A Deoxyuridine-Based Far-Red Emitting Viscosity Sensor
Abstract
:1. Introduction
2. Results
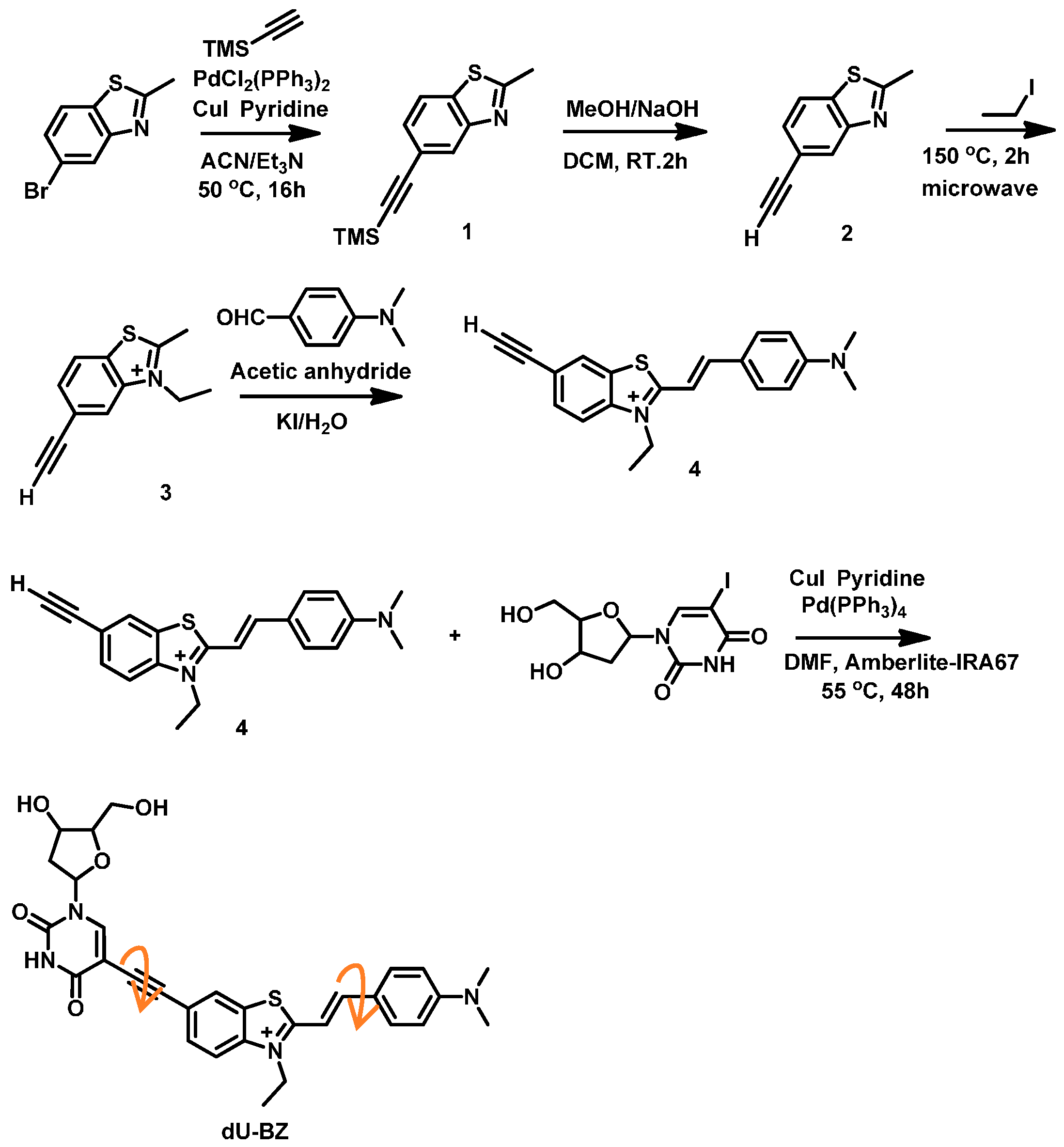
2.1. Synthesis of dU-BZ
2.2. Linear Photophsical Characterization of dU-BZ by Varying Temperature
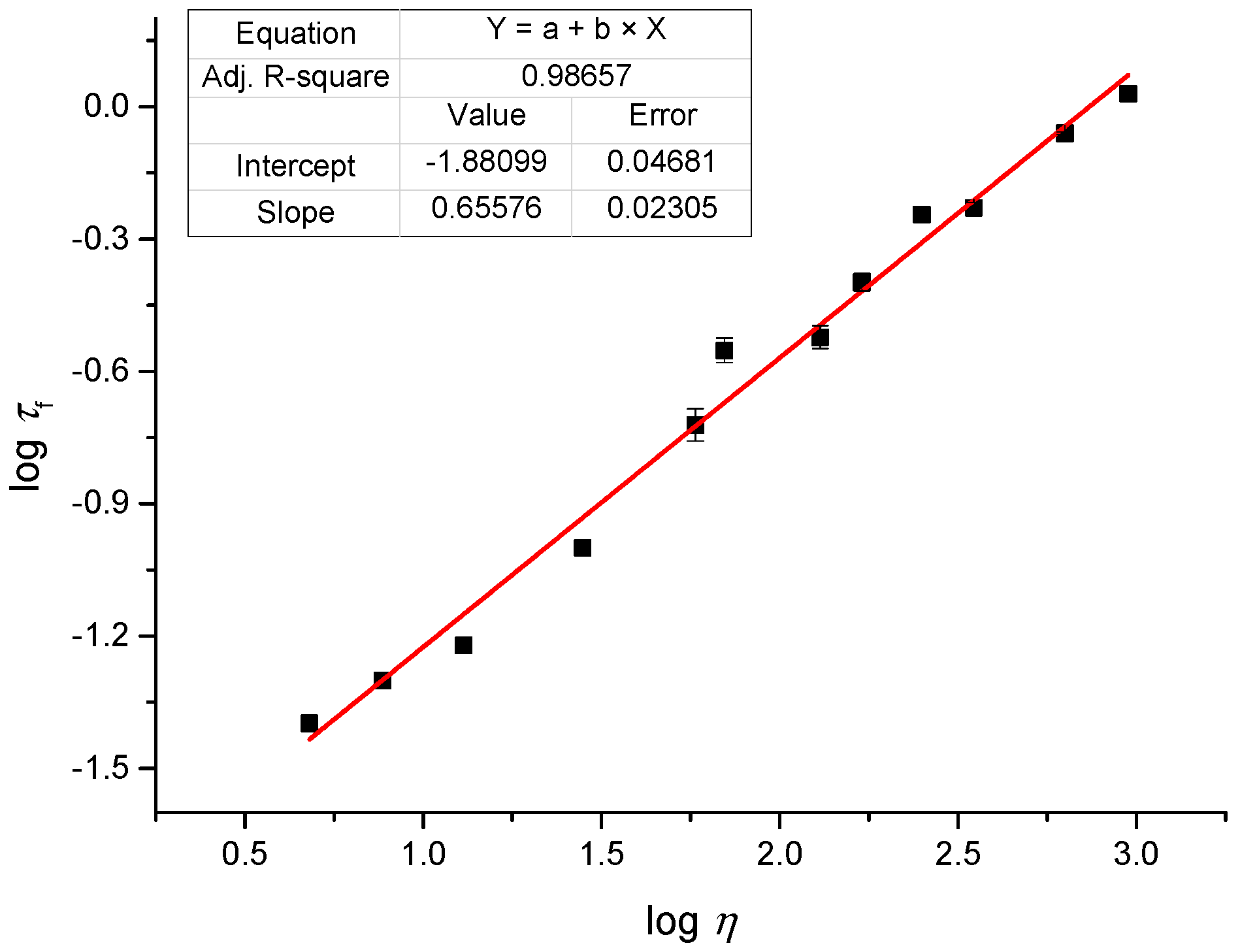
2.3. Linear Photophysical Characterization of dU-BZ as A Function of Viscosity
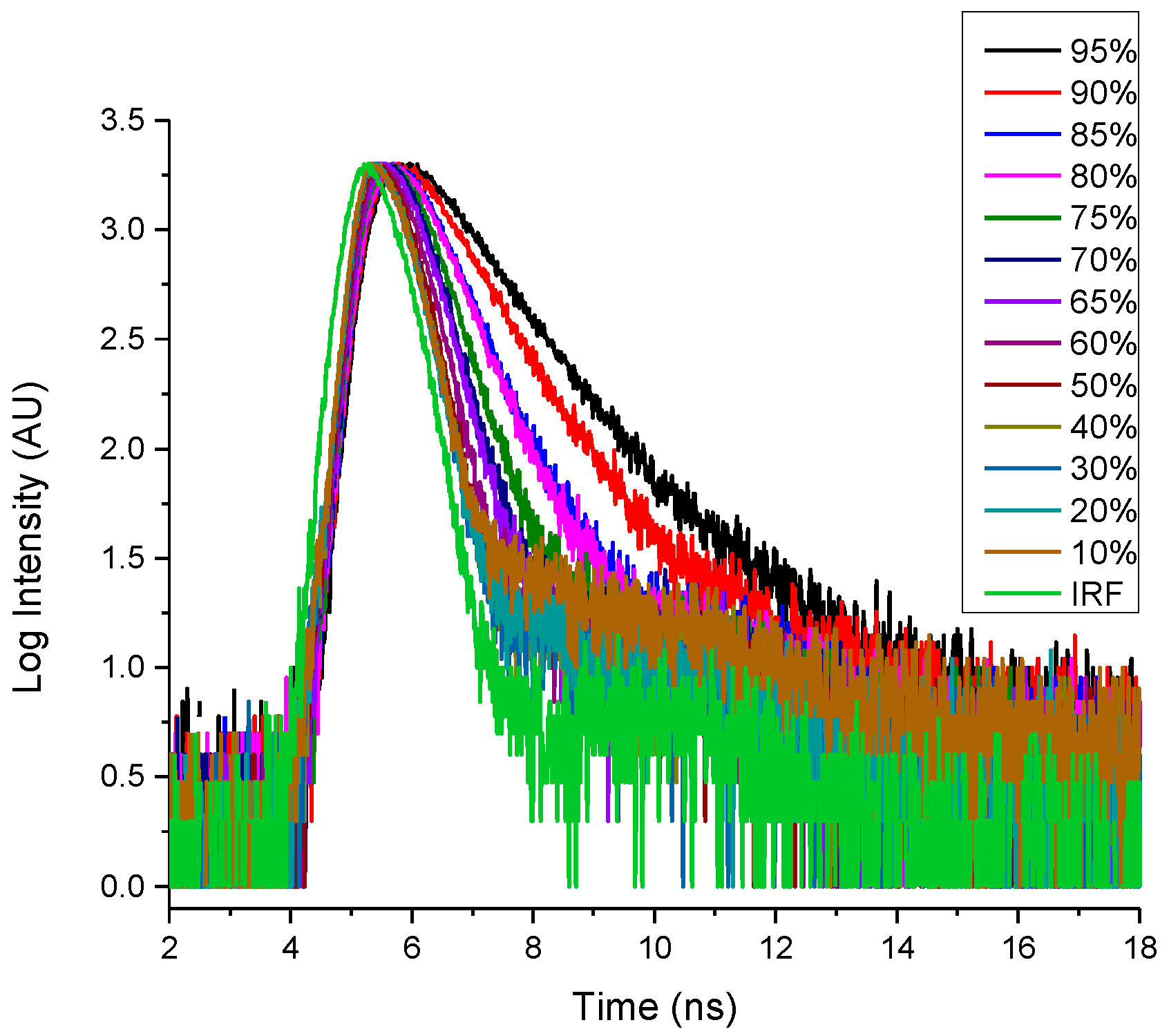
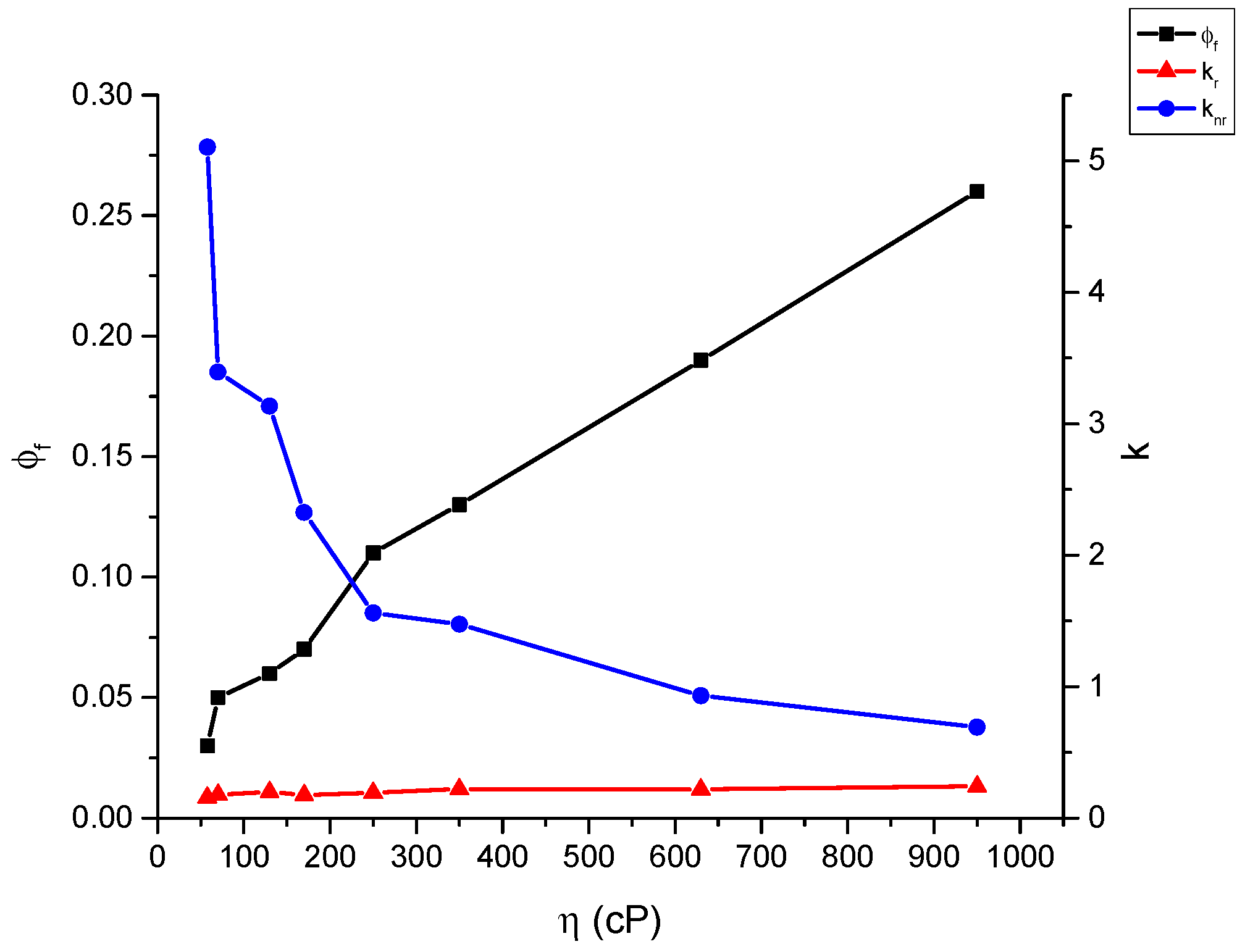
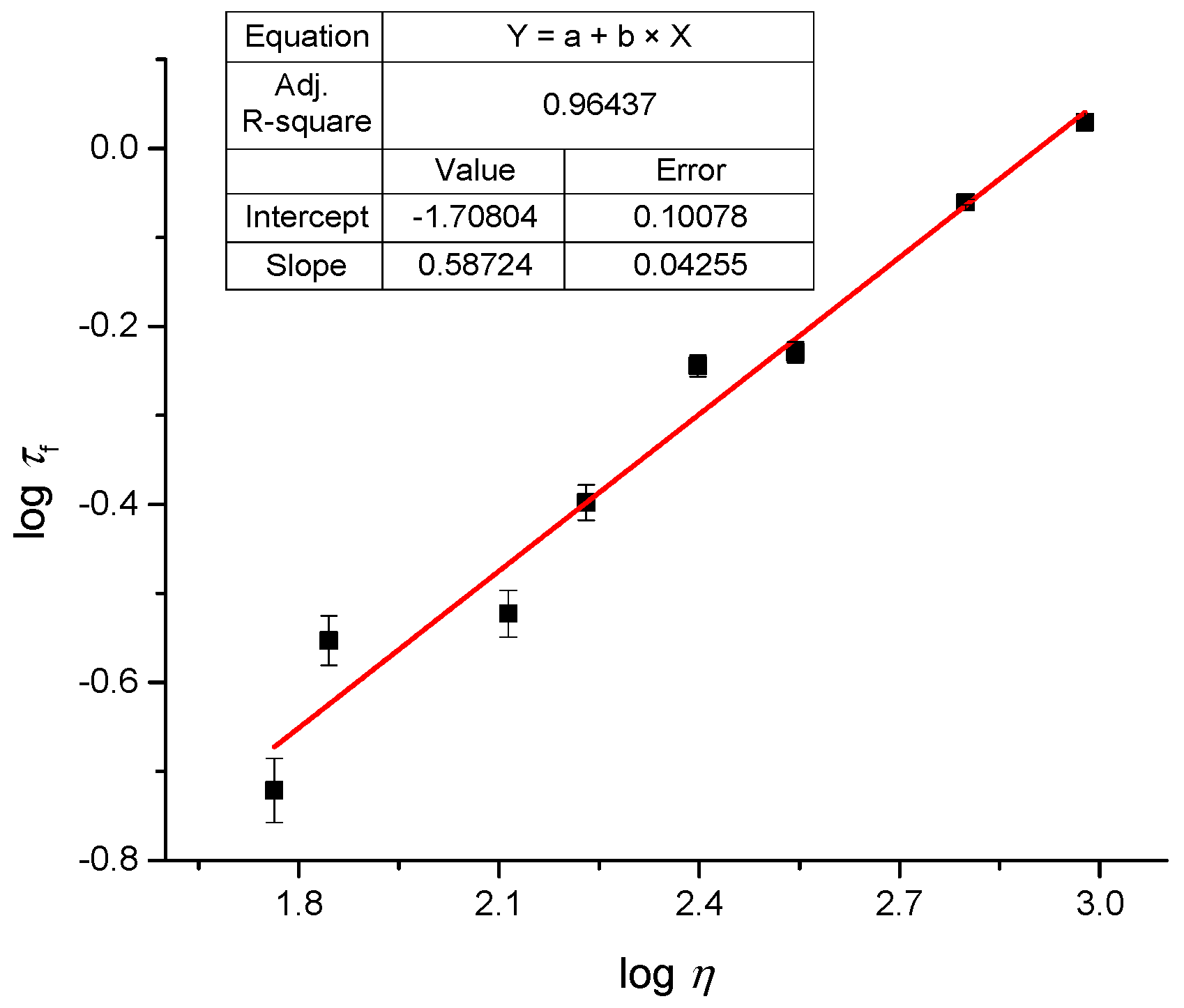
2.4. Fluoresence Lifetime of dU-BZ in Glycerol/Methanol Solutions, Radiative, and Non-Radiative Rate Constants
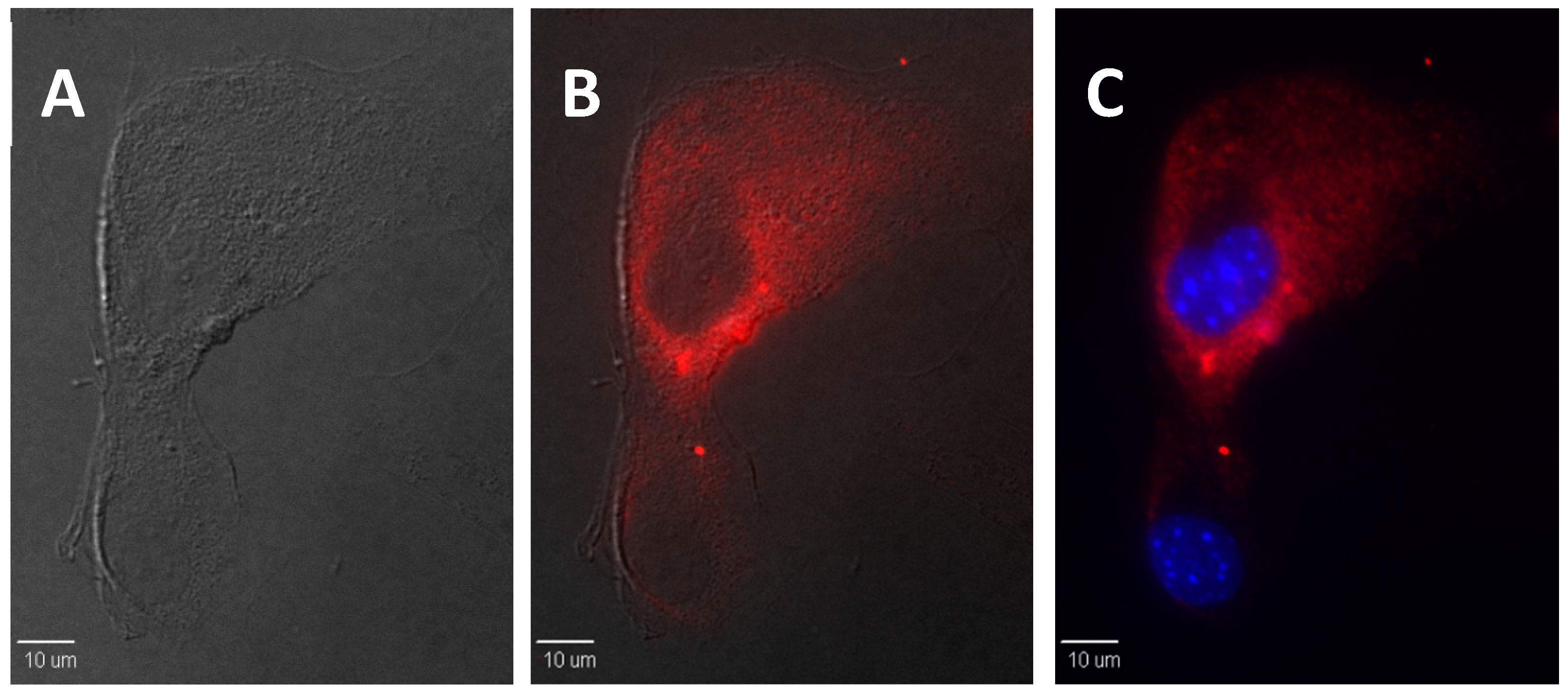
2.5. In Vitro Bioimaging of dU-BZ
3. Discussion
4. Materials and Methods
4.1. Synthesis
4.2. Viscosity Values
4.3. Linear Photophysical Characterization
4.4. In Vitro Bioimaging
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Berg, E.L.; Hsu, Y.; Lee, J.A. Consideration of the cellular microenvironment: Physiologically relevant co-culture systems in drug discovery. Adv. Drug Del. Rev. 2014, 69–70, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Aleardi, A.M.; Benard, G.; Augereau, O.; Malgat, M.; Talbot, J.C.; Mazat, J.P.; Letellier, T.; Dachary-Prigent, J.; Solaini, G.C.; Rossignol, R. Gradual alteration of mitochondrial structure and function by β-amyloids: Importance of membrane viscosity changes, energy deprivation, reactive oxygen species production, and cytochrome c release. J. Bioenerg. Biomembr. 