Developing an Absorption–Based Quality Control Method for Hu–Gan–Kang–Yuan Capsules by UFLC–QTOF–MS/MS Screening and HPLC–DAD Quantitative Determination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Instrumentation and Conditions
2.2.1. UFLC–QTOF 5600+MS/MS
2.2.2. HPLC–DAD
2.3. Animal Administration
2.4. Sample Preparation
2.4.1. Extract of HGKYC
2.4.2. Rat Serum Sample
2.4.3. Preparation of Standard Solutions for Qualitative Identification
2.4.4. Preparation of Standard Solutions for Quantitative Determination
2.4.5. Extract of Negative Control Sample Solution
2.5. Establishment of Peak Identification
2.6. Method Validation for Determination of Baicalein, Wogonin, Paeonol and Emodin in HGKYC
3. Results
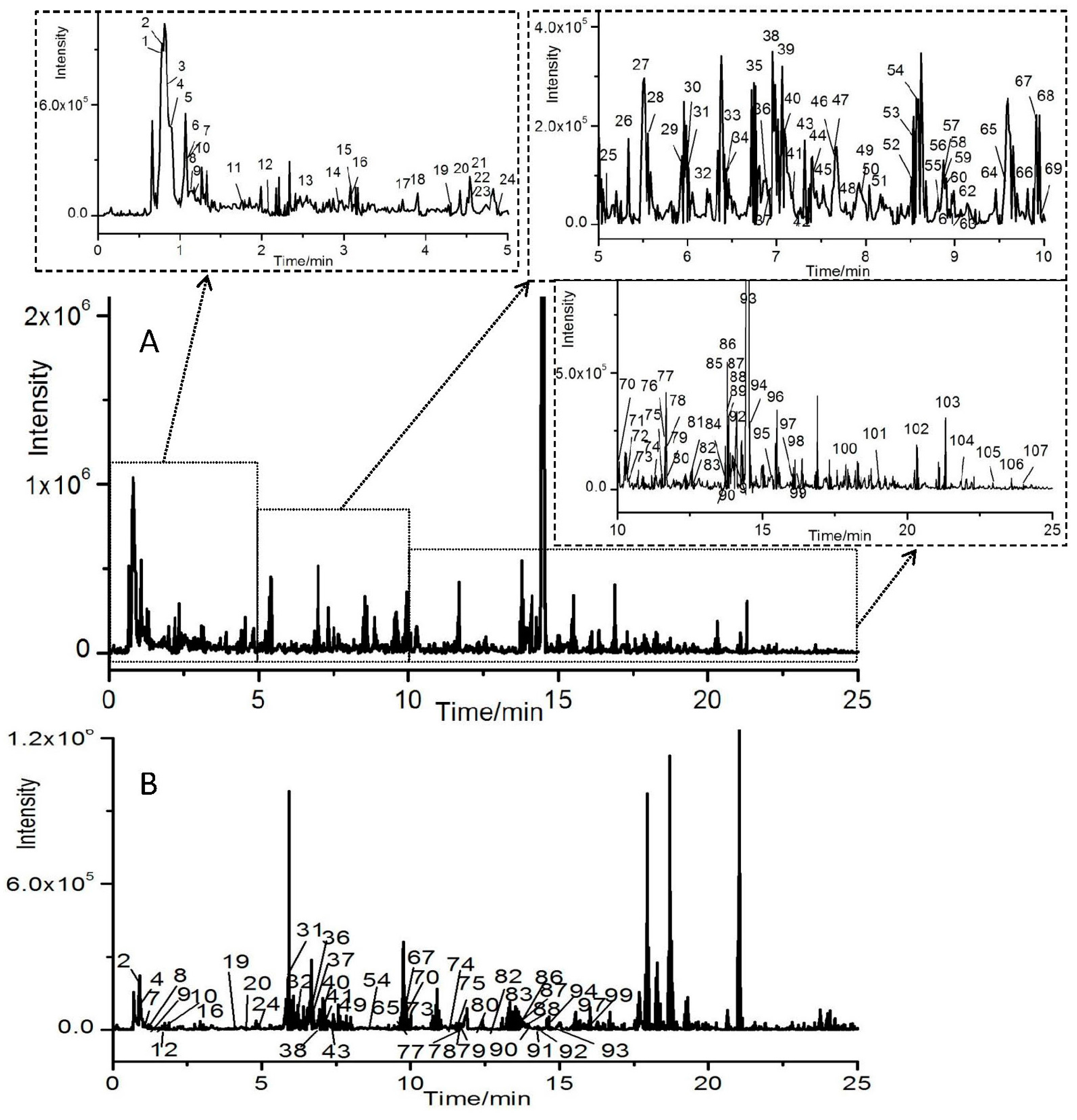
3.1. Optimization of UFLC–QTOF–MS/MS Conditions
3.2. Profiles of Ingredients from HGKYC Extract
3.2.1. Identification of Flavonoids and Isoflavonoids
3.2.2. Identification of Organic Acids
3.2.3. Identification of Phenylpropanoids
3.2.4. Identification of Anthraquinones
3.2.5. Identification of Saponins
3.2.6. Identification of Alkaloids
3.2.7. Identification of Terpenes and Phenols
3.2.8. Identification of Other Compounds
3.3. Profiles of Ingredients in Rat Serum after Oral Administration HGKYC
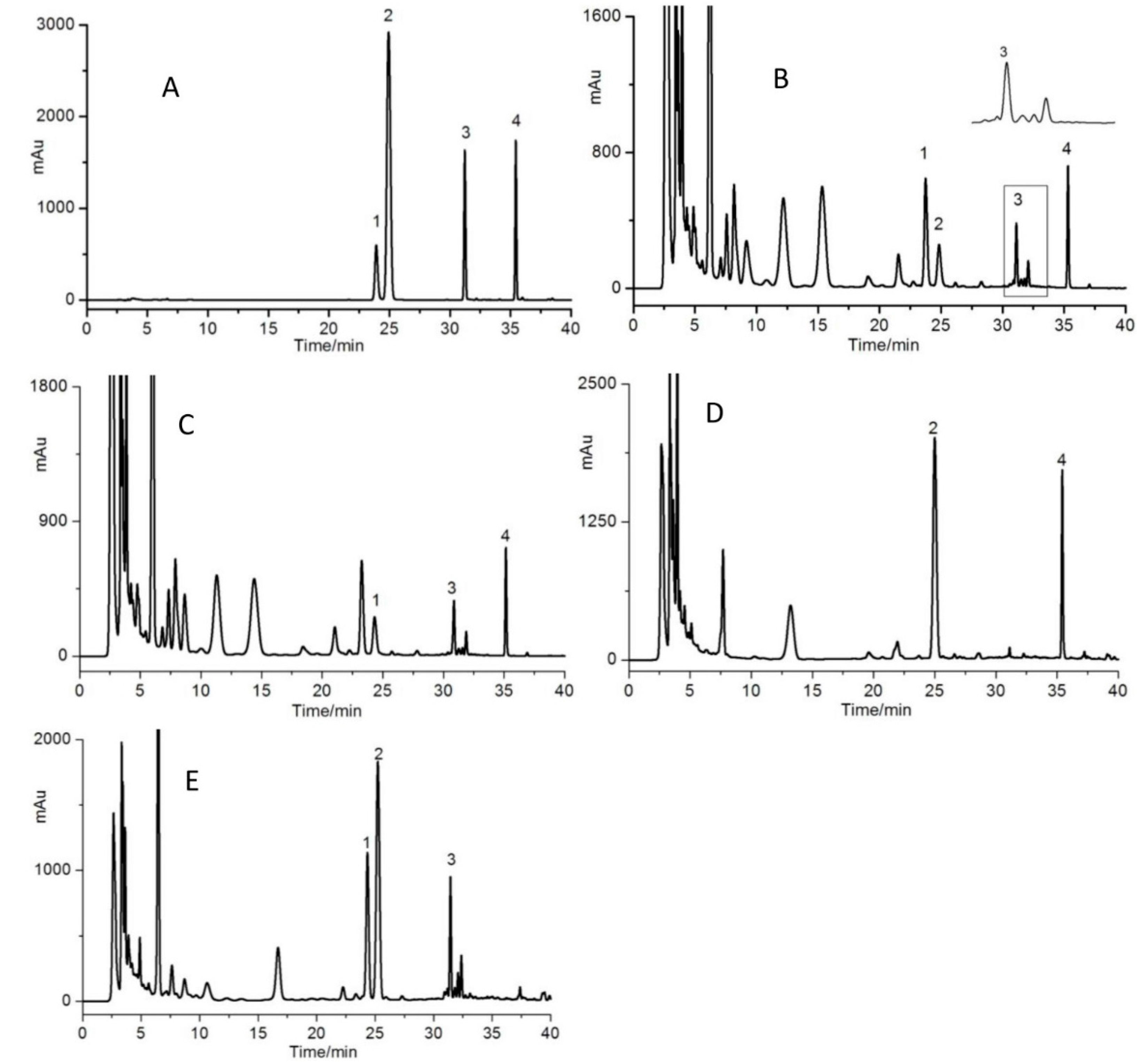
3.4. Optimization of HPLC Conditions
3.5. Optimization of Extraction Conditions
3.6. Method Validation
3.7. Sample Determination
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Editorial Committee of Pharmacopoeia of Ministry of Health, China. The Pharmacopeoia of People’s Republic of China (Part I); Chemical Industry Press: Beijing, China, 2015; pp. 425–, 1749.
- Yin, T.J.; Yang, G.Y.; Ma, Y.; Xu, B.B.; Hu, M.; You, M.; Gao, S. Developing an activity and absorption-based quality control platform for Chinese traditional medicine: Application to Zeng-Sheng-Ping (Antitumor B). J. Ethnopharmacol. 2015, 172, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.H.; Jiang, P.; Wang, S.P.; Yan, S.K.; Xiang, L.; Zhang, W.D.; Liu, R.H. Plasma pharmacochemistry based approach to screening potential bioactive components in Huang-Lian-Jie-Du-Tang using high performance liquid chromatography coupled with mass spectrometric detection. J. Ethnopharmacol. 