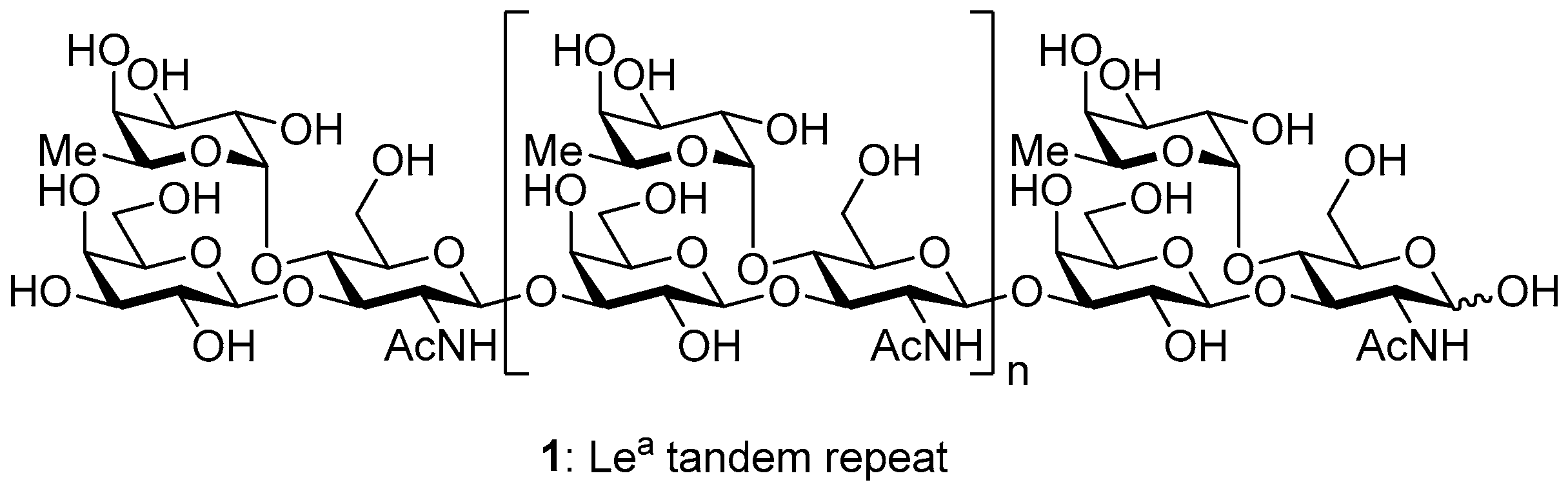
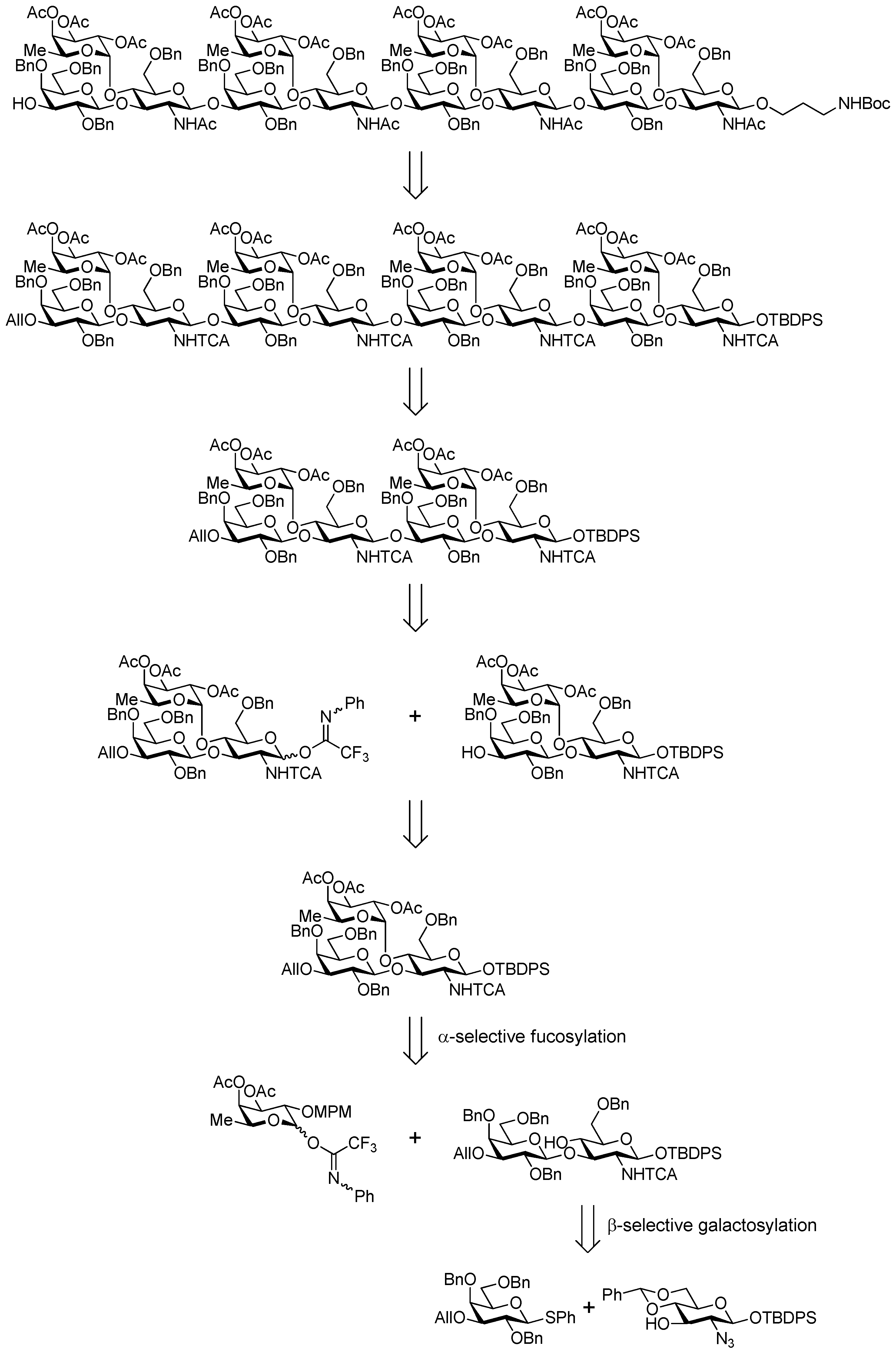
3.2. Physical Data for All New Compounds
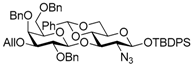
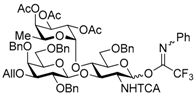
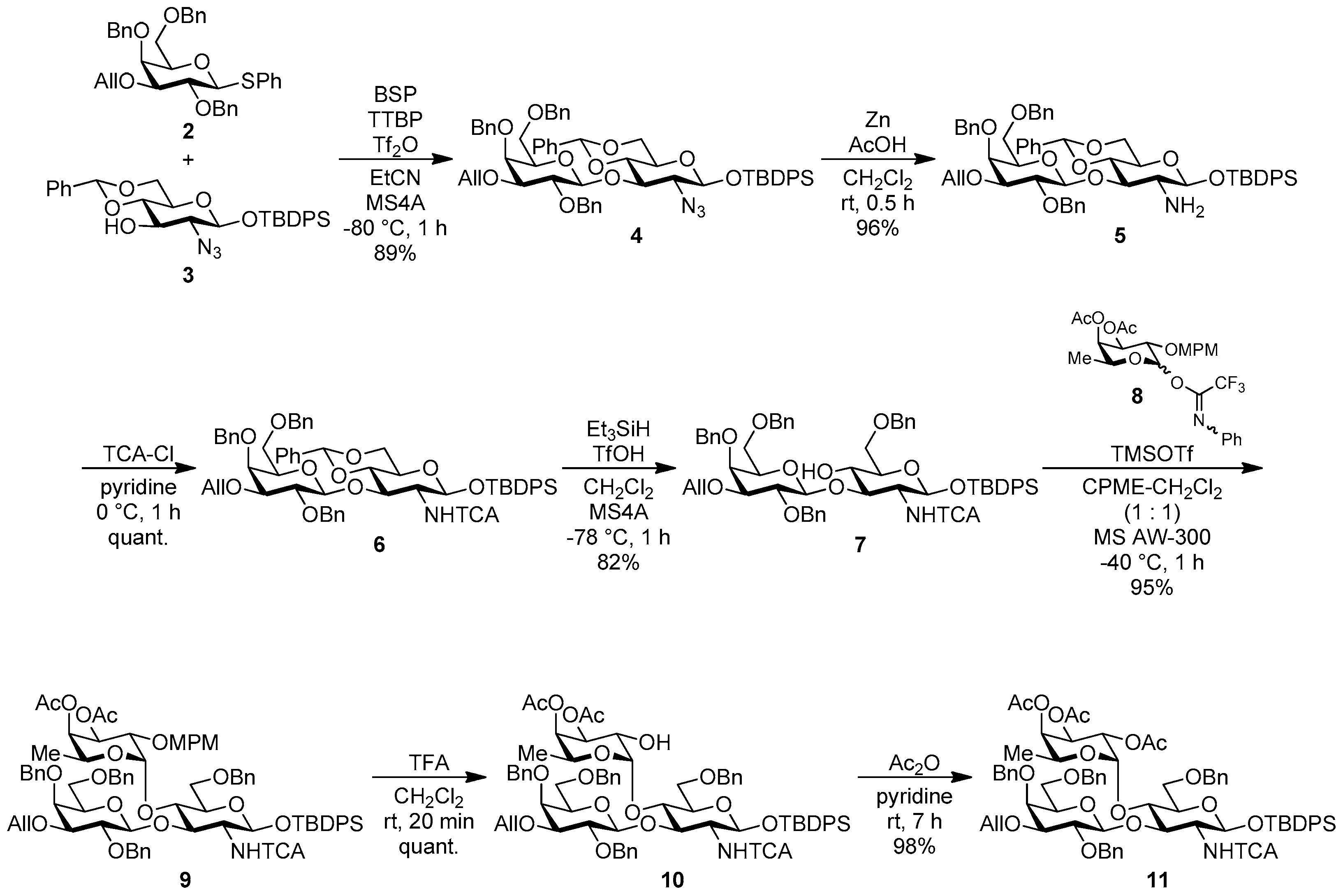
tert-Butyldiphenylsilyl 3-O-allyl-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-2-azido-4,6-O-benzylidene-2-deoxy-β-d-glucopyranoside (4). To a mixture of phenyl 3-O-allyl-2,4,6-tri-O-benzyl-1-thio-β-d-galactopyranoside 2 (438 mg, 0.75 mmol), tert-butyldiphenylsilyl 2-azido-4,6-O-benzylidene-2-deoxy-β-d-glucopyranoside 3 (200 mg, 0.38 mmol), benzenesulfinyl piperidine (237 mg, 1.13 mmol), tri-tert-butylpyrimidine (373 mg, 1.50 mmol), and molecular sieves 4A (1.41 g) in propionitrile (12.5 mL) was added dropwise trifluoromethanesulfonic anhydride (140 μL, 0.83 mmol) at −80 °C under Ar, and stirred for 1 h at −80 °C. The reaction mixture was quenched with sat. NaHCO3 aq., filtered through Celite, and diluted with EtOAc. The organic layer was separated, and the aqueous layer was extracted with EtOAc. The combined organic layer was successively washed with brine, dried over Na2SO4, and concentrated. The crude product was chromatographed on silica gel (PSQ-60B) with toluene–acetone (98:2) to give the title product 4 (338 mg, 89%). [α]D −31.3° (c 1.1, CHCl3); 1H-NMR (500 MHz, CDCl3) δ 7.70–7.15 (m, 30H, Ar), 5.93–5.85 (m, 1H, H2C=CHCH2), 5.39 (s, 1H, >CHPh), 5.31–5.27 (m, 1H, H2C=CHCH2), 5.16–5.13 (m, 1H, H2C=CHCH2), 4.96–4.89 (m, 2H, PhCH2 × 2), 4.80 (d, 1H, Jgem = 10.7 Hz, PhCH2), 4.63 (d, 1H, J1,2 = 7.9 Hz, H-1Gal), 4.57 (d, 1H, Jgem = 11.7 Hz, PhCH2), 4.48 (d, 1H, J1,2 = 7.8 Hz, H-1GlcN), 4.26–4.19 (m, 2H, PhCH2 × 2), 4.15–4.09 (m, 2H, H2C=CHCH2), 3.89 (dd, 1H, J5,6b = 4.9 Hz, Jgem = 10.1 Hz, H-6aGlcN), 3.78–3.69 (m, 4H, H-2Gal, H-4Gal, H-3GlcN, H-4GlcN), 3.59–3.51 (m, 3H, H-2GlcN, H-6bGlcN, H-6aGal), 3.36–3.31 (m, 2H, H-3Gal, H-6bGal), 3.25 (dd, 1H, J5,6a = J5,6b = 6.5 Hz, H-5Gal), 2.96–2.91 (m, 1H, H-5GlcN), 1.16 (s, 9H, tBu); 13C-NMR (125 MHz, CDCl3) δ 138.9, 138.8, 137.9, 137.3, 135.8, 134.9, 133.1, 132.5, 130.0, 129.8, 128.9, 128.4, 128.2, 128.2, 128.2, 128.1, 128.0, 127.9, 127.7, 127.6, 127.5, 127.4, 127.4, 126.0, 116.5, 102.7, 101.1, 97.2, 82.3, 79.9, 79.8, 78.7, 75.2, 74.4, 73.5, 73.1, 72.9, 71.7, 68.9, 68.8, 68.3, 66.2, 26.8, 19.1. HRMS (ESI) m/z: found [M + Na]+ 1026.4337, C59H65N3O10Si calcd. for [M + Na]+ 1026.4337.