2005, 37, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Kaliviotis, E.; Yianneskis, M. On the effect of dynamic flow conditions on blood microstructure investigated with optical shearing microscopy and rheometry. Proc. Inst. Mech. Eng. H 2007, 221, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Shul’man, Z.P.; Mansurov, V.A. Determination of the Rheological Properties of Whole Blood by the Nonstationary Method of Pulsed Changing of the Rate of Shear. J. Eng. Phys. Thermophys. 2005, 78, 1018–1021. [Google Scholar] [CrossRef]
- Kuimova, M.K.; Botchway, S.W.; Parker, A.W.; Balaz, M.; Collins, H.A.; Anderson, H.L.; Suhling, K.; Ogilby, P.R. Imaging intracellular viscosity of a single cell during photoinduced cell death. Nat. Chem. 2009, 1, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Haidekker, M.A.; Theodorakis, E.A. Molecular rotors—Fluorescent biosensors for viscosity and flow. Org. Biomol. Chem. 2007, 5, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, Z.R.; Rotkiewicz, K.; Siemiarczuk, A.; Cowley, D.J.; Baumann, W. Twisted Intramolecular Charge Transfer States. A New Class of Excited States with a Full Charge Separation. Nouv. J. Chimie 1979, 3, 443–454. [Google Scholar]