2012, 141, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jiang, P.; Ye, F.Y.; Shi, R.; Ma, Y.M.; Zhong, J.; Wu, J.S.; Liu, P.; Liu, C.H.; Jia, Y.Q. Identification and pharmacokinetics of multiple constituents in rat plasma after oral administration of yin chen zhu fu decoction. J. Ethnopharmacol. 2014, 153, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Xu, Q.M.; Chen, C.; Song, C.W.; Xu, Y.; Xiang, Y.; Feng, Y.L.; Ouyang, H.; Zhang, Y.; Jiang, H.L. The rapid discovery and identification of physalins in the calyx of Physalisalkekengil.var franchetii (Mast.) Makino using ultra-highperformance liquid chromatography-quadrupole time of flight tandem mass spectrometry together with a novel three-step data mining strategy. J. Chromatogr A 2014, 1361, 139–152. [Google Scholar]
- Li, Z.F.; Wang, Y.W.; Ouyang, H. ; Lu,Y.; Qiu, Y.; Feng,Y.L.; Jiang, H.L.; Zhou, X.; Yang, S.L. A novel dereplication strategy for the identification of two new trace compounds in the extract of Gastrodiaelata using UHPLC/Q–TOF–MS/MS. J. Chromatogr. B 2015, 988, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.D.; Mao, Q.; Shen, H.; Zhu, L.Y.; Li, S.L.; Yan, R. Ultra-high performance liquid chromatography coupled with photo–diode array and quadrupole/time-of-flight mass spectrometry based chemical profiling approach to evaluate the influence of preparation methods on the holistic quality of Qiong-Yu-Gao, a traditional complex herbal medicine. J. Chromatogr. A 2013, 1304, 154–168. [Google Scholar] [PubMed]
- Li, P.L.; Liu, M.H.; Hu, J.H.; Su, W.W. Systematic chemical profiling of Citrus grandis ‘‘Tomentosa’’ byultra-fast liquid chromatography/diode-array detector/quadrupole time-of-flight tandem mass spectrometry. J. Pharm. Biomed. Anal. 2014, 90, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, G.S.; Peng, Y.; Tu, P.F.; Li, X.B. Screening and identification of three typical phenylethanoid glycosides metabolites from Cistanches Herba by human intestinal bacteria using UPLC/Q–TOF–MS. J. Pharm. Biomed. Anal. 2016, 118, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Tong, X.; Wang, J.X.; Zou, W.; Cao, H.; Su, W.W. Rapid separation and identification of multiple constituents in traditional Chinese medicine formula shenqi fuzheng injection by ultra-fast liquid chromatography combined with quadrupole-time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2013, 74, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Gou, Q.; Xia, Z.N. Coordination interaction capillary electrophoresis detection of arginine and proline in radix isatidis and its injection. J. Chongqing Univ. 2009, 32, 1357–1362. [Google Scholar]
