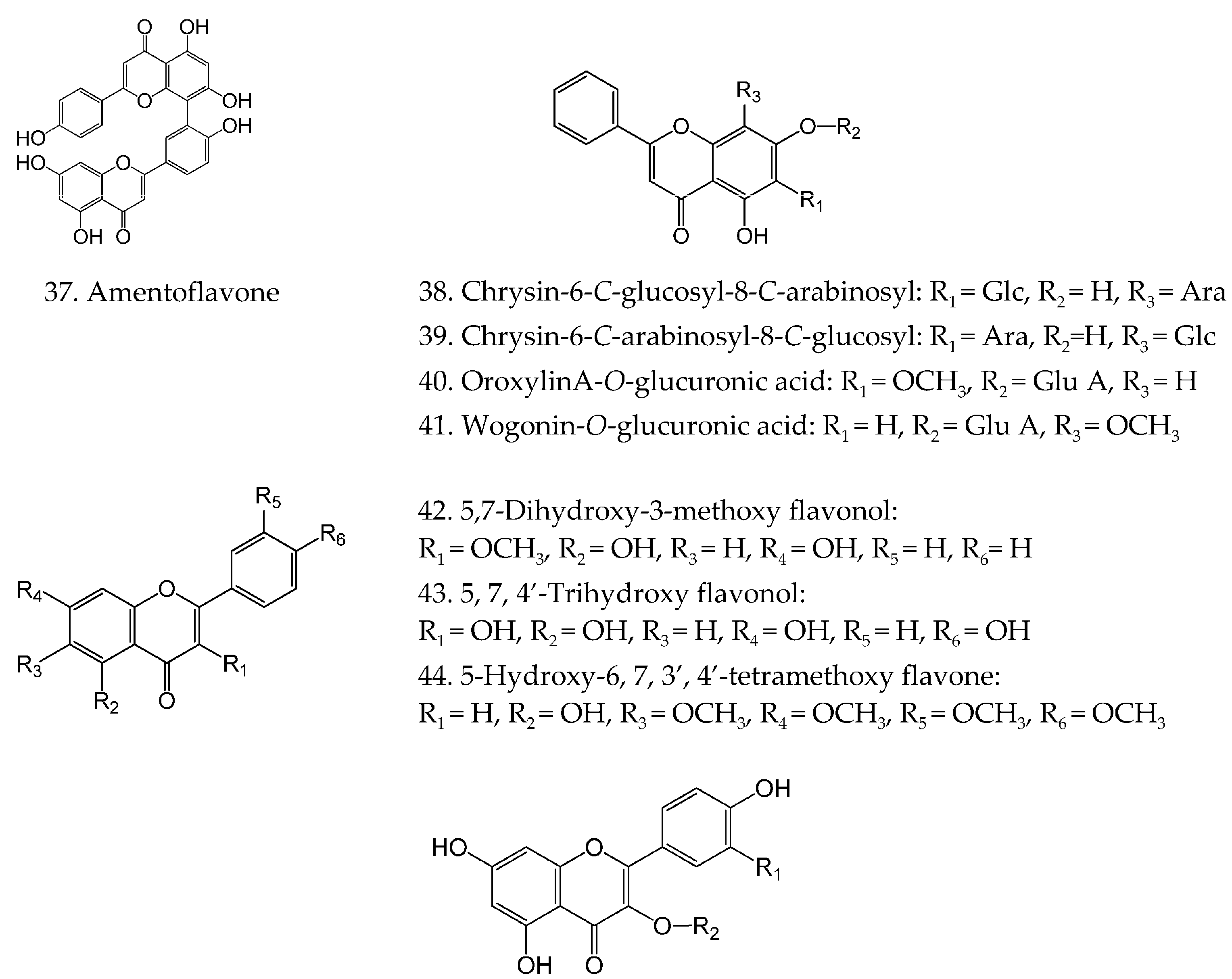
| 37 | Amentoflavone | C30H18O10 | [26,29] |
| 38 | Chrysin-6-C-glucosyl-8-C-arabinosyl | C26H28O13 | [22] |
| 39 | Chrysin-6-C-arabinosyl-8-C-glucosyl | C26H28O13 | [22] |
| 40 | Oroxylin-A-O-glucuronic acid | C22H20O11 | [22] |
| 41 | Wogonin-O-glucuronic acid | C22H20O11 | [22] |
| 42 | 5,7-Dihydroxy-3-methoxy flavonol | C16H12O5 | [13] |
| 43 | 5,7,4′-Trihydroxy flavonol | C15H10O6 | [13] |
| 44 | 5-Hydroxy-6,7,3′,4′-tetramethoxy flavone | C19H18O7 | [21] |
| 45 | Quercetin | C15H10O7 | [17,19,30] |
| 46 | Rutin | C27H30O16 | [15,25,31] |
| 47 | Quercetin-3-O-β-d-glucopyranside | C21H20O12 | [25,32,33] |
| 48 | Quercetin-3-O-β-d-galactopyranoside | C21H20O12 | [32] |
| 49 | Quercetin-3-O-(2-O-glucopyranosyl)-β-d-glucopyranside | C27H30O17 | [15,25,32,33] |
| 50 | Quercetin-3-O-(2-O-glucopyranosyl)-β-d-galactopyranoside | C27H30O17 | [11,34] |
| 51 | Quercetin-3-O-sambubioside | C26H28O16 | [15,25] |
| 52 | Quercetin-3-O-[2-O-(6-O-E-ferloyl)-β-d-glucopyranosyl]-β-d-galactopyranoside | C37H38O20 | [11,34] |
| 53 | Quercetin-3-O-[2-O-(6-O-E-feruloyl)-β-d-glucopyranosyl]-β-d-glucopyanoside | C37H38O20 | [11,15,25] |
| 54 | Quercetin-3-O-[2-O-(6-O-E-sinapoyl)-β-d-glucopyranosyl]-β-d-glucopyanoside | C38H40O21 | [15] |
| 55 | Quercetin-3-O-[2-O-(6-O-E-sinapoyl)-β-d-glucopyranosyl]-β-d-galactopyranoside | C38H40O21 | [25] |
| 56 | Kaempferol | C15H10O6 | [17,35] |
| 57 | Kaempferol-3-O-β-d-glucopyranside | C21H20O11 | [32] |
| 58 | Kaempferol-3-O-β-d-galactopyranoside | C21H20O11 | [32] |
| 59 | Kaempferol-3-O-(2-O-β-d-glucopyranosyl)-β-d-galactopyranoside | C27H30O16 | [11,25,34] |
| 60 | Kaempferol-3-O-(6-O-α-l-rhamnosyl)-β-d-glucopyranside | C27H30O16 | [32] |
| 61 | Kaempferol-3-O-[2-O-(E-6-O-feruloyl)-β-d-glucopyranosyl]-β-d-glucopyranosyl | C37H38O19 | [11,25,33] |
| 62 | Kaempferol-3-O-[2-O-(6-O-E-feruloyl)-β-d-glucopyranosyl]-β-d-galactopyranoside | C37H38O19 | [21,34] |
| Athraquinones | |||
| 63 | 2-Methyl-3-methoxy anthraquinone | C16H12O3 | [19] |
| 64 | 2-Hydroxy-1,3-dimethoxy anthraquinone | C16H12O5 | [29] |
| 65 | 2-Hydroxy-3-methyl-1-methoxy anthraquinone | C16H12O4 | [36] |
| 66 | 2-Hydroxy-3-methyl-4-methoxy anthraquinone | C16H12O4 | [37] |
| 67 | 2-Hydroxy-7-methyl-3-methoxy anthraquinone | C16H12O4 | [36] |
| 68 | 2-Hydroxy-1-methoxy-3-methyl anthraquinone | C16H14O4 | [31] |
| 69 | 2-Hydroxy-3-methyl anthraquinone | C15H10O3 | [17,27] |
| 70 | 2-Hydroxy-1-methoxy anthraquinone | C15H10O4 | [27,29] |
| 71 | 2-Hydroxy-4-methoxy anthraquinone | C15H10O4 | [38] |
| 72 | 2-Hydroxy-3-methoxy-7-methyl anthraquinone | C16H12O4 | [36] |
| 73 | 2-Hydroxy-6-methyl anthraquinone | C15H10O3 | [18] |
| 74 | 2-Hydroxy-3-methoxy-6-methyl anthraquinone | C16H12O4 | [18] |
| 75 | 2,7-Dihydroxy-3-methyl anthraquinone | C15H10O4 | [39] |
| 76 | 3-Hydroxy-2-methyl anthraquinone | C15H10O3 | [19] |
| 77 | 3-Hydroxy-2-methyl-4-methoxy anthraquinone | C16H12O4 | [40] |
| 78 | 2,3-Dimethoxy-6-methyl anthraquinone | C17H14O4 | [18] |
| 79 | 1,3-Dihydroxy-2-methyl anthraquinone | C15H10O4 | [41] |
| 80 | 1,7- Dihydroxy-6-methoxy-2-methyl anthraquinone | C16H12O5 | [41] |
| 81 | 3-Hydroxy-2-methyl-4-methoxy anthraquinone | C16H10O4 | [18] |
| 82 | 2,6-Dihydroxy-3-methyl-4-methoxy anthraquinone | C16H12O5 | [42] |