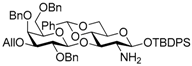
tert-Butyldiphenylsilyl 3-O-allyl-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-2-amino-4,6-O-benzylidene-2-deoxy-β-d-glucopyranoside (5). A mixture of 4 (576 mg, 0.57 mmol), powdered Zn (1.50 g, 23.0 mmol), and AcOH (0.66 ml, 11.5 mmol) in CH2Cl2 (14 mL) was stirred for 30 min at room temperature under Ar. The mixture was diluted with CHCl3 and filtered through Celite. The filtrate was evaporated, and the residue was diluted with CHCl3. The organic layer was successively washed with sat. NaHCO3, water, and brine, dried over Na2SO4, and concentrated. The residue was chromatographed on silica gel with toluene–MeOH (95:5) to give the title product 5 (540 mg, 96%). [α]D −21.8° (c 1.3, CHCl3); 1H-NMR (500 MHz, CDCl3) δ 7.68–7.18 (m, 30H, Ar), 5.94–5.87 (m, 1H, H2C=CHCH2), 5.45 (s, 1H, >CHPh), 5.33–5.28 (m, 1H, H2C=CHCH2), 5.18–5.15 (m, 1H, H2C=CHCH2), 4.93–4.88 (m, 2H, PhCH2 × 2), 4.78 (d, 1H, Jgem = 10.9 Hz, PhCH2), 4.58 (d, 1H, Jgem = 11.6 Hz, PhCH2), 4.49 (d, 1H, J1,2 = 7.9 Hz, H-1Gal), 4.43 (d, 1H, J1,2 = 7.8 Hz, H-1GlcN), 4.31–4.25 (m, 2H, PhCH2 × 2), 4.17–4.08 (m, 2H, H2C=CHCH2), 3.96 (dd, 1H, J5,6a = 4.9 Hz, Jgem = 10.4 Hz, H-6aGlcN), 3.87–3.81 (m, 2H, H-2Gal, H-4Gal), 3.66–3.58 (m, 3H, H-4GlcN, H-6bGlcN, H-6aGlcN), 3.54 (t, 1H, J2,3 = J3.4 = 9.2 Hz, H-3GlcN), 3.41–3.37 (m, 3H, H-3Gal, H-5Gal, H-6bGal), 3.03–2.99 (m, 2H, H-2GlcN, H-5GlcN), 1.20 (s, 9H, tBu); 13C-NMR (125 MHz, CDCl3) δ 138.9, 138.5, 137.9, 137.6, 135.8, 135.8, 134.8, 133.4, 132.9, 129.8, 129.7, 128.6, 128.4, 128.3, 128.3, 128.1, 128.1, 127.8, 127.7, 127.7, 127.5, 127.4, 127.3, 126.1, 116.7, 104.3, 100.8, 99.1, 83.5, 82.7, 79.9, 79.4, 75.7, 74.5, 73.5, 73.1, 73.0, 71.4, 68.4, 68.2, 66.8, 60.1, 27.0, 19.2. HRMS (ESI) m/z: found [M + Na]+ 1000.4432, C59H67NO10Si calcd. for [M + Na]+ 1000.4432.
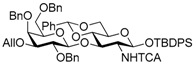
tert-Butyldiphenylsilyl 3-O-allyl-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-4,6-O-benzylidene-2-deoxy-2-trichloroacetamido-β-d-glucopyranoside (6); To a solution of 5 (12.9 g, 13.2 mmol) in pyridine (132 mL) was added dropwise trichloroacetyl chloride (1.76 mL, 15.8 mmol) at 0 °C under Ar, and stirred at 0 °C for 1 h. The mixture was evaporated, and the residue was diluted with CHCl3, successively washed with 2 M HCl, sat. NaHCO3, and brine, dried over Na2SO4, and concentrated. The residue was chromatographed on silica gel with toluene–EtOAc (97:3) to give the title product 6 (14.8 g, quant.). [α]D −9.8° (c 1.3, CHCl3); 1H-NMR (500 MHz, CDCl3) δ 7.67–7.18 (m, 30H, Ar), 7.02 (d, 1H, J2,NH = 7.0 Hz, NH), 5.95–5.87 (m, 1H, H2C=CHCH2), 5.45 (s, 1H, >CHPh), 5.34–5.30 (m, 1H, H2C=CHCH2), 5.24 (d, 1H, J1,2 = 7.9 Hz, H-1GlcN), 5.20–5.17 (m, 1H, H2C=CHCH2), 4.90 (d, 1H, Jgem = 11.6 Hz, PhCH2), 4.82 (d, 1H, Jgem = 10.7 Hz, PhCH2), 4.69 (d, 1H, PhCH2), 4.57 (d, 1H, PhCH2), 4.42 (d, 1H, J1,2 = 7.9 Hz, H-1Gal), 4.39–4.30 (m, 3H, PhCH2 × 2, H-3GlcN), 4.18–4.10 (m, 2H, H2C=CHCH2), 3.95 (dd, 1H, J5,6a = 4.9 Hz, Jgem = 10.4 Hz, H-6aGlcN), 3.82–3.79 (m, 2H, H-2Gal, H-4Gal), 3.65 (t, 1H, J3,4 = J4,5 = 9.2 Hz, H-4GlcN), 3.61–3.54 (m, 2H, H-6bGlcN, H-6aGal), 3.49 (dd, 1H, J5,6b = 5.4 Hz, Jgem = 9.0 Hz, H-6bGal), 3.44–3.39 (m, 2H, H-2GlcN, H-5Gal), 3.31 (dd, 1H, J2,3 = 9.8 Hz, J3,4 = 2.9 Hz, H-3Gal), 3.12–3.07 (m, 1H, H-5GlcN), 1.06 (s, 9H, tBu); 13C-NMR (125 MHz, CDCl3) δ 161.4, 138.9, 138.7, 137.8, 137.3, 135.9, 135.7, 134.8, 133.0, 132.5, 129.8, 129.8, 128.7, 128.5, 128.4, 128.1, 128.1, 128.0, 127.9, 127.9, 127.8, 127.5, 127.4, 127.4, 126.1, 116.8, 103.1, 100.8, 94.1, 92.2, 82.1, 79.9, 79.2, 77.6, 76.5, 75.8, 74.5, 73.6, 73.3, 73.2, 71.4, 68.3, 68.3, 65.9, 62.7, 26.9, 19.1. HRMS (ESI) m/z: found [M + Na]+ 1144.3368, C61H66Cl3NO11Si calcd. for [M + Na]+ 1144.3368.
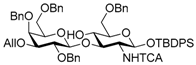
tert-Butyldiphenylsilyl 3-O-allyl-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-6-O-benzyl-2-deoxy-2-tri-chloroacetamido-β-d-glucopyranoside (7). To a mixture of 6 (814 mg, 0.73 mmol) and molecular sieves 4A (4.20 g) in CH2Cl2 (7.3 mL) was added triethylsilane (463 μL, 2.90 mmol) and trifluoromethane sulfonic acid (127 μL, 1.45 mmol) at −78 °C under Ar, and stirred for 1 h at −78 °C, and 1.5 h at −40 °C. The reaction mixture was quenched with triethylamine, filtered through Celite, and diluted with CHCl3. The organic layer was successively washed with sat. NaHCO3, water, and brine, dried over Na2SO4, and concentrated. The crude product was chromatographed on silica gel with toluene–EtOAc (89:11) to give the title product 7 (665 mg, 82%). [α]D +1.5° (c 1.3, CHCl3); 1H-NMR (500 MHz, CDCl3) δ 7.72–7.16 (m, 30H, Ar), 6.70 (d, 1H, J2,NH = 7.1 Hz, NH), 5.93–5.86 (m, 1H, H2C=CHCH2), 5.32–5.29 (m, 1H, H2C=CHCH2), 5.19–5.17 (m, 1H, H2C=CHCH2), 5.11 (d, 1H, J1,2 = 8.0 Hz, H-1GlcN), 4.88 (d, 1H, Jgem = 11.6 Hz, PhCH2), 4.82 (d, 1H, Jgem = 11.3 Hz, PhCH2), 4.72 (d, 1H, PhCH2), 4.53 (d, 1H, PhCH2), 4.42–4.34 (m, 4H, PhCH2 × 4), 4.23 (d, 1H, J1,2 = 7.8 Hz, H-1Gal), 4.19–4.12 (m, 2H, H2C=CHCH2), 4.03 (dd, 1H, J2,3 = 10.1 Hz, J3,4 = 8.4 Hz, H-3GlcN), 3.80 (d, 1H, J3,4 = 2.9 Hz, H-4Gal), 3.75 (dd, 1H, J2,3 = 9.8 Hz, H-2Gal), 3.69 (s, 1H, OH), 3.58–3.47 (m, 6H, H-4GlcN, H-5Gal, H-6aGlcN, H-6aGal, H-6bGlcN, H-6bGal), 3.38–3.33 (m, 1H, H-2GlcN), 3.29 (dd, 1 H, H-3Gal), 3.17–3.13 (m, 1 H, H-5GlcN), 1.06 (s, 9 H, tBu); 13C-NMR (125 MHz, CDCl3) δ 161.5, 139.1, 138.6, 138.4, 137.6, 136.0, 135.8, 134.7, 133.1, 132.6, 129.7, 129.7, 128.4, 128.4, 128.2, 128.2, 127.9, 127.8, 127.8, 127.6, 127.5, 127.4, 127.3, 127.3, 116.8, 103.5, 93.7, 92.1, 81.7, 81.1, 79.5, 75.7, 75.1, 74.6, 73.6, 73.5, 73.4, 73.4, 71.7, 69.2, 69.1, 68.3, 61.3, 26.9, 19.1. HRMS (ESI) m/z: found [M + Na]+ 1146.3525, C61H68Cl3NO11Si calcd. for [M + Na]+ 1146.3525.