- Haidekker, M.A.; Nipper, M.; Mustafic, A.; Lichlyter, D.; Dakanali, M.; Theodorakis, E.A. Dyes with Segmental Mobility: Molecular Rotors. Fundam. Mol. Des. 2010, 8, 267–308. [Google Scholar]
- Loutfy, R.O.; Arnold, B.A. Effect of Viscosity and Temperature on Torsional Relaxation of Molecular Rotors. Phys. Chem. 1982, 86, 4205–4211. [Google Scholar] [CrossRef]
- Fischer, D.; Theodorakis, E.A.; Haidekker, M.A. Synthesis and use of an in-solution ratiometric fluorescent viscosity sensor. Nat. Protoc. 2007, 2, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; He, Y.; Lee, J.H.; Park, N.; Suh, M.; Chae, W.S.; Cao, J.; Peng, X.; Jung, H.; Kang, C.; et al. A self-calibrating bipartite viscosity sensor for mitochondria. J. Am. Chem. Soc. 2013, 135, 9181–9185. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiao, Y.; Tian, W.; Deng, L. Activatable rotor for quantifying lysosomal viscosity in living cells. J. Am. Chem. Soc. 2013, 135, 2903–2906. [Google Scholar] [CrossRef] [PubMed]
- Luby-Phelps, K.; Mujumdar, S.; Mujumdar, R.B.; Ernst, L.A.; Galbraith, W.; Waggoner, A.S. A novel fluorescence ratiometric method confirms the low solvent viscosity of the cytoplasm. Biophys. J. 1993, 65, 236–242. [Google Scholar] [CrossRef]