| 83 | 2,6-Dihydroxy-1-methoxy-3-methyl anthraquinone | C16H12O5 | [31] |
| 84 | 1-Hydroxy-4-methoxy anthraquinone | C15H10O4 | [43] |
| 85 | 2-Hydroxymethy-1-hydroxy anthraquinone | C15H10O4 | [5] |
| 86 | 2-Hydroxymethyl anthraquinone | C15H10O3 | [5] |
| Phenolic acids and their derivatives | |||
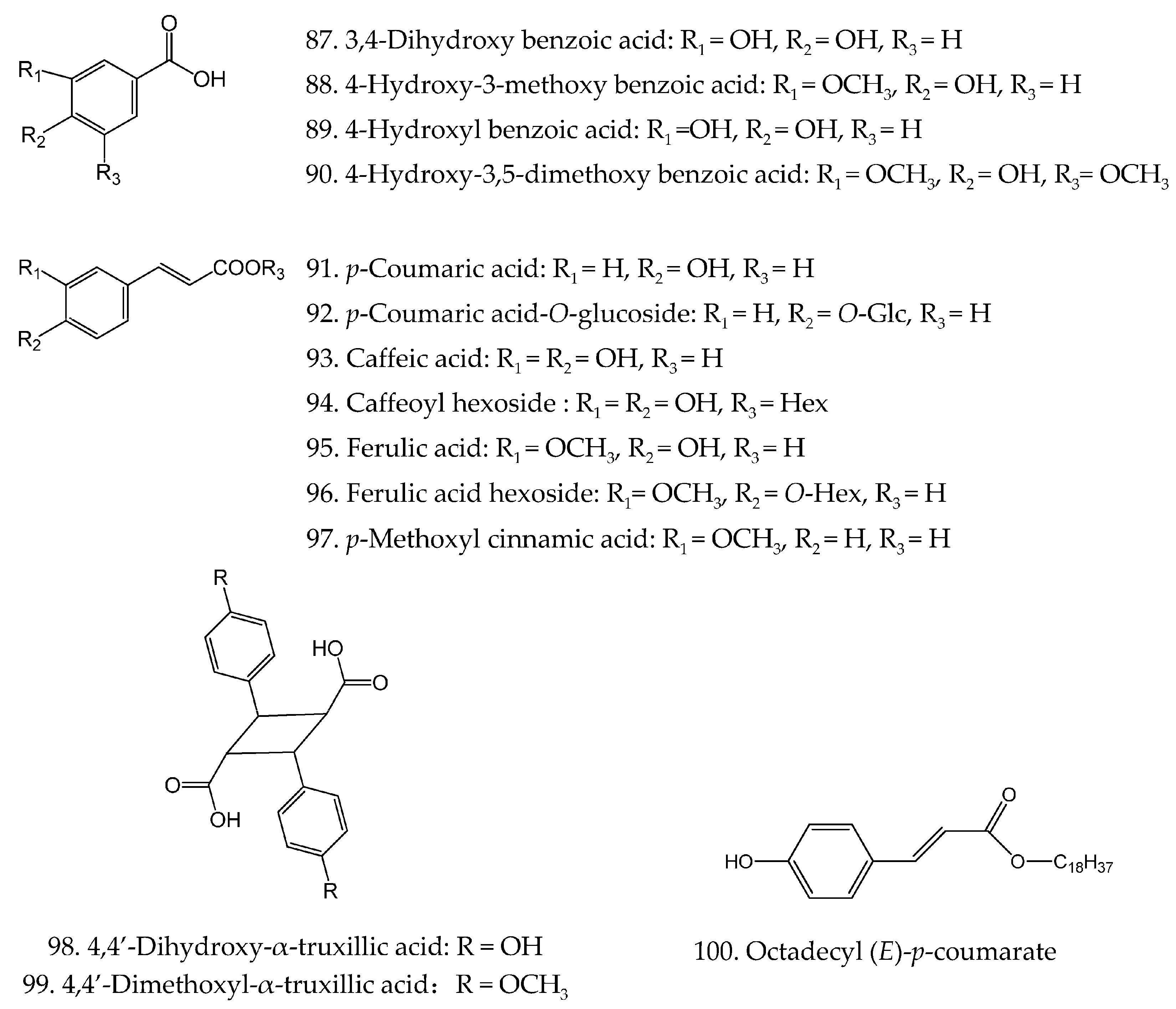
| 87 | 3,4-Dihydroxy benzoic acid | C7H6O4 | [21] |
| 88 | 4-Hydroxy-3-methoxy benzoic acid | C8H8O4 | [30] |
| 89 | trans-Hydroxybenzoic acid | C7H6O3 | [30] |
| 90 | 4-Hydroxy-3,5-dimethoxy benzoic acid | C9H10O5 | [30] |
| 91 | p-Coumaric acid | C9H8O3 | [19,29] |
| 92 | p-Coumaric acid-O-glucopyranside | C15H18O8 | [22] |
| 93 | Caffeic acid | C9H8O4 | [21] |
| 94 | Caffeoyl hexoside | C15H18O9 | [22] |
| 95 | Ferulic acid | C10H10O4 | [41] |
| 96 | Ferulic acid hexoside | C16H20O9 | [22] |
| 97 | p-Methoxy cinnamic acid | C10H10O3 | [44] |
| 98 | 4,4′-Dihydroxy-α-truxillic acid | C18H16O6 | [44] |
| 99 | 4,4′-Dimethoxyl-α-truxillic acid | C19H18O6 | [45] |
| 100 | Octadecyl (E)-p-coumarate | C27H44O3 | [46] |
| 101 | 3-Caffeoyl quinic acid | C16H18O9 | [22] |
| 102 | 4-Caffeoyl quinic acid | C16H18O9 | [22] |
| 103 | 5-Caffeoyl quinic acid | C16H18O9 | [22] |
| 104 | 3-р-Coumaroyl quinic acid | C16H18O8 | [22] |
| 105 | 4-р-Coumaroyl quinic acid | C16H18O8 | [22] |
| 106 | 5-р-Coumaroyl quinic acid | C16H18O8 | [22] |
| 107 | 3-Feruloyl quinic acid | C17H20O9 | [22] |
| 108 | 4-Feruloyl quinic acid | C17H20O9 | [22] |
| 109 | 5-Feruloyl quinic acid | C17H20O9 | [22] |
| Sterols | |||
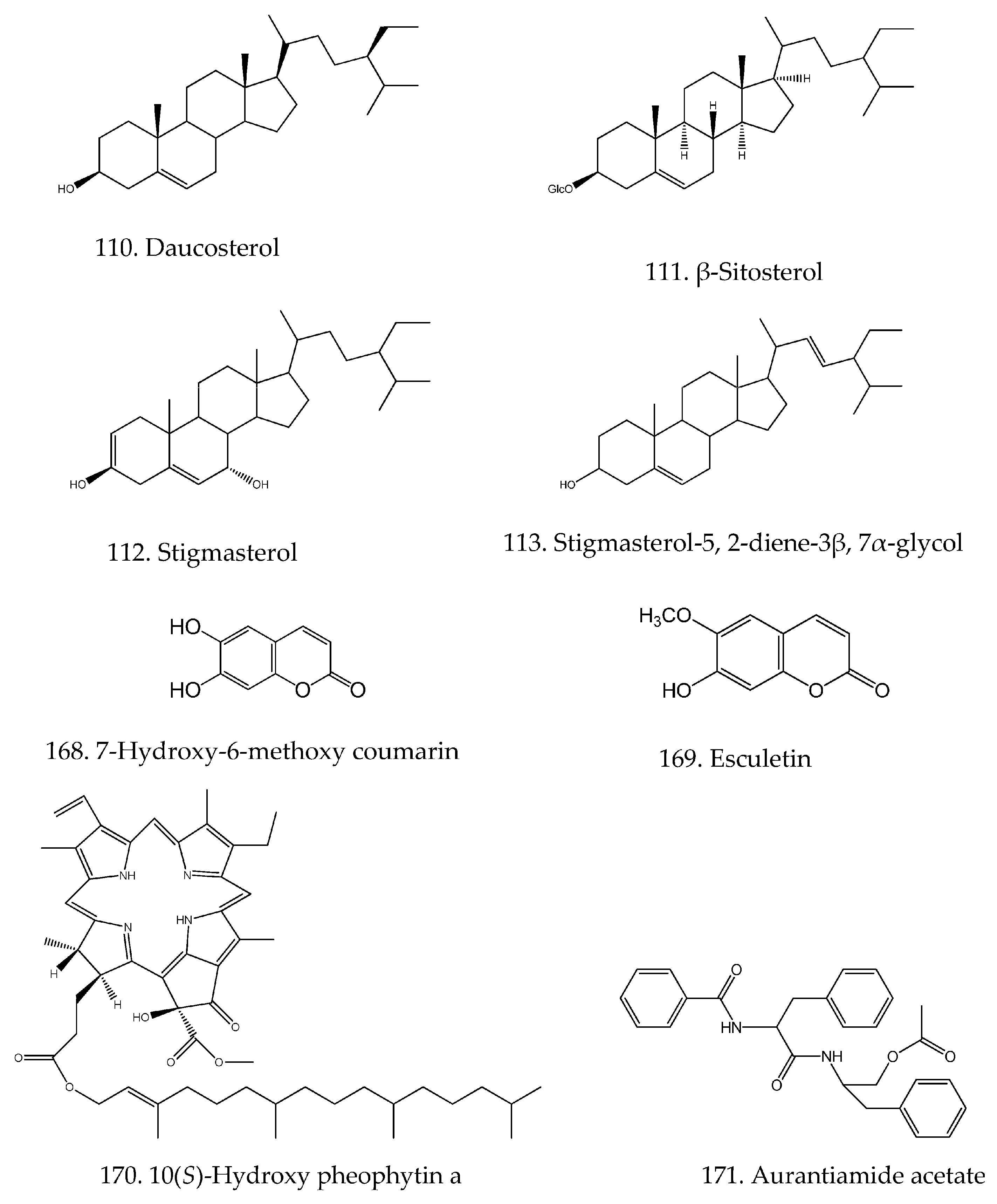
| 110 | Daucosterol | C35H60O6 | [19] |
| 111 | β-Sitosterol | C29H50O | [19] |
| 112 | Stigmasterol | C29H48O | [17,19] |
| 113 | Stigmasterol-5,2-diene-3β, 7α-glycol | C29H48O2 | [47] |
| Volatile oils | |||
| 114 | 6,10,14-Trimethyl-2-pentadecanone | C18H36O | [48] |
| 115 | Phytol | C20H40O | [48] |
| 116 | α-Cedrol | C15H26O | [48] |
| 117 | Tetradecanoic acid | C14H28O2 | [48] |
| 118 | Hexadecanoic acid, methyl ester | C17H34O2 | [48] |
| 119 | Hexadecanoic acid, | C16H32O2 | [48] |
| 121 | 1,2-Benzenediearboxylic acid isobutyl ester | C16H22O4 | [48] |
| 122 | 1,2-Benzenediearboxylic acid, bis(2-methylpropyl)ester | C16H22O4 | [48] |
| 123 | 9,12,15-Octadecatrienoic acid, methyl ester | C19H32O2 | [48] |
| 124 | 9-Octadecenoic acid | C18H34O2 | [48] |
| 125 | 9,12-Octadecenoic acid | C18H32O2 | [48] |
| 126 | Ethyl linoleate | C20H36O2 | [48] |
| 127 | Triethyl phosphate | C6H15O4P | [48] |
| 128 | 4-Vinyl phenol | C8H8O | [48] |
| 129 | 2-Methoxy-4-vinylphenol | C9H10O2 | [48] |
| 130 | n-Pentadecanoic acid | C15H30O2 | [48] |
| 131 | 4,8,12,16-Tetramethyl heptadecan-4-olide | C21H40O2 | [48] |
| 132 | 2,6,10,14,18,22-Tetracosahexaene | C30H50 | [48] |
| 133 | α-Terpineol | C10H18O | [11] |
| 134 | Geranyl acetate | C12H20O2 | [11] |
| 135 | β-Ionone | C13H20O | [11] |
| 136 | Lauric acid | C12H24O2 | [11] |
| 137 | Myristic acid | C14H28O2 | [11] |
| 138 | Palmitic acid | C16H32O2 | [11] |
| 139 | Linoleic acid | C18H32O2 | [11] |
| 140 | β-Linalool | C10H18O | [11] |
| 141 | Isoborneol | C10H18O | [49] |
| 142 | 3-(2-Propenyl)-cyclohexene | C9H14 | [49] |
| 143 | 2-Pentyl-furam | C9H14O | [49] |
| 144 | Cis-2-(2-pentenyl)-furan | C9H12O | [49] |