tert-Butyldiphenylsilyl 3-O-allyl-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[3,4-di-O-acetyl-6-2-O-p-methoxybenzyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-β-d-glucopyranoside (9). To a mixture of 7 (1.96 g, 1.74 mmol), 3,4-di-O-acetyl-2-O-p-methoxybenzyl-l-fucipyranosyl (N-phenyl)-2,2,2-trifluoroacetimidate 8 (1.87 g, 3.47 mmol), and molecular sieves AW-300 (5.22 g) in CPME/CH2Cl2 (1:1, 58.0 mL) was added TMSOTf (15.7 μL, 0.087 mmol) dropwise at −40 °C under Ar, and stirred for 1 h at −40 °C. The reaction mixture was quenched with sat. NaHCO3, filtered through Celite, and diluted with CHCl3. The organic layer was separated, and the aqueous layer was extracted with CHCl3. The combined organic layer was successively washed with water and brine, dried over Na2SO4, and concentrated. The crude product was chromatographed on silica gel with hexane–acetone (80:20) to give the title product 9 (2.44 g, 95%). [α]D −19.6° (c 1.3, CHCl3); 1H-NMR (500 MHz, CDCl3) δ 7.72–7.11 (m, 32H, Ar), 6.93 (d, 1H, J2,NH = 6.8 Hz, NH), 6.81–6.78 (m, 2H, Ar), 5.95–5.87 (m, 1H, H2C=CHCH2), 5.35–5.32 (m, 1H, H2C=CHCH2), 5.21–5.11 (m, 5H, H2C=CHCH2, H-1GlcN, H-1Fuc, H-3Fuc, H-4Fuc), 5.09–5.05 (m, 1H, H-5Fuc), 4.88 (d, 1H, Jgem = 10.5 Hz, ArCH2), 4.74–4.69 (m, 2H, ArCH2 × 2), 4.55–4.49 (m, 3H, ArCH2 × 3), 4.46–4.39 (m, 3H, ArCH2 × 2, H-1Gal), 4.34 (d, 1H, Jgem = 12.6 Hz, ArCH2), 4.24–4.08 (m, 4H, H2C=CHCH2 × 2, ArCH2, H-3GlcN), 3.87–3.77 (m, 7H, OMe, H-4GlcN, H-4Gal, H-6aGal, H-2Fuc), 3.73–3.68 (m, 2H, H-6aGlcN, H-6bGal), 3.59 (dd, 1H, J1,2 = 8.0 Hz, J2,3 = 9.7 Hz, H-2Gal), 3.34 (dd, 1H, J5,6a = 4.9 Hz, J5,6b = 8.9 Hz, H-5Gal), 3.30–3.27 (m, 2H, H-2GlcN, H-3Gal), 3.09 (dd, 1H, J5,6b = 1.5 Hz, Jgem = 11.7 Hz, H-6bGlcN), 2.98–2.96 (m, 1H, H-5GlcN), 2.11 (s, 3H, Ac), 1.97 (s, 3H, Ac), 1.06 (s, 9H, tBu), 0.78 (d, 3H, J5,6 = 6.5 Hz, H-6Fuc); 13C-NMR (125 MHz, CDCl3) δ 170.3, 169.1, 160.9, 159.3, 138.9, 138.5, 138.5, 138.4, 135.8, 135.7, 134.9, 133.4, 132.6, 130.1, 129.7, 129.6, 128.8, 128.5, 128.5, 128.3, 128.3, 128.2, 128.1, 128.0, 127.6, 127.5, 127.4, 127.4, 127.3, 116.8, 113.7, 103.0, 97.1, 93.4, 92.2, 81.7, 79.9, 77.6, 76.0, 75.7, 75.0, 74.6, 73.5, 73.4, 73.3, 73.2, 73.1, 72.6, 72.3, 71.2, 70.8, 67.9, 67.0, 64.2, 55.2, 26.9, 20.9, 20.8, 19.2, 15.4. HRMS (ESI) m/z: found [M + Na]+ 1496.4890, C79H90Cl3NO18Si calcd. for [M + Na]+ 1496.4890.
tert-Butyldiphenylsilyl 3-O-allyl-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[3,4-di-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-β-d-glucopyranoside (10). A solution of 9 (29.3 mg, 19.9 μmol) in trifluoroacetic acid/CH2Cl2 (1:9, 0.80 mL) was stirred for 20 min at room temperature. The reaction mixture was diluted with toluene, and evaporated. The residue was dissolved with CHCl3, successively washed with sat. NaHCO3, water, and brine, dried over Na2SO4, and concentrated. The crude product was chromatographed on silica gel with hexane–EtOAC (80:20) to give the title product 10 (27.0 g, quant.). [α]D −33.2° (c 1.1, CHCl3); 1H-NMR (500 MHz, CDCl3) δ 7.69–7.15 (m, 30H, Ar), 6.98 (d, 1H, J2,NH = 6.7 Hz, NH), 5.97–5.89 (m, 1H, H2C=CHCH2), 5.36 (dd, 1H, Jtrans = 17.3 Hz, Jgem = 1.6 Hz, H2C=CHCH2), 5.23–5.19 (m, 2H, H2C=CHCH2, H-1GlcN), 5.12–5.10 (m, 2H, H-1Fuc, H-4Fuc), 5.02–4.95 (m, 2H, H-5Fuc, H-3Fuc), 4.91 (d, 1H, Jgem = 10.4 Hz, PhCH2), 4.75 (d, 1H, Jgem = 11.1 Hz, PhCH2), 4.68 (d, 1H, PhCH2), 4.53 (d, 1H, PhCH2), 4.47–4.35 (m, 5H, PhCH2 × 4, H-1Gal), 4.22–4.11 (m, 3H, H-3GlcN, H2C=CHCH2 × 2), 3.92 (t, 1H, J3,4 = J4,5 = 9.4 Hz, H-4GlcN), 3.85–3.80 (m, 2H, H-2Fuc, H-4Gal), 3.70–3.56 (m, 4H, H-6aGal, H-6bGal, H-6aGlcN, H-2Gal), 3.34 (dd, 1H, J5,6a = 4.9 Hz, J5,6b = 8.7 Hz, H-5Gal), 3.30 (dd, 1H, J2,3 = 9.8 Hz, J3,4 = 2.8 Hz, H-3Gal), 3.22–3.19 (m, 2H, H-6bGlcN, H-2GlcN), 2.95–2.93 (m, 1H, H-5GlcN), 2.13 (s, 3H, Ac), 2.05 (s, 3H, Ac), 1.75 (d, 1H, J2,OH = 10.8 Hz, OH), 1.03 (s, 9H, tBu), 0.81 (d, 3H, J5,6 = 6.5 Hz, H-6Fuc); 13C-NMR (125 MHz, CDCl3) δ 170.4, 170.2, 161.0, 138.8, 138.4, 138.2, 138.0, 135.9, 135.7, 134.8, 133.2, 132.5, 129.7, 129.7, 128.7, 128.6, 128.5, 128.3, 128.3, 128.2, 128.2, 127.7, 127.6, 127.5, 127.3, 116.9, 103.0, 97.5, 93.2, 92.1, 81.8, 80.1, 77.6, 76.2, 75.9, 74.6, 73.4, 73.3, 73.0, 72.9, 72.4, 71.8, 71.3, 67.9, 67.3, 67.2, 64.6, 63.4, 29.7, 26.9, 21.0, 20.7, 19.2, 15.4. HRMS (ESI) m/z: found [M + Na]+ 1376.4315, C71H82Cl3NO17Si calcd. for [M + Na]+ 1376.4315.
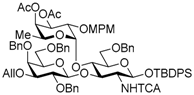
tert-Butyldiphenylsilyl 3-O-allyl-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-β-d-glucopyranoside (11). To a solution of 10 (9.32 g, 6.87 mmol) in pyridine (458 mL) was added acetic anhydride (458 mL) at 0 °C under Ar, and stirred for 7 h at room temperature. The reaction mixture was concentrated. The crude product was chromatographed on silica gel with hexane–EtOAc (67:33) to give the title product 11 (9.45 g, 98%). [α]D −37.1° (c 1.2, CHCl3); 1H-NMR (500 MHz, CDCl3) δ 7.69–7.14 (m, 30H, Ar), 6.99 (d, 1H, J2,NH = 6.7 Hz, NH), 5.97–5.90 (m, 1H, H2C=CHCH2), 5.35 (dd, 1H, Jtrans = 17.3 Hz, Jgem = 1.7 Hz, H2C=CHCH2), 5.22–5.11 (m, 7H, H2C=CHCH2, H-1GlcN, H-1Fuv, H-2Fuc, H-3Fuc, H-4Fuc, H-5Fuc), 4.91 (d, 1H, Jgem = 10.3 Hz, PhCH2), 4.75 (d, 1H, Jgem = 11.0 Hz, PhCH2), 4.67 (d, 1H, PhCH2), 4.53–4.50 (m, 2H, PhCH2 × 2), 4.46–4.43 (m, 2H, PhCH2, H-1Gal), 4.38 (d, 1H, Jgem = 12.5 Hz, PhCH2), 4.31 (d, 1H, PhCH2), 4.25 (t, 1H, J2,3 = J3,4 = 9.4 Hz, H-3GlcN), 4.19–4.11 (m, 2H, H2C=CHCH2), 3.90 (t, 1H, J4,5 = 9.4 Hz, H-4GlcN), 3.84 (d, 1H, J3,4 =2.5 Hz, H-4Gal), 3.79–3.71 (m, 2H, H-6aGal, H-6bGal), 3.60 (dd, 1H, J1,2 = 8.0 Hz, J2,3 = 9.7 Hz, H-2Gal), 3.37–3.34 (m, 1H, H-5Gal), 3.31 (dd, 1H, H-3Gal), 3.24–3.15 (m, 3H, H-2GlcN, H-6aGlcN, H-6bGlcN), 2.99–2.97 (m, 1H, H-5GlcN), 2.15 (s, 3H, Ac), 1.97 (s, 3H, Ac), 1.96 (s, 3H, Ac), 1.03 (s, 9 H, tBu), 0.82 (d, 3H, J5,6 = 6.5 Hz, H-6Fuc); 13C-NMR (125 MHz, CDCl3) δ 170.5, 170.3, 169.3, 160.9, 138.7, 138.5, 138.4, 138.0, 135.9, 135.8, 134.9, 133.3, 132.7, 129.6, 128.7, 128.6, 128.5, 128.3, 128.3, 128.2, 127.6, 127.5, 127.4, 127.4, 127.3, 116.9, 103.1, 95.3, 93.1, 92.1, 81.8, 80.1, 76.2, 75.9, 74.7, 74.5, 73.3, 73.1, 73.1, 72.6, 72.5, 71.7, 71.2, 68.2, 68.1, 68.0, 66.8, 64.3, 63.6, 26.9, 20.8, 20.8, 20.7, 19.2, 15.3. HRMS (ESI) m/z: found [M + Na]+ 1418.4421, C73H84Cl3NO18Si calcd. for [M + Na]+ 1418.4421.