- Liu, E.H.; Qi, L.W.; Peng, Y.B.; Cheng, X.L.; Wu, Q.; Li, P.; Li, C.Y. Rapid separation and identification of 54 major constituents in Bu yang huan wu decoction by ultra-fast HPLC system coupled with DAD-TOF/MS. Biomed. Chromatogr. 2009, 23, 828–842. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Shao, Q.; Xiang, Y.H.; Ge, Z.W.; Fan, X.H. Identification of phenylpropanoids in Ciwujia injection by HPLC–MS. China J. Chin. Mater. Med. 2014, 39, 2513–2520. [Google Scholar]
- Gan, J.S.; Ma, Y.; Wang, Z.Y.; Liu, X.S.; Liu, Y. Analysis on chemical constituents in Epimedii Herba by UPLC/Q–TOF–MS. Drugs Clin. 2014, 29, 351–352. [Google Scholar]
- Jiang, Y.J.; Yao, W.F.; Zhang, L.; Ding, A.W. Anaylsis of chemical components of Ligustrum lucidum by ultra-performance liquid chromatography-electrospray ionization-quadrupole-time of flight-mass spectrometry. China J. Chin. Mater. Med. 2012, 37, 2304–2308. [Google Scholar]
- Sun, H.; Han, Y.; Zhang, A.H.; Meng, X.C.; Wang, Z.Y.; Sun, W.J.; Sun, H.F.; Wang, X.J. UPLC–MS based metabolic profiling of the phenotypes of Acanthopanax senticosus reveals the changes in active metabolites distinguishing the diversities of the plant grown in northeast area of China. Chin. J. Nat. Med. 2012, 10, 196–206. [Google Scholar] [CrossRef]
- Yang, L.; Ge, H.S.; Wang, W.J.; Zu, Y.G.; Yang, F.J.; Zhao, C.J.; Zhang, L.; Zhang, Y. Development of sample preparation method for eleutheroside B and E analysis in Acanthopanax senticosus by ionic liquids-ultrasound based extraction and high-performance liquid chromatography detection. Food Chem. 2013, 141, 2426–2433. [Google Scholar] [CrossRef] [PubMed]
- P. Yu, H. Zhang, Simultaneous analysis of 17 compounds from the extract of Giant knotweed R. by HPLC–ESI–MS. J. Shenyang. Pharm. Univ. 2011, 28, 963–968. [Google Scholar]
- Chen, T.; Tian, F.; Tang, Y.N.; Liu, Y.; Lin, Z.Y.; Wang, Y.C. Quick separation and identification of 24 chemical constituents in Radix Astragali by HPLC–ESI–TOF/MS. China Pharm. 2014, 17, 593–596. [Google Scholar]