- Haidekker, M.A.; Brady, T.P.; Lichlyter, D.; Theodorakis, E.A. A ratiometric fluorescent viscosity sensor. J. Am. Chem. Soc. 2006, 128, 398–399. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Yang, Z.; Wang, J.; Fan, J.; He, Y.; Song, F.; Wang, B.; Sun, S.; Qu, J.; Qi, J.; et al. Fluorescence ratiometry and fluorescence lifetime imaging: Using a single molecular sensor for dual mode imaging of cellular viscosity. J. Am. Chem. Soc. 2011, 133, 6626–6635. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Duarte, I.; Vu, T.T.; Izquierdo, M.A.; Bull, J.A.; Kuimova, M.K. A molecular rotor for measuring viscosity in plasma membranes of live cells. Chem. Commun. 2014, 50, 5282–5284. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, M.S. Fluorescent labeling of biomolecules with organic probes. Chem. Rev. 2009, 109, 190–212. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.Y.; Yao, S.; Wang, X.; Belfield, K.D. Near-infrared-emitting squaraine dyes with high 2PA cross-sections for multiphoton fluorescence imaging. ACS Appl. Mater. Interfaces 2012, 4, 2847–2854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yue, X.; Kim, B.; Yao, S.; Bondar, M.V.; Belfield, K.D. Bovine serum albumin nanoparticles with fluorogenic near-IR-emitting squaraine dyes. ACS Appl. Mater. Interfaces 2013, 5, 8710–8717. [Google Scholar] [CrossRef] [PubMed]