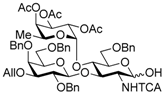
3-O-Allyl-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-d-glucopyranose (12). To a solution of 11 (1.67 g, 1.19 mmol) in THF (11.9 mL) were added acetic acid (0.68 mL, 11.9 mmol) and 1 M tetra-n-butylammonium fluoride in THF (4.76 mL, 4.76 mmol) at 0 °C under Ar, and stirred for 1 d at room temperature. The reaction mixture was concentrated. The residue was diluted with EtOAc and water, and extracted with EtOAc. The combined organic layer was successively washed with sat. NaHCO3, water, and brine, dried over Na2SO4, and concentrated. The crude product was purified by silica gel column chromatography with hexane–EtOAc (60:40) and gel permeation chromatography (LH-20, CHCl3-MeOH (50:50)) to give the title product 12 (1.38 g, quant.). 1H-NMR (500 MHz, CDCl3) δ 7.41–7.20 (m, 20H, Ar), 6.76 (d, 1H, J2,NH = 9.7 Hz, NH), 5.87–5.80 (m, 1H, H2C=CHCH2), 5.30–5.12 (m, 7H, H2C=CHCH2 × 2, H-1GlcN, H-1Fuc, H-2Fuc, H-3Fuc, H-4Fuc), 4.97 (dd, 1H, J4,5 = 12.8 Hz, J5,6 = 6.5 Hz, H-5Fuc), 4.87 (d, 1H, Jgem = 12.1 Hz, PhCH2), 4.72 (d, 1H, Jgem = 11.4 Hz, PhCH2), 4.63–4.47 (m, 6H, PhCH2 × 5, H-1Gal), 4.44 (d, 1H, Jgem = 11.6 Hz, PhCH2), 4.34–4.28 (m, 1H, H-2GlcN), 4.19–4.00 (m, 4H, H2C=CHCH2 × 2, H-3GlcN, H-6aGlcN), 3.96–3.91 (m, 1H, H-4GlcN), 3.81–3.76 (m, 2H, H-4Gal, H-6aGal), 3.70 (dd, 1H, J5,6b = 7.4 Hz, Jgem = 9.3 Hz, H-6bGal), 3.64–3.46 (m, 3H, H-2Gal, H-5GlcN, H-6bGlcN), 3.41–3.39 (m, 1H, H-5Gal), 3.22 (dd, 1H, J2,3 = 9.7 Hz, J3,4 = 2.7 Hz, H-3Gal), 3.09–3.08 (m, 1H, OH), 2.13 (s, 3H, Ac), 2.00 (s, 3H, Ac), 1.99 (s, 3H, Ac), 0.75 (d, 3H, H-6Fuc); 13C-NMR (125 MHz, CDCl3) δ 170.5, 170.3, 169.5, 161.3, 139.0, 138.6, 138.3, 137.5, 135.0, 134.7, 129.2, 128.8, 128.5, 128.5, 128.4, 128.3, 128.2, 128.2, 128.1, 127.9, 127.8, 127.8, 127.6, 127.5, 127.1, 116.9, 116.6, 103.8, 95.8, 92.7, 91.2, 81.9, 78.6, 77.6, 74.8, 74.3, 74.2, 73.7, 73.3, 73.2, 72.8, 72.3, 71.7, 71.7, 71.1, 68.7, 68.2, 68.1, 68.1, 67.4, 64.6, 64.5, 55.8, 20.8, 20.8, 20.7, 15.4, 15.4. HRMS (ESI) m/z: found [M + Na]+ 1180.3243, C57H66Cl3NO18 calcd. for [M + Na]+ 1180.3243.
3-O-Allyl-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-d-glucopyranosyl (N-phenyl)-2,2,2-trifuoroacetimidate (13). A mixture of 12 (1.35 g, 1.16 mmol), (N-phenyl)-2,2,2-trifluoroacetoimidoyl chloride (482 mg, 2.32 mmol), and K2CO3 (802 mg, 5.80 mmol) in acetone (23.2 mL) was stirred for 1 h at room temperature. The reaction mixture was filtered through Celite, and concentrated. The crude product was purified by gel permeation chromatography [S-X3, toluene–EtOAc (75:25)] and silica gel column chromatography with hexane–EtOAc (71:29) to give the title product 13 (1.31 g, 85%). [α]D −0.7° (c 1.4, CHCl3); 1H-NMR (500 MHz, CDCl3) δ 7.38–7.08 (m, 23H, Ar), 6.75–6.71 (m, 3H, Ar, NH), 6.32 (br, 1H, H-1GlcN), 5.88–5.80 (m, 1H, H2C=CHCH2), 5.31–5.21 (m, 5H, H2C=CHCH2, H-1Fuc, H-2Fuc, H-3Fuc, H-4Fuc), 5.14 (dd, 1H, Jtrans = 10.5 Hz, Jgem = 1.4 Hz, H2C=CHCH2), 4.95 (dd, 1H, J4,5 = 10.5 Hz, J5,6 = 6.4 Hz, H-5Fuc), 4.84 (d, 1H, Jgem = 11.9 Hz, PhCH2), 4.73 (d, 1H, Jgem = 11.4 Hz, PhCH2), 4.64–4.49 (m, 7H, PhCH2 × 5, H-2GlcN, H-1Gal), 4.44 (d, 1H, Jgem = 11.6 Hz, PhCH2), 4.16 (t, 1H, J2,3 = J3,4 = 9.2 Hz, H-3GlcN), 4.10–4.05 (m, 3H, H2C=CHCH2 × 2, H-4GlcN), 3.87–3.81 (m, 3H, H-4Gal, H-6aGal, H-5GlcN), 3.70 (dd, 1H, J5,6b = 7.5 Hz, Jgem = 9.2 Hz, H-6bGal), 3.62–3.56 (m, 3H, H-2Gal, H-6aGlcN, H-6bGlcN), 3.46–3.44 (m, 1H, H-5Gal), 3.24 (dd, 1H, J2,3 = 9.7 Hz, J3,4 = 2.7 Hz, H-3Gal), 2.14 (s, 3H, Ac), 2.02 (s, 3H, Ac), 2.00 (s, 3H, Ac), 0.78 (d, 3 H, H-6Fuc); 13C-NMR (125 MHz, CDCl3) δ 170.4, 120.2, 169.5, 161.2, 142.9, 138.7, 138.4, 138.2, 137.5, 134.9, 129.2, 129.0, 128.8, 128.5, 128.4, 128.3, 128.2, 128.1, 128.0, 127.8, 127.8, 127.7, 127.6, 127.3, 124.6, 119.3, 116.6, 103.8, 95.9, 92.4, 81.9, 78.5, 77.6, 74.9, 74.8, 74.2, 73.7, 73.7, 73.3, 73.2, 72.2, 71.9, 71.7, 71.6, 68.7, 68.1, 68.0, 66.7, 64.8, 54.7, 20.8, 20.8, 20.7, 15.4. HRMS (ESI) m/z: found [M + Na]+ 1351.3539, C65H70Cl3F3N2O18 calcd. for [M + Na]+ 1351.3539.
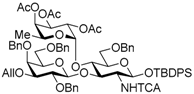
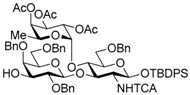
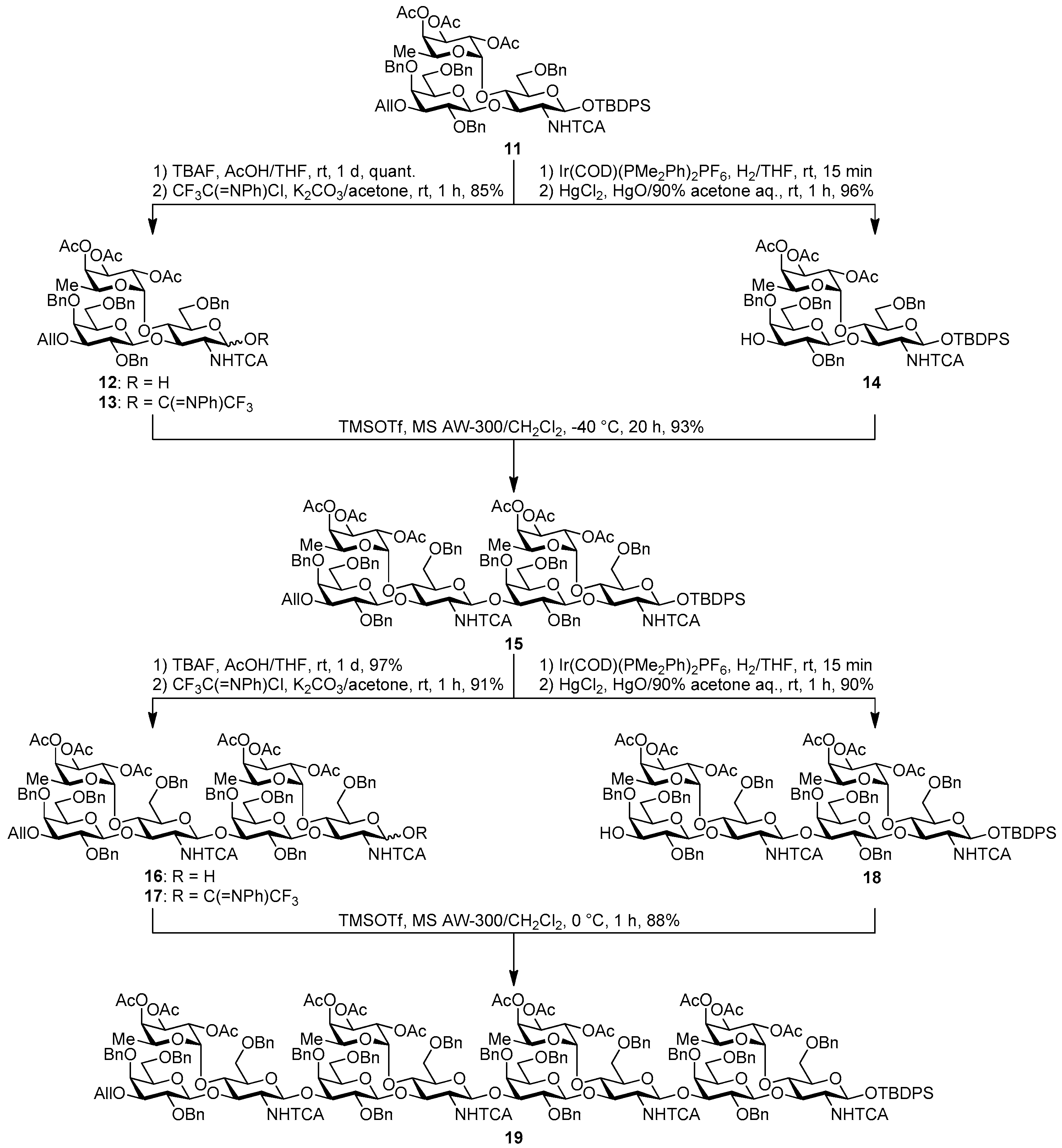
tert-Butyldiphenylsilyl 2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-β-d-glucopyranoside (14). A mixture of Ir(COD)(PMe2Ph)2PF6 (14.2 mg, 16.8 μmol) in THF (14.0 mL) was stirrede at room temperature for 15 min under H2, and the atmosphere was replaced by Ar. To the mixture of activated Ir complex in THF was added a solution of 11 (781 mg, 0.56 mmol) in THF (14.0 mL) under Ar, and stirred for 30 min at room temperature. The reaction mixture was concentrated. The residue was dissolved with 90% acetone aq. (28.0 mL). To the solution were added HgCl2 (380 mg, 1.40 mmol) and HgO (48.5 mg, 0.22 mmol), and stirred for 1 h at room temperature. The reaction mixture was diluted with CHCl3 and water, and extracted with CHCl3. The combined organic layer was successively washed with 10% KI aq., water, and brine, dried over Na2SO4, and concentrated. The crude product was purified by silica gel column chromatography with hexane–EtOAc (75:25) and gel permeation chromatography (LH-20, CHCl3–MeOH (50:50)) to give the title product 14 (727 mg, 96%). [α]D −41.9° (c 1.1, CHCl3); 1H-NMR (500 MHz, CDCl3) δ 7.68–7.14 (m, 30H, Ar), 6.85 (d, 1H, J2,NH = 7.2 Hz, NH), 5.24–5.18 (m, 4H, H-1Fuc, H-2Fuc, H-3Fuc, H-4Fuc), 5.10 (dd, 1H, J4,5 = 12.9 Hz, J5,6 = 6.4 Hz, H-5Fuc), 5.05 (d, 1H, J1,2 = 6.7 Hz, H-1GlcN), 4.80 (d, 1H, Jgem = 11.1 Hz, PhCH2), 4.76 (d, 1H, PhCH2), 4.64 (d, 1H, Jgem = 11.2 Hz, PhCH2), 4.58–4.52 (m, 3H, PhCH2 × 3), 4.47 (d, 1H, J1,2 = 7.8 Hz, H-1Gal), 4.38 (d, 1H, Jgem = 12.5 Hz, PhCH2), 4.32 (d, 1H, PhCH2), 4.22 (t, 1H, J2,3 = J3,4 = 9.3 Hz, H-3GlcN), 3.91 (t, 1H, J4,5 = 9.3 Hz, H-4GlcN), 3.83–3.79 (m, 3H, H-4Gal, H-6aGal, H-6bGal), 3.51–3.43 (m, 2H, H-3Gal, H-5Gal), 3.36–3.32 (m, 2H, H-2GlcN, H-2Gal), 3.25 (dd, 1H, J5,6a =2.5 Hz, Jgem = 11.0 Hz, H-6aGlcN), 3.17 (dd, 1H, J5,6b = 1.4 Hz, H-6bGlcN), 2.98–2.96 (m, 1H, H-5GlcN), 2.17 (s, 3H, Ac), 2.10 (d, 1H, J3,OH = 6.8 Hz, OH), 1.99 (s, 3H, Ac), 1.96 (s, 3H, Ac), 1.16 (s, 9 H, tBu), 0.89 (d, 3H, H-6Fuc); 13C-NMR (125 MHz, CDCl3) δ 170.5, 170.2, 169.4, 161.1, 138.8, 138.4, 138.1, 137.9, 135.9, 135.8, 133.2, 132.6, 129.7, 129.7, 128.9, 128.6, 128.5, 128.3, 128.3, 128.3, 128.1, 128.1, 127.9, 127.6, 127.5, 127.5, 127.3, 102.8, 95.4, 93.6, 92.2, 81.2, 77.6, 75.8, 75.4, 75.3, 75.3, 74.6, 74.1, 73.4, 73.2, 73.2, 72.7, 71.7, 68.1, 68.1, 66.8, 64.3, 62.8, 26.9, 20.8, 20.8, 20.8, 19.2, 15.6. HRMS (ESI) m/z: found [M + Na]+ 1378.4108, C70H80Cl3NO18Si calcd. for [M + Na]+ 1378.4108.