| 145 | Limonene | C10H18 | [49] |
| 146 | 3,7-Dimethyl-1,6-octadiem-3-ol | C10H18O | [49] |
| 147 | trans-5-Methyl-2-(1-methylethyl)-cyclohexanope | C10H18O | [49] |
| 148 | (1S-endo)-1,7,7-Trimethyl-bicyclo[2,2,1]heptan-2-ol | C10H18O | [49] |
| 149 | p-Menth-1-en-8-ol | C10H18O | [49] |
| 150 | Pulegone | C10H16O | [49] |
| 151 | 4-(2,6,6-Trimethyl-1-cyclohexen-1-yl)-3-buten-2-one | C13H20O | [49] |
| 152 | Hexadecanal | C16H32O | [49] |
| 153 | 2,6,10,14-Tetramethyl-hexadecane | C20H42 | [49] |
| 154 | (Z,Z)-9,12-octadecadienoic acid | C18H32O2 | [49] |
| 155 | (Z)-9,17-octadecadienal | C18H32O | [49] |
| 156 | Cis,cis,cis-7,10,13-hexadecatrienal | C16H26O | [49] |
| 157 | Oleic acid | C18H34O2 | [49] |
| 158 | Hexaldehyde | C6H12O | [49] |
| 159 | Borneol | C10H18O | [49] |
| 160 | Docosane | C22H46 | [49] |
| 161 | Tetracosane | C24H50 | [49] |
| 162 | Hexacosane | C26H54 | [49] |
| 163 | Heptacosane | C27H56 | [49] |
| Polysaccharides | |||
| 164 | ODP-1 | [50] | |
| Cyclotides | |||
| 165 | CD1 | [51] | |
| 166 | CD2 | [51] | |
| 167 | CD3 | [51] | |
| Coumarins | |||
| 168 | 7-Hydroxy-6-methoxy-Coumarin | C10H8O4 | [17] |
| 169 | Esculetin | C9H6O4 | [46] |
| Alkaloids | |||
| 170 | 10(S)-hydroxy pheophytin a | C55H74N4O6 | [52] |
| 171 | Aurantiamide acetate | C27H28N2O4 | [46] |
| Activities | Model | Formulation/Dosage/Extract | Reference | |
|---|---|---|---|---|
| Anti-tumor activity | ||||
| Colorectal cancer | HT-29 cells | Ethanol extract | The extract suppressed HT-29 cell growth and induced apoptosis via inactivation of the IL-6/STAT3-signaling pathway. | [2] |
| HT-29 cells | Ethanol extract | The extract reduced HT-29 cell viability and survival. It could suppress cancer cell proliferation by blocking the cell cycle, preventing G1 to S progression, and reducing mRNA expression of pro-proliferative PCNA, Cyclin D1 and CDK4, but increasing that of anti-proliferative p21. | [55] | |
| HT-29 cells | Ethanol extract | The extract induced the HT-29 cell morphological changes and reduced cell viability. In addition, the extract treatment resulted in DNA fragmentation, loss of plasma membrane asymmetry, collapse of mitochondrial membrane potential, activation of caspase-9 and caspase-3 and increase of the ratio of pro-apoptotic Bax to anti-apoptotic Bcl-2. | [56] | |
| HT-29 cells | Ethanol extract | The extract treatment downregulated the mRNA and protein expression levels of VEGF-A in HT-29 human colon carcinoma cells. | [57] | |
| HT-29 cells | Ethanol extract | The extract inhibits colorectal cancer growth in vivo via inhibition of SHH-mediated tumor angiogenesis. | [58] | |
| CRC mouse xenograft model | Ethanol extract | The extract inhibited the expression of the gene VEGF-A and VEGFR2, thus, suppressed the activation of Sonic hedgehog (SHH)-signaling in CRC xenograft tumors; it inhibits colorectal cancer growth. | [58] | |
| CRC mouse xenograft model | Ethanol extract | The extract suppressed the STAT3 pathway by suppressing STAT3 phosphorylation in tumor tissues, altering the expression pattern of target genes of Cyclin D1, CDK4 and Bcl-2, as well as upregulating p21 and Bax. | [59] | |
| CT-26 cells | Ethanol extract | The extract can inhibit the proliferation of CT-26 colon cancer cells from BALB/c mice in a time- and dose- dependent manner. | [60] | |