- Wu, H.; Zhu, Z.Y.; Zhang, G.Q.; Zhao, L.; Zhang, H.; Zhu, D.L.; Chai, Y.F. Comparative pharmacokinetic study of paeoniflorin after oral administration of pure paeoniflorin, extract of Cortex Moutan and Shuang-Dan prescription to rats. J. Ethnopharmacol. 2009, 125, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Guo, Q.S.; Wang, H.L.; Gao, J.P.; Xu, X. Simultaneous determination of resveratrol and polydatin in Polygonum Cuspidatum by quantitative nuclear magnetic resonance spectroscopy. Chin. J. Anal. Chem. 2015, 43, 69–74. [Google Scholar]
- Ye, X.L.; Song, L.; Ren, G.F.; Ye, W.F.; Wang, J. Pharmacokinetics study of bioactive constituents of Polygonumcuspidatum in rat by HPLC–MS. Chin. J. Pharm. Anal. 2013, 33, 749–754. [Google Scholar]
- Chen, Z.W.; Tong, L.; Li, S.M.; Li, D.X.; Liu, X.L.; Ding, L.; Zhu, Y.H.; Zhou, S.P.; Sun, H. Identification of major parent compounds and metabolites in bile, plasma and urine of rats after oral administration of Radix Scutellariae extract by UFLC–IT–TOF/MS. J. Chin. Pharm. Sci. 2013, 22, 319–328. [Google Scholar] [CrossRef]
- Gong, J.R.; Wang, S.F. Chemical constituents of Acanthopanax senticosus. Chin. Tradit. Herb. Drugs. 2012, 43, 2337–2341. [Google Scholar]
- Zhou, X.Q.; Liang, H.; Lu, X.H.; Cai, S.Q.; Wang, B.; Zhao, Y.Y. Flavonoids from Scutellaria baicalensis and their bioactivities. J. Peking. Univ. Health. Sci. 2009, 41, 578–584. [Google Scholar]
- Lu, L.; Zhang, H.; Zhao, L.; Jia, J.; Li, Y.Y.; Zhang, G.Q. RRLC–TOF/MS in rapid identification of 43 chemical constituents of epimedium. Acad. J. Second. Mil. Med. Univ. 2011, 32, 306–310. [Google Scholar] [CrossRef]
- Huang, W.; Su, Z.R.; Bi, W.C.; Li, J.; Shi, L.Q.; Wen, Y.Q.; Zhan, H.Q. HPLC determination of nuezhenide in Fructus Ligustri Lucidi. Chin. J. Pharm. Anal. 2009, 29, 824–826. [Google Scholar]
- Lau, C.H.; Chan, C.M.; Chan, Y.W.; Lau, K.M.; Lau, T.W.; Lam, F.C.; Law, W.T.; Che, C.T.; Leung, P.C.; Fung, K.P.; et al. Pharmacological investigations of the anti-diabetic effect of Cortex Moutan and its active component paeonol. Phytomedicine 2007, 14, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Rui, W.F.; Yi, F.; Shi, Z.F.; Jiang, M.M. UPLC/Q–TOF–MS study on fingerpint of Astragalus membranaceus obtained from 7 different origin. Chin. J. Pharm. Anal. 2012, 32, 607–611. [Google Scholar]