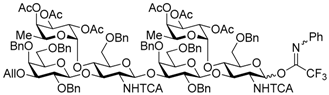
tert-Butyldiphenylsilyl 3-O-allyl-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-β-d-glucopyranosyl-(1→3)-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-β-d-glucopyranoside (15). To a mixture of the glycosyl donor 13 (343 mg, 0.26 mmol), the glycosyl acceptor 14 (234 mg, 0.17 mmol), and molecular sieves AW-300 (516 mg) in CH2Cl2 (5.7 mL) was added dropwise TMSOTf (3.1 μL, 17.2 μmol) at −40 °C under Ar, and stirred for 20 h at −40 °C. The reaction mixture was quenched with sat. NaHCO3, filtered through Celite, and diluted with CHCl3. The organic layer was separated, and the aqueous layer was extracted with CHCl3. The combined organic layer was successively washed with water and brine, dried over Na2SO4, and concentrated. The crude product was purified by gel permeation chromatography [S-X1, toluene–EtOAc (75:25)] and silica gel column chromatography with toluene–EtOAc (89:11) to give the title product 15 (399 mg, 93%). [α]D −58.2° (c 1.4, CHCl3); 1H-NMR (500 MHz, CDCl3) δ 7.65–6.98 (m, 50H, Ar), 6.62–6.60 (m, 2H, NH × 2), 5.92–5.84 (m, 1H, H2C=CHCH2), 5.47 (d, 1H, J1,2 = 7.2 Hz, H-1GlcN), 5.32–4.99 (m, 13H, H-1GlcN, H-1Fuc × 2, H-2Fuc × 2, H-3Fuc × 2, H-4Fuc × 2, H-5Fuc × 2, H2C=CHCH2 × 2), 4.78–4.70 (m, 5H, PhCH2 × 5), 4.64 (d, 1H, Jgem = 10.7 Hz, PhCH2), 4.60–4.52 (m, 3H, PhCH2 × 3), 4.50–4.40 (m, 6H, PhCH2 × 5, H-1Gal), 4.36–4.32 (m, 2H, PhCH2, H-1Gal), 4.29–4.17 (m, 3H, PhCH2, H-3GlcN × 2), 4.14–4.06 (m, 2H, H2C=CHCH2 × 2), 4.00 (t, 1H, J3,4 = J4,5 = 9.4 Hz, H-4GlcN), 3.90 (d, 1H, J3,4 = 2.3 Hz, H-4Gal), 3.85–3.77 (m, 5H, H-4Gal, H-4GlcN, H-3Gal, H-6aGal × 2), 3.70–3.69 (m, 2H, H-6bGal × 2), 3.61–3.57 (m, 3H, H-2Gal × 2, H-6aGlcN), 3.50–3.38 (m, 5H, H-6bGlcN, H-2GlcN, H-5GlcN, H-5Gal × 2), 3.26 (dd, 1H, J2,3 = 9.7 Hz, J3,4 = 2.6 Hz, H-3Gal), 3.22 (dd, 1H, J5,6a = 2.4 Hz, Jgem = 11.3 Hz, H-6aGlcN), 3.12–3.10 (m, 1H, H-6bGlcN), 2.98–2.90 (m, 2H, H-2GlcN, H-5GlcN), 2.16 (s, 3H, Ac), 2.13 (s, 3H, Ac), 2.08 (s, 3H, Ac), 2.02 (s, 3H, Ac), 1.97 (s, 3H, Ac), 1.93 (s, 3H, Ac), 1.00 (s, 9H, tBu), 0.79 (d, 3H, J5,6 = 6.5 Hz, H-6Fuc), 0.74 (d, 3H, J5,6 = 6.5 Hz, H-6Fuc); 13C-NMR (125 MHz, CDCl3) δ 170.5, 170.3, 170.2, 169.4, 169.3, 161.0, 160.8, 139.2, 138.7, 138.5, 138.4, 138.3, 138.0, 137.5, 135.8, 135.7, 134.8, 133.2, 132.6, 129.7, 129.1, 129.0, 128.9, 128.7, 128.4, 128.3, 128.3, 128.2, 128.2, 128.1, 127.9, 127.8, 127.7, 127.6, 127.5, 127.4, 127.3, 116.7, 103.2, 103.2, 95.6, 95.5, 92.2, 92.1, 81.8, 81.2, 79.3, 77.6, 76.1, 75.6, 75.4, 75.2, 75.1, 74.9, 74.6, 74.5, 73.5, 73.3, 73.1, 73.0, 72.9, 72.8, 72.4, 71.7, 71.7, 71.4, 68.2, 68.2, 68.1, 68.0, 66.8, 66.7, 64.5, 64.3, 26.9, 20.9, 20.8, 20.8, 20.7, 19.1, 15.3. HRMS (ESI) m/z: found [M + Na]+ 2517.7349, C127H144Cl6N2O35Si calcd. for [M + Na]+ 2517.7348.
3-O-Allyl-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-β-d-glucopyranosyl-(1→3)-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-d-glucopyranose (16). Compound 15 (453 mg, 0.18 mmol) was desilylated with 1 M TBAF/THF (0.72 mL, 0.72 mmol) and AcOH (0.10 mL, 1.81 mmol) in THF (3.6 mL) as described for 12. The crude product was purified by gel permeation chromatography [S-X1, toluene–EtOAc (75:25)] and silica gel column chromatography with toluene-EtOAc (67:33) to give the title product 16 (395 mg, 97%). Analysis of compound 16 was too difficult for anomeric isomer, so the product was analyzed in next step. HRMS (ESI) m/z: found [M + Na]+ 2279.6171, C111H126Cl6N2O35 calcd for [M + Na]+ 2279.6170.
3-O-Allyl-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-β-d-glucopyranosyl-(1→3)-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-d-gluco-pyranosyl (N-phenyl)-2,2,2-trifuoroacetimidate (17). Compound 16 (97 mg, 42.9 μmol) was reacted with (N-phenyl)-2,2,2-trifluoroacetoimidoyl chloride (17.8 mg, 85.8 μmol) and K2CO3 (29.7 mg, 215 μmol) in acetone (1.7 mL) as described for 13. The crude product was purified by silica gel column chromatography with hexane–EtOAc (83:17) and gel permeation chromatography [S-X1, toluene–EtOAc (75:25)] to give the title product 17 (94.7 mg, 91%). [α]D −35.2° (c 1.3, CHCl3); 1H-NMR (500 MHz, CDCl3) δ 7.38–7.07 (m, 43H, Ar), 6.82 (d, 1H, J2,NH = 9.4 Hz, NH), 6.71–6.69 (m, 2H, Ar), 6.26 (br, 1H, H-1GlcN), 6.00 (d, 1H, J2,NH = 4.7 Hz, NH), 5.88–5.81 (m, 1H, H2C=CHCH2), 5.30–5.14 (m, 10H, H2C=CHCH2 × 2, H-1Fuc × 2, H-2Fuc × 2, H-3Fuc × 2, H-4Fuc × 2), 5.07 (d, 1H, J1,2 = 8.1 Hz, H-1GlcN), 5.03–5.00 (m, 2H, H-5Fuc, PhCH2), 4.94 (dd, 1H, J4,5 = 12.7 Hz, J5,6 = 6.4 Hz, H-5Fuc), 4.78 (d, 1H, Jgem = 11.6 Hz, PhCH2), 4.71 (d, 1H, Jgem = 11.2 Hz, PhCH2), 4.63 (d, 1H, Jgem = 11.9 Hz, PhCH2), 4.60–4.37 (m, 13H, PhCH2 ×11, H-1Gal, H-2GlcN), 4.32 (d, 1H, J1,2 = 7.8 Hz, H-1Gal), 4.20 (t, 1H, J2,3 = J3,4 = 9.2 Hz, H-3GlcN), 4.07–4.01 (m, 3H, H2C=CHCH2 × 2, H-4GlcN), 3.93–3.77 (m, 8H, H-4GlcN, H-2GlcN, H-6aGal, H-6aGal, H-4Gal, H-5GlcN, H-4Gal, H-6bGal), 3.76–3.65 (m, 3H, H-3Gal, H-3GlcN, H-2Gal), 3.62–3.52 (m, 5H, H-6aGlcN, H-6bGlcN, H-6aGlcN, H-6bGal, H-2Gal), 3.49–3.44 (m, 2H, H-5Gal, H-6bGlcN), 3.38 (dd, 1H, J5,6a = 5.5 Hz, J5,6b = 7.5 Hz, H-5Gal), 3.21–3.18 (m, 2H, H-3Gal, H-5GlcN), 2.16 (s, 3H, Ac), 2.15 (s, 3H, Ac), 2.14 (s, 3H, Ac), 2.05 (s, 3H, Ac), 2.01 (s, 6H, Ac × 2), 0.85 (d, 3H, H-6Fuc), 0.69 (d, 3H, J5,6 = 6.4 Hz, H-6Fuc); 13C-NMR (125 MHz, CDCl3) δ 170.4, 170.3, 170.2, 170.1, 169.4, 160.9, 160.7, 142.7, 139.0, 138.8, 138.4, 138.3, 138.2, 138.1, 137.8, 137.6, 137.3, 134.9, 129.3, 129.0, 129.0, 128.8, 128.5, 128.4, 128.4, 128.3, 128.2, 128.2, 128.2, 128.1, 128.1, 127.9, 127.8, 127.7, 127.7, 127.6, 127.5, 127.3, 127.3, 125.2, 124.6, 119.2, 117.0, 116.6, 114.8, 103.4, 103.2, 99.4, 96.0, 95.6, 93.3, 92.6, 92.4, 81.8, 80.8, 78.5, 78.1, 77.6, 76.5, 75.1, 74.9, 74.7, 74.6, 74.4, 74.3, 74.1, 73.4, 73.3, 73.2, 72.9, 72.8, 72.4, 72.0, 71.6, 71.5, 71.4, 68.8, 68.5, 68.2, 68.1, 68.0, 67.9, 67.1, 66.7, 64.8, 64.5, 58.7, 54.6, 30.9, 29.6, 21.4, 20.9, 20.8, 20.8, 20.7, 20.7, 15.7, 15.2, 14.1. HRMS (ESI) m/z: found [M + Na]+ 2450.6466, C119H130Cl6F3N3O35 calcd. for [M + Na]+ 2450.6466.