| HCT-8/5-FU cells | Ethanol extracts | The extract treatment significantly reduced the cell viability of HCT-8/5-FU cells by downregulating the expression of P-gp and ABCG2. | [61] | |
| Caco-2 cells | Aqueous extracts | The decoction of H. diffusa and its fraction 9 contained sufficient ursolic acid and oleanolic acid to possibly induce apoptosis of Caco-2 cells. | [62] | |
| Caco-2 cells | Nine pure compounds isolated from H. diffusa | 2-Hydroxymethy-1-hydroxy anthraquinone (IC50 45 mM) and ursolic acid (IC50 71 mM) exhibited the highest inhibition of Caco-2 cell proliferation. | [5] | |
| Leukemia | CEM cells | Aqueous extract | The extract inhibited Leukemia CEM cells growth in time- and concentration-dependent manners. And the inhibition mechanism has greater correlation with the upregulation of P53 expression. | [63] |
| BALB/c mice | Aqueous extract | The extract had anti-leukemia effects on WEHI-3 cell-induced leukemia in vivo. | [64] | |
| HL-60 cells | H. diffusa injection | The extract could induce HL-60 cells differentiation, and suppress the expression of the anti-apoptosis-related gene to inhibit the growth of HL-60 cells. | [65] | |
| HL-60 cells, WEHI-3 cells | Ethanol extract | The extract inhibited the cell proliferation of HL-60 cells. It triggered an arrest of HL-60 cells at the G0/G1 phase and sub-G1 population, provoked DNA condensation and DNA damage, but the activities of caspase-3, caspase-8, and caspase-9 were elevated in H. diffusa-treated HL-60 cells. | [66] | |
| U937 cells | 2-Hydroxy-3-methyl anthraquinone | 2-Hydroxy-3-methyl anthraquinone enhanced apoptosis of U937 cells through the activation of p-p38MAPK and downregulation of p-ERK1/2. | [67] | |
| THP-1 Cells | 2-Hydroxy-3-methyl anthraquinone | 2-Hydroxy-3-methyl anthraquinone induced THP-1 cell apoptosis, which was associated with a more prominent induction expression of Fas/FasL, DR4 and TRAIL. Moreover, 2-Hydroxy-3-methylanthraquinone treatment resulted in activation of caspase-8. | [68] | |
| Liver cancer | H22 mice | Aqueous extract | The extract had an inhibitory effect on the metastasis of hepatocarcinoma in blood. | [69] |
| HepG2 cells | Aqueous extract | The extract remarkably inhibited HepG2 cell proliferation via arrest of HepG2 cells at the G0/G1 phase and induction of S phase delay. In addition, the extract potentiated the anticancer effect of low-dose 5-FU in the absence of overt toxicity by downregulating the mRNA and protein levels of CDK2, cyclin E and E2F1. | [70] | |
| MHCC97-H cells | Total flavones extract | The extract treatment reduced the level of E-cadherin protein and increased the expression of vimentin protein in TGF-β1-induced MHCC97-H. | [71] | |
| HepG2 cells | 1,3-Dihydroxy-2-Methylanthraquinone Ethyl acetate extract | Both 1,3-Dihydroxy-2-Methylanthraquinone and ethyl acetate extract exhibited an inhibitory effect on HepG2 cells, resulting in in upregulation of Bax, p53, Fas, FasL, p21 and cytoplasmic cytochrome C levels and caspase-3, -8, -9 proteases activities, while downregulating Bcl-2, mitochondrial cytochrome C, cyclin E and CDK 2 in a dose-dependent manner. | [72] | |