- Cristofoli, W.A.; Wiebe, L.I.; de Clercq, E.; Andrei, G.; Snoeck, R.; Balzarini, J.; Knaus, E.E. 5-alkynyl analogs of arabinouridine and 2′-deoxyuridine: Cytostatic activity against herpes simplex virus and varicella-zoster thymidine kinase gene-transfected cells. J. Med. Chem. 2007, 50, 2851–2857. [Google Scholar] [CrossRef] [PubMed]
- Sinkeldam, R.W.; Greco, N.J.; Tor, Y. Fluorescent analogs of biomolecular building blocks: Design, properties, and applications. Chem. Rev. 2010, 110, 2579–2619. [Google Scholar] [CrossRef] [PubMed]
- Srivatsan, S.G.; Tor, Y. Fluorescent pyrimidine ribonucleotide: Synthesis, enzymatic incorporation, and utilization. J. Am. Chem. Soc. 2007, 129, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, H.; Dodo, K.; Palonpon, A.; Ando, J.; Fujita, K.; Kawata, S.; Sodeoka, M. Alkyne-tag Raman imaging for visualization of mobile small molecules in live cells. J. Am. Chem. Soc. 2012, 134, 20681–20689. [Google Scholar] [CrossRef] [PubMed]
- Herdewijn, P. Modified Nucleosides: In Biochemistry Biotechnology and Medicine; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Zhang, Y.; Yue, X.; Kim, B.; Yao, S.; Belfield, K.D. Deoxyribonucleoside-modified squaraines as near-IR viscosity sensors. Chem. Eur. J. 2014, 20, 7249–7253. [Google Scholar] [CrossRef] [PubMed]