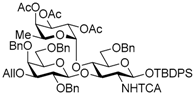
tert-Butyldiphenylsilyl 2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-β-d-glucopyranosyl-(1→3)-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-β-d-glucopyranoside (18). Compound 15 (104 mg, 41.6 μmol) was deallylated by Ir(COD)(PMe2Ph)2PF6 (1.1 mg, 1.25 μmol) in THF (1.0 mL × 2) and deprotected by HgCl2 (28.2 mg, 104 μmol) and HgO (3.6 mg, 16.6 μmol) with 90% acetone aq. (2.1 mL) as described for 14. The crude product was purified by silica gel column chromatography with hexane–EtOAc (83:17) and gel permeation chromatography [S-X1, toluene–EtOAc (75:25)] to give the title product 18 (92.2 mg, 90%). [α]D −46.2° (c 1.0, CHCl3); 1H-NMR (500 MHz, CDCl3) δ 7.65–7.12 (m, 50H, Ar), 6.63 (d, 1H, J2,NH = 7.3 Hz, NH), 6.27 (br, 1H, NH), 5.33–5.08 (m, 10H, H-1GlcN, H-1Fuc × 2, H-2Fuc × 2, H-3Fuc × 2, H-4Fuc × 2, H-5Fuc), 5.00 (dd, 1H, J4,5 = 12.3 Hz, J5,6 = 6.4 Hz, H-5Fuc), 4.91 (br, 1H, H-1GlcN), 4.84–4.82 (m, 2H, PhCH2 × 2), 4.74 (d, 1H, Jgem = 10.9 Hz, PhCH2), 4.67 (d, 1H, Jgem = 11.3 Hz, PhCH2), 4.62–4.53 (m, 6H, PhCH2 × 6), 4.51–4.46 (m, 3H, PhCH2 × 3), 4.43–4.28 (m, 5H, H-1Gal × 2, PhCH2 × 3), 4.15–4.12 (m, 1H, H-3GlcN), 4.04–4.00 (m, 2H, H-3GlcN, H-4GlcN), 3.90–3.87 (m, 2H, H-4Gal, H-6aGal), 3.85–3.81 (m, 3H, H-6bGal, H-4Gal, H-4GlcN), 3.79–3.68 (m, 4H, H-3Gal, H-6aGal, H-6bGal, H-2GlcN), 3.64–3.60 (m, 2H, H-2Gal, H-6aGlcN), 3.52 (dd, 1H, J5,6b = 2.0 Hz, Jgem = 10.7 Hz, H-6bGlcN), 3.49–3.40 (m, 3H, H-5Gal × 2, H-3Gal), 3.36–3.32 (m, 2H, H-5GlcN, H-2Gal), 3.24–3.12 (m, 3H, H-6GlcN × 2, H-2GlcN), 2.92 (d, J4,5 = 9.6 Hz, H-5GlcN), 2.18 (s, 3H, Ac), 2.14 (s, 3H, Ac), 2.09 (s, 3H, Ac), 2.03 (s, 3H, Ac), 2.00 (d, 1H, J3,OH = 5.2 Hz, OH), 1.98 (s, 3H, Ac), 1.93 (s, 3H, Ac), 1.00 (s, 9H, tBu), 0.84 (d, 3H, J5,6 = 6.4 Hz, H-6Fuc), 0.76 (d, 3H, H-6Fuc); 13C-NMR (125 MHz, CDCl3) δ 170.5, 170.3, 170.2, 170.1, 169.5, 169.3, 161.0, 160.7, 139.2, 138.6, 138.5, 138.4, 138.2, 138.1, 137.9, 137.8, 137.4, 135.8, 135.7, 133.1, 132.5, 129.7, 129.6, 129.1, 129.0, 129.0, 128.7, 128.4, 128.4, 128.3, 128.3, 128.2, 128.2, 128.1, 128.0, 128.0, 127.8, 127.8, 127.8, 127.7, 127.6, 127.5, 127.4, 127.4, 127.3, 125.3, 103.1, 99.0, 95.7, 95.5, 93.6, 92.4, 92.3, 81.5, 80.1, 77.6, 77.5, 76.2, 75.3, 75.2, 75.1, 75.1, 75.0, 74.9, 74.9, 74.7, 74.1, 73.9, 73.7, 73.5, 73.1, 73.1, 72.9, 72.9, 72.8, 71.6, 71.6, 68.2, 68.1, 68.1, 68.0, 67.9, 66.8, 66.6, 64.4, 64.3, 62.2, 59.3, 31.9, 29.6, 29.3, 26.8, 26.7, 22.6, 21.4, 20.9, 20.8, 20.8, 20.7, 20.7, 19.1, 18.8, 15.6, 15.4, 14.1. HRMS (ESI) m/z: found [M + Na]+ 2477.7035, C124H140Cl6N2O35Si calcd. for [M + Na]+ 2477.7035.
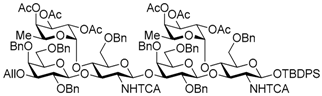
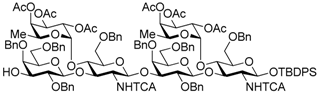
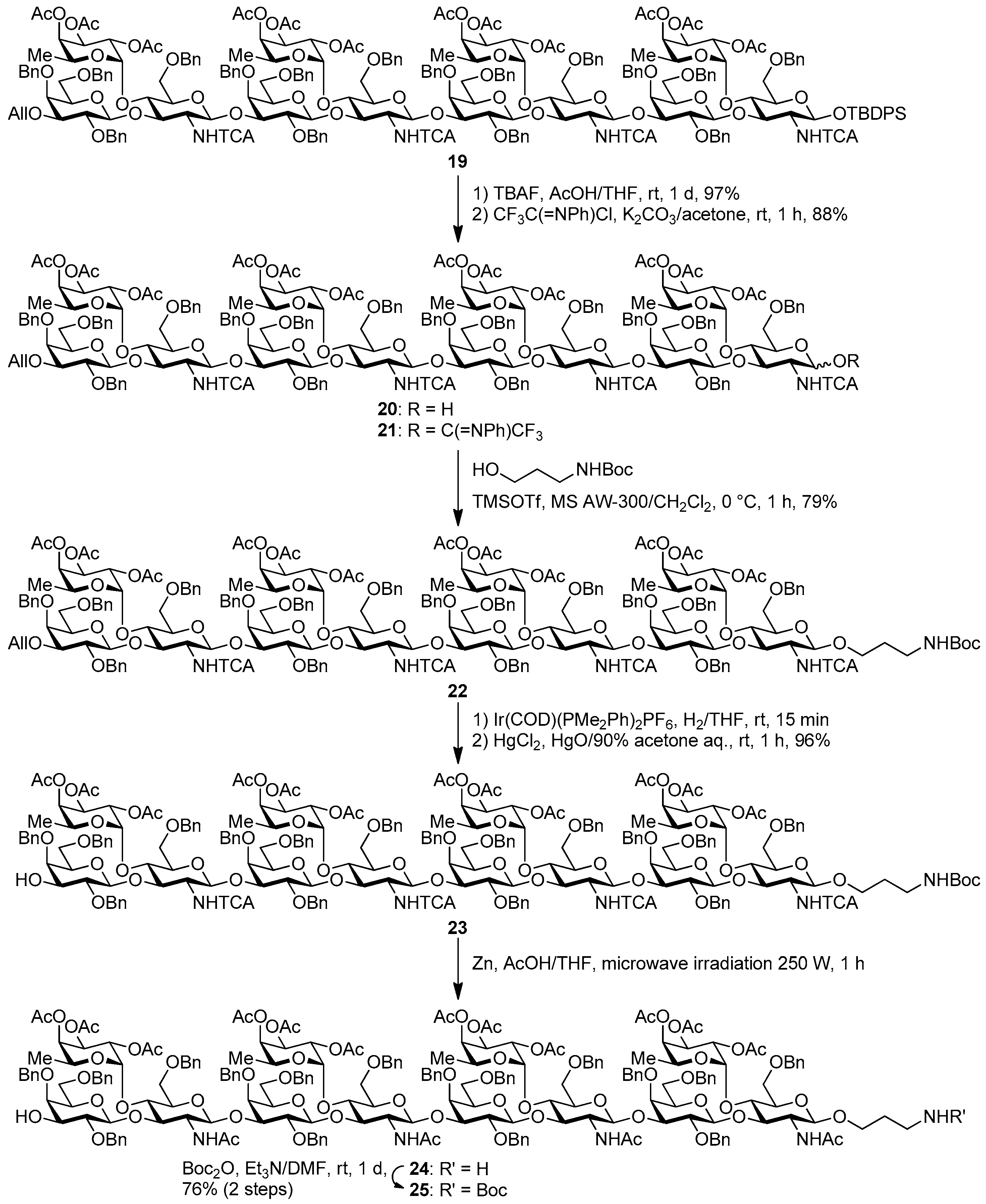
tert-Butyldiphenylsilyl 3-O-allyl-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-β-d-glucopyranosyl-(1→3)-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-β-d-glucopyranosyl-(1→3)-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-β-d-glucopyranosyl-(1→3)-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-β-d-glucopyranoside (19). To a mixture of the glycosyl donor 17 (276 mg, 113 μmol), the glycosyl acceptor 18 (210 mg, 85.0 μmol), and molecular sieves AW-300 (255 mg) in CH2Cl2 (2.8 mL) was added dropwise TMSOTf (3.0 μL, 17.0 μmol) at 0 °C under Ar, and stirred for 1 h at 0 °C. The reaction mixture was quenched with sat. NaHCO3, filtered through Celite, and diluted with CHCl3. The organic layer was separated, and the aqueous layer was extracted with CHCl3. The combined organic layer was successively washed with brine, dried over Na2SO4, and concentrated. The crude product was purified by gel permeation chromatography [S-X1, toluene–EtOAc (75:25)] and silica gel column chromatography with toluene–EtOAc (83:17) to give the title product 19 (352 mg, 88%). [α]D −50.5° (c 1.0, CHCl3); 1H-NMR (500 MHz, CDCl3) δ 7.65–7.02 (m, 90H, Ar), 6.67 (d, 1H, J2,NH = 7.1 Hz, NH), 6.27 (brs, 1H, NH), 5.97 (m, 2H, NH × 2), 5.86-5.78 (m, 1H, H2C=CHCH2), 5.43–4.87 (m, 26H, H-1Fuc × 4, H-2Fuc × 4, H-3Fuc × 4, H-4Fuc × 4, H-5Fuc × 4, H-1GlcN × 4, H2C=CHCH2 × 2), 4.84–4.27 (m, 37H, H-1Gal × 4, H-2GlcN, PhCH2 × 32), 4.22–3.12 (m, 48H, H-2Gal × 4, H-3Gal × 4, H-4Gal × 4, H-5Gal × 4, H-6aGal × 4, H-6bGal × 4, H-2GlcN × 3, H-3GlcN × 4, H-4GlcN × 4, H-5GlcN × 3, H-6aGlcN × 4, H-6bGlcN × 4, H2C=CHCH2 × 2), 2.93 (d, 1H, J4,5 = 9.6 Hz, H-5GlcN), 2.21–1.88 (m, 36H, Ac × 12), 1.01 (s, 9H, tBu), 0.89–0.67 (m, 12H, H-6Fuc × 4); 13C-NMR (125 MHz, CDCl3) δ 171.1, 170.5, 170.4, 170.3, 170.2, 170.2, 169.6, 169.5, 169.4, 160.9, 160.7, 139.4, 139.2, 138.9, 138.6, 138.6, 138.5, 138.5, 138.4, 138.0, 137.9, 137.7, 137.6, 137.5, 136.0, 135.8, 135.0, 133.2, 132.6, 130.0, 129.8, 129.3, 129.2, 129.2, 129.1, 128.8, 128.7, 128.6, 128.5, 128.5, 128.4, 128.4, 128.3, 128.3, 128.2, 128.1, 128.1, 128.0, 127.9, 127.8, 127.8, 127.8, 127.7, 127.6, 127.6, 127.4, 125.4, 116.7, 103.5, 103.3, 103.1, 99.2, 99.1, 98.9, 96.0, 95.9, 95.7, 95.6, 93.7, 92.8, 92.6, 92.4, 81.9, 81.5, 81.4, 78.7, 77.6, 77.4, 76.6, 76.2, 75.6, 75.6, 75.4, 75.3, 75.3, 75.1, 75.0, 75.0, 74.8, 74.5, 74.1, 74.0, 73.9, 73.6, 73.3, 73.3, 73.3, 73.1, 73.0, 73.0, 72.9, 72.6, 72.8, 71.8, 71.7, 68.6, 68.5, 68.3, 68.2, 68.1, 67.0, 66.8, 64.6, 64.5, 64.4, 62.4, 60.4, 59.4, 58.9, 32.0, 29.7, 29.4, 27.0, 22.8, 21.5, 21.1, 21.0, 20.9, 20.9, 20.8, 20.8, 19.2, 15.8, 15.6, 15.4, 14.3, 14.2. HRMS (ESI) m/z: found [1/2M + Na]+ 2369.6540, C235H264Cl12N4O69Si calcd for [1/2M + Na]+ 2369.6544.