| HepG2 cells | Nine pure compounds isolated from H. diffusa | Ursolic acid exhibited a strong inhibition of cell survival with C50 37 mM. | [5] | |
| HepG2 cells | 2-Hydroxy-3-methyl anthraquinone 1-Methoxy-2-hydroxy anthraquinone | Both compounds showed inhibitory activity against protein tyrosine kinases v-src and pp60src and arrested the growth of HepG2 cancer cells. | [38] | |
| Lung cancer | A549 cells, H1355 cells, LLC cells | Ethanol extract | The extract suppressed the cell proliferation of A549 and H1355 cells as well as reduced cell viability in a concentration-dependent manner. | [66] |
| SPC-1-A cells | 2-Hydroxy-3-methyl anthraquinone 1-Methoxy-2-hydroxy anthraquinone | Both compounds showed inhibitory activity against protein tyrosine kinases v-src and pp60src and arrested the growth of SPC-1-A. | [38] | |
| Breast cancer | MCF-7 cells | Compounds of anthraquinones, iridoid glucosides, stigmasterols and alkaloids/flavonoids | Alkaloids/flavonoids possessed antitumor activity against the human breast cancer cell line MCF7 | [73] |
| MCF-7 cells | Methyl anthraquinone | Methyl anthraquinone-induced MCF-7 cells apoptosis via Ca2+/calpain/caspase-4 pathway. | [74] | |
| Bcap37 cells | 2-Hydroxy-3-methyl anthraquinone, 1-Methoxy-2-hydroxy anthraquinone | Both compounds showed inhibitory activity against protein tyrosine kinases v-src and pp60src and arrested the growth of Bcap37 cells. | [38] | |
| Cervical tumor | Nude mouse model | Aqueous extract | The extract had an inhibitory effect on cervical cancer cells with the expression of Ki-67 protein significantly decreased, and the mean survival time of the mice was significantly extended. | [3] |
| HeLa cells | Nine pure compounds isolated from H. diffusa | 2-Hydroxymethy-1-hydroxy anthraquinone exhibited the strongest inhibitory effect on cell viability. | [5] | |
| Prostate Cancer | DU145 cells, PC-3 cells LNCaP cells | Nine pure compounds isolated from H. diffusa | 2-Methyl-3-methoxy anthraquinone, 2-hydroxy-3-methyl anthraquinone and ursolic acid exhibited inhibitory effects on prostate cancer cell survival. | [5] |
| PC3 cells LNCaP cells | 6-O-(E)-p-Coumaroyl scandoside methyl ester 10(S)-Hydroxy pheophytin | Two compounds showed a moderate anti-proliferation effect on PC3 human androgen-independent prostate cancer cells, while 10(S)-hydroxy pheophytin also showed a strong anti-proliferation effect on LNCaP human androgen-sensitive prostate cancer cells. | [52] | |
| Multiple myeloma | RPMI 8226 cells | Nine pure compounds isolated from H. diffusa | 2-Hydroxymethy-1-hydroxy anthraquinone exhibited the strongest inhibition of RPMI 8226cells growth. | [5] |
| RPMI 8226 cells | Polysaccharides extracts | Polysaccharides extracts suppressed the growth of RPMI 8226 cells in a dose- and time-dependent manner. | [75] | |
| RPMI 8226 cells | H. diffusa injection | H. diffusa injection could inhibit the proliferation of RPMI 8226 cells. | [76] | |
| Others | B16F10 cells | Ethanol extract | The extract suppressed the cell proliferation of B16F10 cells as well as reducing cell viability in a concentration-dependent manner. | [66] |
| S180 cells | Decoction, lipophilic extract, crude polysaccharide | Lipophilic extract and crude polysaccharide showed anti-tumor activities and a protective effect on chemotherapeutic damage. However, the aqueous extract had no marked anti-tumor effect on S-180 cells. | [77] | |