- Teo, Y.N.; Kool, E.T. DNA-multichromophore systems. Chem. Rev. 2012, 112, 4221–4245. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, A.J. Applicability of the Free Volume Concept on Relaxation Phenomena in the Glass Transition Range. Rheol. Acta. 1966, 5, 262–269. [Google Scholar] [CrossRef]
- Förester, T.; Hoffmann, G.Z. The viscosity dependence of fluorescence quantum yields of some dye systems. Phys. Chem. 1971, 75, 63–76. [Google Scholar]
- Hoegger, M.J.; Fishcer, A.J.; McMenimen, J.D.; Ostedgaard, L.S.; Tucker, A.J.; Awadalla, M.A.; Moninger, T.O.; Michalski, A.S.; Hoffman, E.A.; Zabner, J.; et al. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science 2014, 345, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Levitt, J.A.; Chung, P.H.; Kuimova, M.K.; Yahioglu, G.; Wang, Y.; Qu, J.; Suhling, K. Fluorescence anisotropy of molecular rotors. Chem. Phys. Chem. 2011, 12, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 2nd ed.; Kluwer: New York, NY, USA, 1999. [Google Scholar]
- Yanez, C.O.; Morales, A.R.; Yue, X.; Urakami, T.; Komatsu, M.; Jarvinen, T.A.H.; Belfield, K.D. Deep vascular imaging in wounds by two-photon fluorescence microscopy. PLoS ONE 2013, 8, e67559. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds not available.