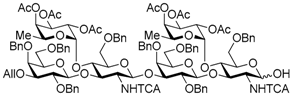
3-O-Allyl-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-β-d-glucopyranosyl-(1→3)-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-β-d-glucopyranosyl-(1→3)-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-β-d-glucopyranosyl-(1→3)-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-d-glucopyranose (20). Compound 19 (324 mg, 68.9 μmol) was desilylated with 1 M TBAF/THF (0.28 mL, 0.28 mmol) and AcOH (39.0 μL, 0.69 mmol) in THF (1.4 mL) as described for 12. The crude product was purified by gel permeation chromatography [S-X1, toluene–EtOAc (75:25)] and silica gel column chromatography with toluene–EtOAc (71:29) to give the title product 20 (298 mg, 97%). [α]D −42.5° (c 1.0, CHCl3); 1H-NMR (500 MHz, CDCl3) δ 7.65–7.02 (m, 83H, Ar, NH × 3), 6.70 (d, 1H, J2,NH = 9.9 Hz, NH), 5.86–5.79 (m, 1H, H2C=CHCH2), 5.63–3.11 (m, 112H, H-1Gal × 4, H-2Gal × 4, H-3Gal × 4, H-4Gal × 4, H-5Gal × 4, H-6aGal × 4, H-6bGal × 4, H-1GlcN × 4, H-2GlcN × 4, H-3GlcN × 4, H-4GlcN × 4, H-5GlcN × 4, H-6aGlcN × 4, H-6bGlcN × 4, H-1Fuc × 4, H-2Fuc × 4, H-3Fuc × 4, H-4Fuc × 4, H-5Fuc × 4, H2C=CHCH2 × 2, H2C=CHCH2 × 2, PhCH2 × 32), 2.17–1.99 (m, 36H, Ac × 12), 0.89–0.67 (m, 12H, H-6Fuc × 4); 13C-NMR (125 MHz, CDCl3) δ 170.4, 170.3, 170.3, 170.3, 170.2, 170.2, 169.5, 169.5, 169.4, 160.7, 160.3, 139.5, 139.5, 139.4, 138.9, 138.5, 138.4, 138.4, 138.4, 138.3, 138.2, 138.1, 137.8, 137.6, 137.5, 137.5, 137.4, 134.9, 129.1, 129.1, 129.0, 128.7, 128.6, 128.5, 128.4, 128.4, 128.4, 128.3, 128.3, 128.2, 128.2, 128.2, 128.1, 128.1, 128.1, 128.0, 127.8, 127.8, 127.7, 127.7, 127.7, 127.7, 127.6, 127.6, 127.6, 127.4, 127.3, 127.3, 127.3, 127.2, 125.3, 116.5, 103.5, 103.4, 103.1, 99.4, 99.3, 99.0, 96.0, 95.8, 95.6, 93.0, 92.9, 92.8, 92.8, 92.6, 91.2, 82.0, 81.8, 81.6, 78.3, 77.6, 76.6, 76.5, 75.7, 75.5, 75.4, 75.3, 75.1, 75.0, 74.8, 74.8, 74.7, 74.7, 74.6, 74.3, 74.2, 74.1, 74.0, 73.4, 73.3, 73.2, 73.2, 73.0, 72.8, 72.8, 72.4, 71.7, 71.6, 71.6, 70.8, 68.6, 68.5, 68.2, 68.1, 68.0, 67.2, 66.9, 66.8, 64.6, 64.4, 57.8, 57.6, 55.6, 29.7, 29.3, 29.3, 21.4, 20.9, 20.9, 20.8, 20.8, 15.8, 15.7, 15.6, 15.5, 15.2, 14.1. HRMS (ESI) m/z: found [1/2M + Na]+ 2250.5955, C219H246Cl12N4O69 calcd for [1/2M + Na]+ 2250.5955.
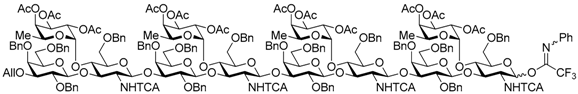
3-O-Allyl-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-β-d-glucopyranosyl-(1→3)-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-β-d-glucopyranosyl-(1→3)-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-β-d-glucopyranosyl-(1→3)-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-d-glucopyranosyl (N-phenyl)-2,2,2-trifuoroacetimidate (21). Compound 20 (761 mg, 0.17 mmol) was reacted with (N-phenyl)-2,2,2-trifluoroacetoimidoyl chloride (55.0 μL, 0.34 mmol) and K2CO3 (117 mg, 0.85 mmol) in acetone (6.8 mL) as described for 13. The crude product was purified by silica gel column chromatography with hexane–EtOAc (83:17) to give the title product 21 (693 mg, 88%). Analysis of compound 21 was too difficult for a few isomers, so the product was analyzed in the next step. 1H-NMR of product 21 could not be assigned because all peaks were shown as broad peaks in all range. 13C-NMR (125 MHz, CDCl3) δ 170.4, 170.3, 170.2, 170.2, 170.2, 170.2, 169.5, 169.5, 160.8, 160.7, 160.4, 142.7, 139.4, 139.3, 139.1, 138.8, 138.5, 138.4, 138.4, 138.3, 138.3, 138.2, 138.1, 137.8, 137.6, 137.5, 137.5, 137.3, 134.9, 129.2, 129.1, 129.0, 129.0, 128.8, 128.6, 128.5, 128.4, 128.4, 128.3, 128.3, 128.2, 128.2, 128.1, 127.9, 127.8, 127.7, 127.6, 127.6, 127.6, 127.6, 127.4, 127.4, 127.3, 127.3, 127.2, 125.3, 124.7, 119.2, 117.0, 116.5, 114.8, 103.5, 103.4, 103.1, 99.3, 99.2, 99.0, 96.1, 95.8, 95.8, 95.6, 93.4, 92.9, 92.7, 92.4, 81.8, 81.6, 78.4, 77.6, 76.6, 76.5, 76.4, 75.6, 75.5, 75.4, 75.3, 75.2, 75.0, 74.8, 74.8, 74.7, 74.3, 74.1, 74.1, 74.0, 73.4, 73.3, 73.2, 73.0, 72.9, 72.9, 72.8, 72.8, 72.4, 72.0, 71.7, 71.6, 71.5, 68.5, 68.1, 68.1, 68.0, 67.9, 67.0, 66.9, 66.8, 66.6, 64.7, 64.5, 64.4, 58.3, 57.9, 54.7, 31.9, 30.9, 29.7, 29.3, 22.7, 21.4, 20.9, 20.9, 20.8, 20.8, 20.7, 15.8, 15.7, 15.6, 15.2, 14.1. HRMS (ESI) m/z: found [1/2M + Na]+ 2336.1103, C227H250Cl12F3N5O69 calcd for [1/2M + Na]+ 2329.1103.