| MG-63cells | H. diffusa injection | H. diffusa injection could inhibit the proliferation of MG-63 cells, and Bax gene expression was significantly increased. | [78] | |
| MG-63 cells | H. diffusa injection | H. diffusa injection could induce the apoptosis of MG-63 cells by increasing Bax gene expression in a concentration-dependent manner. | [79] | |
| MG-63 cells | Aqueous extract | H. diffusa, combined with cisplatin, had a stronger inhibitory effect than the single agents in MG-63 cells with IC50164.6 and 5.0 μL/mL, respectively. As a result, H. diffusa could alter anti-apoptotic (Bax and Bad) and pro-apoptotic protein (Bcl-xl and Bcl-2) expression, and it elevated the levels of caspase-3 and caspase-8. | [80] | |
| U87 cells | Aqueous extract | The extract suppressed U87 cells growth in a dose- and time-dependent manner. | [4] | |
| Angiogenesis | 1.Breast tumor-bearing BALB/c mice 2. Zebrafish embryo model 3. Human endothelial cells 4. C57BL/6 mice | 4-Vinyl phenol | 4-Vinyl phenol was demonstrated with anti-angiogenic activity in vitro and in vivo. | [81] |
| Immunomodulatory effect | ||||
| Normal BALB/c mice | Ethanol Extract | The extract has promoted immune responses in normal BALB/c mice. | [82] | |
| Immunosuppression mice induced by cyclophosphamide | Polysaccharides extracts | The extract could improve the clearance index, phagocytic index, and the index of the thymus and spleen of immunosuppression mice. | [50] | |
| Inmmunosuppressed mice induced by cyclophosphamide | Total flavonoids extract | The extract enhanced specific and non-specific immunity. | [83] | |
| Antioxidant effects | ||||
| The extract from methanol, acetone and 80% alcohol | The extraction with 80% alcohol has the strongest antioxidant activity on DPPH assay. | [84] | ||
| The extract from water, ethanol, acetone, chloroform, ether, petroleum benzine | Acetone extract had the strongest antioxidant effect. | [85] | ||
| LO2 cells | Aqueous extract | The aqueous extract exerted a good antioxidant effect in DPPH assay with a 50% scavenging concentration at 0.153 mg/mL. Aqueous extract treatment reversed H2O2-induced activation of the MEK/ERK pathway and H2O2-induced inhibition of the P13-K/AKT/GSK3b pathway in LO2 cells. This may be due to the improvement activity of the aqueous extract of H. diffusa on the antioxidant defense system. | [86] | |
| Twelve pure compounds isolated from H. diffusa | All compounds showed antioxidant effects on xanthine oxidase inhibition, xanthine-xanthine oxidase cytochrome c and TBA-MDA systems. | [33] | ||
| Anti-inflammatory effect | ||||
| Lipopolysaccharide-induced renal inflammation mice | Aqueous extract | The extract protected renal tissues, significantly suppressed the production of TNF-α, IL-1, IL-6 and MCP-1, as well as significantly promoted the production of IL-10 in serum and renal tissues. | [87] | |
| RAW 264.7 cells | Total flavonoids extract | The extract treatment on LPS-stimulated RAW 264.7 cells, reduced expression of iNOS, TNF-α, IL-6 and IL-1β, as well as suppressing phosphorylation of IκB p38, JNK and ERK1/2 in a concentration-dependent manner, indicating that the anti-inflammatory activity of total flavonoids had a close relationship with the NF-κB and MAPK signaling pathways. | [88] | |