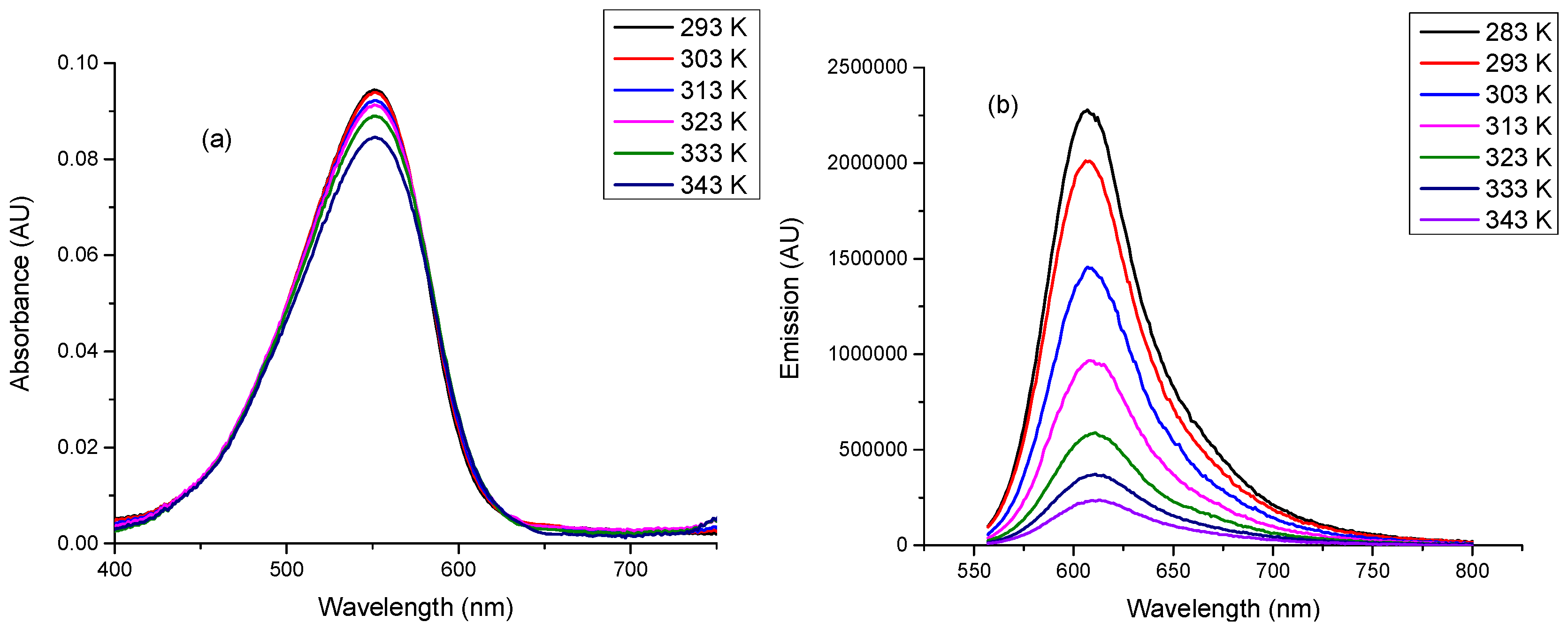
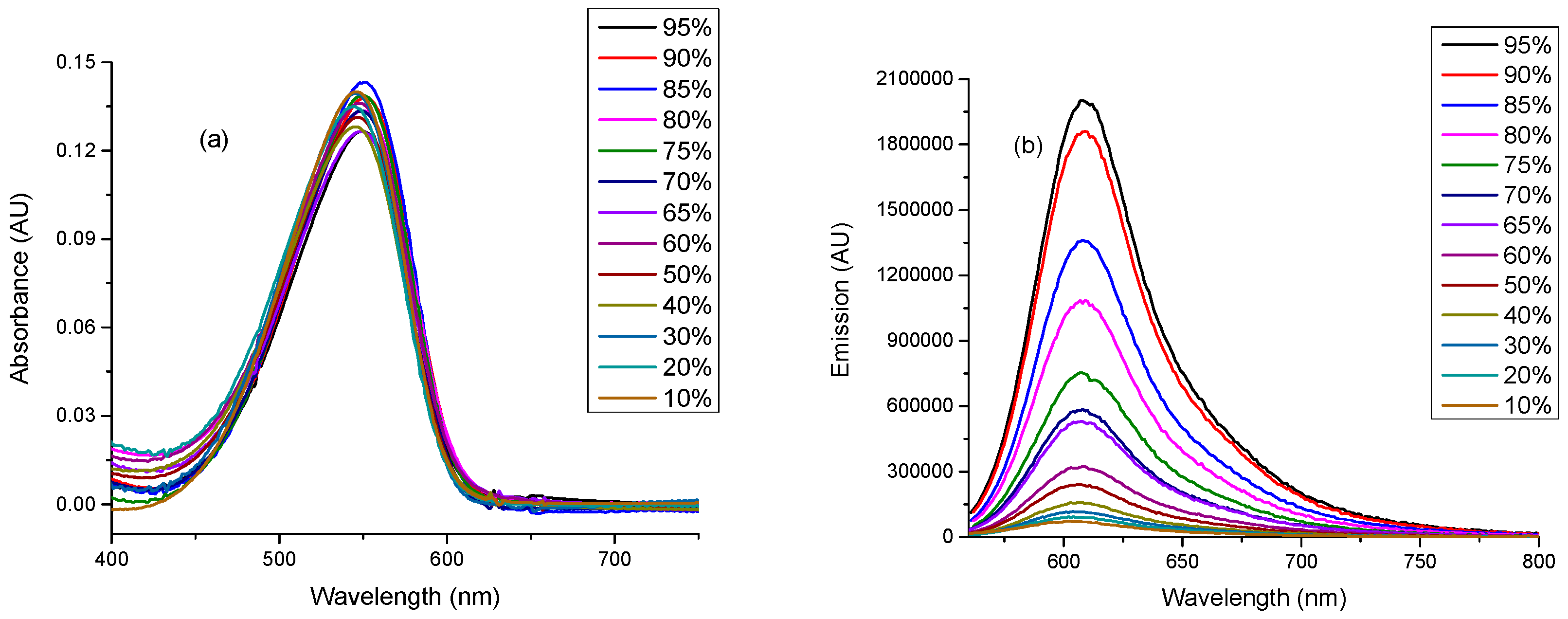
| Temperature (T)/K | Viscosity (η)/cP | Fluorescence Quantum Yield (Φf) |
|---|---|---|
| 343 | 50.6 | 0.04 ± 4 × 10−3 |
| 333 | 81.3 | 0.07 ± 7 × 10−3 |
| 323 | 142 | 0.10 ± 1 × 10−2 |
| 313 | 284 | 0.17 ± 2 × 10−2 |
| 303 | 612 | 0.24 ± 2 × 10−2 |
| 293 | 1412 | 0.34 ± 3 × 10−2 |
| % Glycerol | Viscosity (η)/cP | Fluorescence Quantum Yield (Φf) | Fluorescence Lifetime (τf)/ns * | kr/ns−1 | knr/ns−1 |
|---|---|---|---|---|---|
| 95 | 950 | 0.26 ± 2.5 × 10−2 | 1.07 | 0.243 | 0.692 |
| 90 | 630 | 0.19 ± 2 × 10−2 | 0.87 | 0.218 | 0.931 |
| 85 | 350 | 0.13 ± 1.5 × 10−2 | 0.59 | 0.220 | 1.475 |
| 80 | 250 | 0.11 ± 1 × 10−2 | 0.57 | 0.193 | 1.561 |
| 75 | 170 | 0.07 ± 7 × 10−3 | 0.40 | 0.175 | 2.325 |
| 70 | 130 | 0.06 ± 6 × 10−3 | 0.30 | 0.200 | 3.133 |
| 65 | 70 | 0.06 ± 6 × 10−3 | 0.28 | 0.179 | 3.393 |
| 60 | 58 | 0.03 ± 3 × 10−3 | 0.19 | 0.158 | 5.105 |