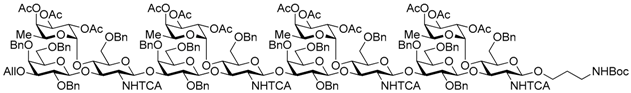
N-(tert-Butoxycarbonyl)-3-aminopropyl 3-O-allyl-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-β-d-glucopyranosyl-(1→3)-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-β-d-glucopyranosyl-(1→3)-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-β-d-glucopyranosyl-(1→3)-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-β-d-glucopyranoside (22). To a mixture of the glycosyl donor 21 (144 mg, 31.0 μmol), N-Boc-3-amino-1-propanol (53.0 μL, 310 μmol), and molecular sieves AW-300 (93.0 mg) in CH2Cl2 (1.0 mL) was added dropwise 1% TMSOTf in CH2Cl2 solution (56.0 μL, 3.1 μmol) at 0 °C under Ar, and stirred for 1 h at 0 °C. The reaction mixture was quenched with sat. NaHCO3, filtered by Celite, and diluted with CHCl3. The organic layer was separated, and the aqueous layer was extracted with CHCl3. The combined organic layer was successively washed with brine, dried over Na2SO4, and concentrated. The crude product was purified by gel permeation chromatography [S-X1, toluene–EtOAc (75:25)] and silica gel column chromatography with toluene–EtOAc (71:29) to give the title product 22 (113 mg, 79%). [α]D −51.8° (c 1.0, CHCl3); 1H-NMR (500 MHz, CDCl3) δ 7.65–7.02 (m, 80H, Ar), 6.67 (d, 1H, J2,NH = 6.5 Hz, NH), 6.19 (brs, 1H, NH), 6.02–5.78 (m, 3H, NH × 2, H2C=CHCH2), 5.31–4.87 (m, 26H, H-1Fuc × 4, H-2Fuc × 4, H-3Fuc × 4, H-4Fuc × 4, H-5Fuc × 4, H-1GlcN × 4, H2C=CHCH2 × 2), 4.84–4.27 (m, 46H, H-1Gal × 4, H-2GlcN × 3, H-3GlcN × 3, H-4GlcN × 3, PhCH2 × 32, NHCH2CH2CH2O), 4.20–3.25 (m, 38H, H-2Gal × 4, H-3Gal × 3, H-4Gal × 4, H-5Gal × 4, H-6aGal × 4, H-6bGal × 4, H-3GlcN, H-4GlcN, H-5GlcN × 3, H-6aGlcN × 3, H-6bGlcN × 3, H2C=CHCH2 × 2, NHCH2CH2CH2O × 2), 3.27–3.01 (m, 7H, H-3Gal, H-2GlcN, H-5GlcN, H-6GlcN × 2, NHCH2CH2CH2O × 2), 2.21–1.95 (m, 36H, Ac × 12), 1.71–1.59 (m, 2H, NHCH2CH2CH2O × 2), 1.42 (s, 9H, tBu), 0.84–0.67 (m, 12H, H-6 Fuc × 4). 13C-NMR (125 MHz, CDCl3) δ 170.4, 170.3, 170.3, 170.2, 169.5, 169.5, 169.4, 161.2, 160.8, 160.7, 160.6, 156.0, 139.3, 139.1, 139.0, 138.9, 138.5, 138.5, 138.5, 138.4, 138.4, 138.4, 138.3, 137.7, 137.5, 137.5, 135.0, 129.2, 129.1, 129.0, 128.8, 128.6, 128.5, 128.5, 128.4, 128.3, 128.3, 128.2, 128.2, 128.2, 128.2, 128.1, 128.1, 128.0, 127.8, 127.8, 127.8, 127.7, 127.7, 127.7, 127.6, 127.5, 127.4, 116.6, 103.5, 103.1, 99.1, 98.5, 95.9, 95.9, 95.7, 92.7, 92.5, 92.3, 81.9, 81.3, 81.2, 79.2, 78.7, 77.9, 77.6, 76.5, 76.2, 75.5, 75.3, 75.2, 75.1, 75.1, 75.0, 74.9, 74.9, 74.4, 73.9, 73.8, 73.5, 73.3, 73.2, 73.2, 73.1, 73.0, 72.9, 72.5, 71.8, 71.7, 71.6, 68.5, 68.4, 68.3, 68.2, 68.1, 68.1, 67.3, 66.8, 64.6, 64.5, 37.3, 29.7, 28.5, 20.9, 20.8, 20.8, 20.8, 20.8, 15.7, 15.7, 15.5, 15.3. HRMS (ESI) m/z: found [1/2M + Na]+ 2329.1507, C227H261Cl12N5O71 calcd. for [1/2M + Na]+ 2329.1506.
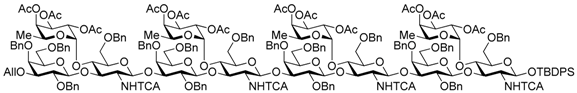
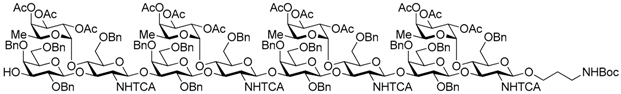
N-(tert-Butoxycarbonyl)-3-aminopropyl 2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-β-d-glucopyranosyl-(1→3)-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-β-d-glucopyranosyl-(1→3)-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-β-d-glucopyranosyl-(1→3)-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-6-O-benzyl-2-deoxy-2-trichloroacetamido-β-d-glucopyranoside (23). Compound 22 (231 mg, 50.0 μmol) was deallylated by Ir(COD)(PMe2Ph)2PF6 (1.3 mg, 1.50 μmol) in THF (2.5 mL × 2) and deprotected by HgCl2 (34.0 mg, 125 μmol) and HgO (4.3 mg, 20.0 μmol) with 90% acetone aq. (5.0 mL) as described for 14. The crude product was purified by silica gel column chromatography with hexane–EtOAc (75:25) to give the title product 23 (219 mg, 96%). [α]D −59.0° (c 1.0, CHCl3); 1H-NMR (500 MHz, CDCl3) δ 7.65–7.02 (m, 80H, Ar), 6.82 (d, 1H, J2,NH = 7.0 Hz, NH), 6.22 (brs, 1H, NH), 5.78–5.63 (m, 2H, NH × 2), 5.31–4.88 (m, 23H, H-1Fuc × 4, H-2Fuc × 4, H-3Fuc × 4, H-4Fuc × 4, H-5Fuc × 4, H-1GlcN × 4), 4.87–4.27 (m, 38 H, H-1Gal × 4, H-1GlcN, PhCH2 × 32, NHCH2CH2CH2O), 4.20 (m, 1H, H-3GlcN), 4.03–3.02 (m, 52H, H-2Gal × 4, H-3Gal × 4, H-4Gal × 4, H-5Gal × 4, H-6aGal × 4, H-6bGal × 4, H-2GlcN × 4, H-3GlcN × 3, H-4GlcN × 4, H-5GlcN × 4, H-6aGlcN × 4, H-6bGlcN × 4, NHCH2CH2CH2O × 2, NHCH2CH2CH2O × 2), 2.21–1.95 (m, 37H, Ac × 12, OH), 1.71–1.59 (m, 2H, NHCH2CH2CH2O × 2), 1.42 (s, 9H, tBu), 0.90–0.73 (m, 12H, H-6Fuc × 4); 13C-NMR (125 MHz, CDCl3) δ 170.5, 170.3, 170.3, 170.2, 170.2, 169.6, 169.6, 169.5, 169.4, 167.8 161.3, 161.0, 160.7, 160.5, 156.0, 139.5, 139., 139.0, 138.9, 138.6, 138.5, 138.5, 138.4, 138.3, 138.3, 138.3, 137.9, 137.7, 137.7, 137.6, 137.5, 132.5, 130.9, 129.2, 129.2, 129.1, 129.0, 128.8, 128.8, 128.6, 128.6, 128.5, 128.5, 128.4, 128.4, 128.3, 128.3, 128.3, 128.2, 128.2, 128.1, 128.1, 127.8, 127.8, 127.8, 127.8, 127.7, 127.7, 127.6, 127.5, 127.4, 125.3, 103.3, 103.0, 99.6, 99.2, 98.6, 95.9, 95.8, 95.7, 92.8, 92.7, 92.4, 81.9, 81.5, 81.3, 79.6, 79.2, 77.9, 76.3, 76.1, 75.5, 75.3, 75.2, 75.2, 75.0, 74.9, 74.7, 74.2, 74.1, 73.8, 73.8, 73.3, 73.3, 73.2, 73.2, 73.1, 73.0, 72.9, 71.7, 71.6, 68.7, 68.6, 68.6, 68.2, 68.1, 67.3, 66.8, 64.6, 64.5, 38.8, 37.2, 30.4, 29.7, 29.0, 28.5, 23.8, 23.0, 21.5, 21.0, 20.9, 20.9, 20.9, 20.8, 20.8, 15.8, 15.6, 14.1, 14.1. HRMS (ESI) m/z: found [1/2M + Na]+ 2309.1352, C224H257Cl12N5O71 calcd. for [1/2M + Na]+ 2309.1350.
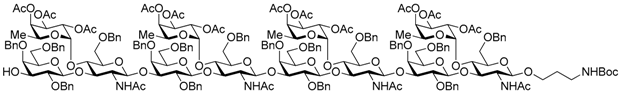
N-(tert-Butoxycarbonyl)-3-aminopropyl 2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-2-acetamido-6-O-benzyl-2-deoxy-β-d-glucopyranosyl-(1→3)-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-2-acetamido-6-O-benzyl-2-deoxy-β-d-glucopyranosyl-(1→3)-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-2-acetamido-6-O-benzyl-2-deoxy-β-d-glucopyranosyl-(1→3)-2,4,6-tri-O-benzyl-β-d-galactopyranosyl-(1→3)-[2,3,4-tri-O-acetyl-α-l-fucopyranosyl-(1→4)]-2-acetamido-6-O-benzyl-2-deoxy-β-d-glucopyranoside (25). A mixture of 23 (60.0 mg, 13.1 μmol), powdered Zn (1.71 g, 26.2 mmol), and AcOH (1.89 mL, 32.8 mmol) in THF (1.3 mL) was stirred under microwave irradiation at 250 W for 1 h under Ar. The mixture was diluted with CHCl3 and filtered through Celite. The filtrate was successively washed with sat. NaHCO3, water, and brine, dried over Na2SO4, and concentrated. The residue was chromatographed on silica gel with CHCl3–MeOH (92:8). The crude product was dissolved in DMF (1.3 mL), and stirred with Boc2O (4.3 μL, 20.0 μmol) and Et3N (5.4 μL, 39.0 μmol) at room temperature for 1 d. The reaction mixture was concentrated, and purified by gel permeation chromatography [S-X1, toluene–EtOAc (75:25)] and silica gel column chromatography with CHCl3–acetone (80:20–67:33) to give the title product 25 (41.0 mg, 76% in 2steps). 1H-NMR of the product 25 could not be assigned because all peaks were shown as broad peaks in all range. [α]D −6.5° (c 1.0, CHCl3); 13C-NMR (125 MHz, CDCl3) δ 170.6, 170.4, 170.3, 170.2, 169.5, 169.4, 169.4, 156.0, 139.1, 139.0, 138.8, 138.7, 138.6, 138.6, 138.2, 137.9, 137.8, 137.7, 137.6, 135.9, 129.4, 129.2, 129.1, 129.0, 129.0, 129.0, 128.7, 128.6, 128.6, 128.5, 128.4, 128.3, 128.2, 128.2, 128.2, 128.1, 128.1, 128.0, 128.0, 127.9, 127.7, 127.7, 127.6, 127.6, 127.6, 127.4, 127.3, 127.1, 127.1, 125.3, 103.4, 103.3, 103.2, 100.9, 100.5, 99.0, 95.8, 95.5, 80.5, 79.8, 79.3, 77.6, 76.4, 75.4, 75.2, 75.0, 74.7, 74.6, 74.3, 73.3, 73.3, 73.2, 73.1, 71.8, 68.2, 67.6, 66.7, 64.4, 37.1, 33.7, 32.8, 31.9, 30.2, 30.1, 29.7, 29.5, 29.4, 28.5, 27.1, 23.4, 23.2, 23.2, 22.7, 21.5, 20.8, 20.8, 15.6, 15.5, 15.4, 15.3, 14.1. HRMS (ESI) m/z: found [1/2M + Na]+ 2105.3693, C224H269N5O71 calcd for [1/2M + Na]+ 2105.3688.