| Neuroprotective effect | ||||
| Rat cortical cells damaged by l-glutamate | Methanolic extract, five flavonoids and four O-acylated iridoid glycosides | All compounds exhibited significant neuroprotective activity in primary cultures of rat cortical cells damaged by l-glutamate. | [34] | |
| Anti-fibrosis effect | ||||
| Ras oncogene-transformed R6 cells | Oleanolic acid | Oleanolic acid inhibits the growth of ras oncogene-transformed R6 cells. Oleanolic acid-mediated growth inhibition of transformed cells does not require direct cell–cell contact between normal and ras-transformed cells. | [89] | |
| Analytes | Method | Results | Reference |
|---|---|---|---|
| Deacetyl asperulosidic acid methyl ester | HPLC | The contents of deacetyl asperulosidic acid methyl ester of 22 batches were from 0.31 to 3.34 mg/g. | [93] |
| Oleanolic acid | TLC | The contents of oleanolic acid of 3 batches were from 1.63% to 1.72% | [94] |
| Isoscutellarein | HPLC | The contents of isoscutellarein have a close relationship with the collecting times and were also different in leaves (1.11–2.72 mg/g) and stem (0.35–0.94 mg/g). | [95] |
| p-Coumaric acid | HPLC | The contents of p-coumaric acid in the injection of H. diffusa from four manufacturers ranged from 0.34 to 0.49 mg/mL. | [96] |
| p-Coumaric acid | HPLC | The contents of p-coumaric acid of 13 batches were from 0.46 to1.88 mg/mL | [97] |
| 3,4-Dihydroxy methyl benzoate | HPLC | The contents of 3,4-dihydroxy methyl benzoate of 8 batches were from 40.8 to 87.0 μg/g. | [98] |
| Polysassharides | UV | Polysassharides have been determined by the phenol-sulfuric acid method by spectrophosured at 490 nm, and the content was 15.10%. | [99] |
| Ursolic acid Oleanolic acid | HPLC | Six batches have been determined with the contents of 1.75–3.37 mg/g for ursolic acid and 0.50–0.80 mg/g for oleanolic acid, indicating that the ursolic acid and oleanolic acid content in the samples from different sources were significantly different. | [100] |
| Ursolic acid Oleanolic acid | HPLC | The contents of ursolic acid and oleanolic acid have a close relationship with the collecting time. The range of contents was 1.17–3.75 and 0.19–0.96 mg/g for ursolic acid and oleanolic acid, respectively. | [101] |
| Ursolic acid Oleanolic acid | HPLC | The contents of ursolic acid and oleanolic acid were 0.51%–0.58% and 0.11%–0.14%, respectively. And the contents of the whole herb were slightly lower than those of the overground part for both of the two compounds. | [102] |
| Ursolic acid Oleanolic acid | HPLC-MS/MS | The contents of ursolic acid and oleanolic acid for 10 batches were 0.15%–0.65% and 0.06%–0.17%, respectively. | [103] |
| 2-Hydroxy-3-methoxy-7-methyl anthraquinone 2-Hydroxy-1-methoxy anthraquinone | HPLC | The contents were 0.16–0.51 and 0.22–0.49 mg/g for 2-hydroxy-3-methoxy-7-methyl anthraquinone and 2-hydroxy-1-methoxyanthraquinone, respectively. | [104] |