| 50 | 28 | 0.02 ± 2 × 10−3 | 0.10 | 0.200 | 9.800 |
| 40 | 13 | 0.015 ± 1.5 × 10−3 | 0.06 | 0.250 | 16.417 |
| 30 | 7.7 | 0.010 ± 1 × 10−3 | 0.05 | 0.200 | 19.800 |
| 20 | 4.8 | 0.008 ± 8 × 10−4 | 0.04 | 0.200 | 24.800 |
| 10 | 1.8 | 0.006 ± 6 × 10−4 | 0.05 | 0.120 | 19.880 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zhang, Y.; Yue, X.; Yao, S.; Bondar, M.V.; Belfield, K.D. A Deoxyuridine-Based Far-Red Emitting Viscosity Sensor. Molecules 2016, 21, 709. https://doi.org/10.3390/molecules21060709
Wang M, Zhang Y, Yue X, Yao S, Bondar MV, Belfield KD. A Deoxyuridine-Based Far-Red Emitting Viscosity Sensor. Molecules. 2016; 21(6):709. https://doi.org/10.3390/molecules21060709
Chicago/Turabian StyleWang, Mengyuan, Yuanwei Zhang, Xiling Yue, Sheng Yao, Mykhailo V. Bondar, and Kevin D. Belfield. 2016. "A Deoxyuridine-Based Far-Red Emitting Viscosity Sensor" Molecules 21, no. 6: 709. https://doi.org/10.3390/molecules21060709