| Asperuloside E-6-O-p-Coumaroyl scandoside methyl ester E-6-O-p-Coumaroyl scandoside methyl ester-10-methyl ether | HPLC | The contents of asperuloside, E-6-O-p-coumaroyl scandoside methyl ester and E-6-O-p-coumaroyl scandoside methyl ester-10-methyl ether have been determined in twenty-three batches. The result was that the contents of the compounds were significantly varied among the different samples. The concentration ranges were 0–7.885, 1.104–7.159 and 0–1.795 mg/g for asperuloside, E-6-O-p-coumaroyl scandoside methyl ester and E-6-O-p-coumaroyl scandoside methyl ester-10-methyl ether, respectively. | [105] |
| 3,4-Dihydroxy methyl benzoate p-Coumaric acid Ferulic acid (E)-6-O-p-Coumaroyl scandoside methyl ester | HPLC | Four compounds have been quantified in the injection of H. diffusa with contents of 2.25–2.63, 7.02–7.15, 0.96–1.17 and 7.16–7.33 g/L for 3,4-dihydroxy methyl benzoate, p-coumaric acid, ferulic acid and (E)-6-O-p-coumaroyl scandoside methyl ester, respectively. | [106] |
| Geniposidic acid Ursolic acid Quercetin p-Coumaric acid | CE | Four compounds have been quantified in the injection of H. diffusa with contents of 1.004, 1.182, 0.110 and 0.067 mg/g for ursolic acid, geniposidic acid, quercetin and p-coumaric acid, respectively. | [107] |
| Asperuloside acid Asperuloside (E)-6-O-Feruloyl scandoside methyl ester (E)-6-O-p-Coumaroyl scandoside methyl ester Scandoside methyl ester | HPLC | The contents were 1.57–5.93, 1.45–3.86, 1.82–3.23, 1.54–3.82 and 1.49–4.11 mg/g for asperuloside acid, asperuloside, (E)-6-O-feruloyl scandoside methyl ester, (E)-6-O-p-coumaroyl scandoside methyl ester and scandoside methyl ester, respectively, and they were very different in different batches. | [108] |
| Quercetin-3-O-sambubioside Quercetin-3-O-β-d-glucopyranside Kaempferol-3-O-β-d-glucopyranside Rutin Quercetin Kaempferol | HPLC | Six compounds from eight batches of H. diffusa have been quantified with contents of 1.36–6.32, 0.98–10.23, 0.79–7.98, 4.92–15.78, 0.52–1.72 and 0.75–2.15 mg/g for quercetin-3-O-sambubioside, quercetin-3-O-β-d-glucopyranside, kaempferol-3-O-β-d-glucopyranside, rutin, quercetin and kaempferol, respectively, indicating that the contents for these compounds were quite different from different regions. | [109] |
| Desacetyl asperulosidic acid Asperuloside Aesculetin Coumaric acid Ferulic acid Quercetin Kaempferol | HPLC | Seven compounds from six batches of H. diffusa have been quantified with contents of 42.48 ± 1.43, 63.76 ± 1.01, 1765 ± 0.69, 881.9 ± 0.74, 86.99 ± 1.65, 1395 ± 0.731 and 902.2 ± 0.82 μg/g for desacetyl asperulosidic acid, asperuloside, aesculetin, coumaric acid, ferulic acid, quercetin, kaempferol, respectively. | [110] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; He, J.; Tong, X.; Tang, L.; Liu, M. The Hedyotis diffusa Willd. (Rubiaceae): A Review on Phytochemistry, Pharmacology, Quality Control and Pharmacokinetics. Molecules 2016, 21, 710. https://doi.org/10.3390/molecules21060710
Chen R, He J, Tong X, Tang L, Liu M. The Hedyotis diffusa Willd. (Rubiaceae): A Review on Phytochemistry, Pharmacology, Quality Control and Pharmacokinetics. Molecules. 2016; 21(6):710. https://doi.org/10.3390/molecules21060710
Chicago/Turabian StyleChen, Rui, Jingyu He, Xueli Tong, Lan Tang, and Menghua Liu. 2016. "The Hedyotis diffusa Willd. (Rubiaceae): A Review on Phytochemistry, Pharmacology, Quality Control and Pharmacokinetics" Molecules 21, no. 6: 710. https://doi.org/10.3390/molecules21060710