- Liu, X.F.; Lou, Z.Y.; Zhu, Z.Y.; Zhang, H.; Zhao, L.; Chai, Y.F. HPLC–TOF/MS in rapid identification of chemical compositions in Xiao chai hu decoction. Acad. J. Second. Mil. Med. Univ. 2009, 30, 941–946. [Google Scholar] [CrossRef]
- Huang, X.; Song, F.R.; Liu, Z.Q.; Liu, S.Y. Studies on the lignans in extract of the fruits of Schisandra chinensis and Schisandra sphenanthera by high performance liquid chromatography-electrospray ionization mass spectrometry. Acta Chim. Sin. 2008, 66, 1059–1066. [Google Scholar]
- Abad-Garcia, B.; Garmon-Lobato, S.; Berrueta, B.; Gallo, L.A.; Vicente, F. On line characterization of 58 phenolic compounds in Citrus fruit juices from Spanish cultivars by high-performance liquid chromatography with photodiode-array detection coupled to electrospray ionization triple quadrupole mass spectrometry. Talanta 2012, 99, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Huang, H.Z.; Chen, J.W.; Li, X. In vitro antioxidant and anti-inflammatory activities of Radix Isatidis extract and bioaccessibility of six bioactive compounds after simulated gastro-intestinal digestion. J. Ethnopharmacol. 2014, 157, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shan, L.; Hua, Y.P.; Wang, D.; Zeng, H.W.; Liu, R.H.; Zhang, W.D.; Hu, Z.L. Baicalein selectively induces apoptosis in activated lymphocytes and ameliorates concanavalin A-induced hepatitis in mice. PLoS ONE 2013, 8, e69592. [Google Scholar]
- Wang, W.P.; Xia, T.S.; Yu, X.P. Wogonin suppresses inflammatory response and maintains intestinal barrier function via TLR4-MyD88-TAK1-mediated NF-B pathway in vitro. Inflamm. Res. 2015, 64, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, W. Anti-inflammatory effect of wogonin on RAW 264.7 mouse macrophages induced with polyinosinic-polycytidylicacid. Molecules 2015, 20, 6888–6900. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yao, J.; Wu, X.P.; Zhao, L.; Zhou, Y.X.; Zhang, Y.; You, Q.D.; Guo, Q.L.; Lu, N. Wogonin suppresses human alveolar adenocarcinoma cell A549 migration in inflammatory microenvironment by modulating the IL–6/STAT3 signaling pathway. Mol. Carcinog. 2015, 54, E81–E93. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chang, E.J.; Lee, Y.; Kim, J.S.; Kang, S.S.; Kim, H.H. A genome–Wide microarray analysis reveals anti-inflammatory target genes of paeonol in macrophages. Inflamm. Res. 2008, 57, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Liu, D.; Soromou, L.W.; Sun, J.; Zhong, W.; Guo, W.; Huo, M.; Li, H.; Guan, S.; Chen, Z.; et al. Paeonol suppresses lipopolysaccharide-induced inflammatory cytokines in macrophage cells and protects mice from lethal endotoxin shock. Fundam. Clin. Pharmacol. 2014, 28, 268–76. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhao, L.; Mei, H.; Zhang, S.L.; Huang, Z.H.; Du, Y.Y.; Ye, P. Exploration of e modin to treat alpha-naphthylisothiocyanate-induced cholestatic hepatitis via anti-inflammatory pathway. Eur. J. Pharmacol. 2008, 590, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.H.; Chen, F.; Wang, J.; Wu, S.S.; Zheng, M.; Zhu, H.H.; Liu, Y.N.; He, J.L.; Chen, Z. Emodin protects against concanavalin A-Induced hepatitis in mice through inhibiting activation of the p38 MAPK–NF–κB signaling pathway. Cell. Physiol. Biochem. 2015, 35, 1557–1570. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
| NC Samples | Preparation Method | Specificity Evaluation |
|---|---|---|
| Without Scutellariae radix | ① | baicalein and wogonin |
| Without Moutan cortex | ② | paeonol |
| Without Polygoni cuspidate rhizome et radix | ③ | emodin |
| No. | TR (min) | Formula | [M + H]+ (Error, ppm) | [M − H]− (Error, ppm) | Fragment Ions in | Identified Compounds | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| Positive (+) Ion Mode | Negative (−) Ion Mode | In Extract | In Serum | ||||||
| 1 | 0.77 | C6H14N4O2 | 173.1046 (1.2) | 131.0829 | Arginine s | − | [10] | ||
| 2 | 0.78 | C5H13NO | 104.1070 (0.7) | 60.0832 [M + H − H − C2H5O]+ | Choline s | + | [10] | ||
| 58.0680 | |||||||||
| 3 | 0.85 | C5H9NO2 | 116.0705 (−0.7) | 70.0671 [M + H − HCOOH]+ | Proline | − | [11] | ||
| 4 | 0.9 | C5H11NO2 | 118.0861 (−0.7) | 116.0721 (3.5) | 72.0818 [M + H − HCOOH]+ | Betaine s | + | [10] | |
| 58.0676, 55.0566 | |||||||||
| 5 | 1.07 | C5H5N5 | 136.0717 (−0.2) | 119.0353 [M + H − H − NH3]+ | Adenine s | − | [10] | ||
| 92.0251 [M + H − H − NH3 − HCNN]+ | |||||||||
| 6 | 1.08 | C6H5NO2 | 124.0392 (−0.5) | 106.0278 [M + H − H2O]+ | Nicotinic acid s | − | |||
| 79.0425, 78.0348 | |||||||||
| 7 | 1.08 | C5H7NO3 | 130.0499 (−0.6) | 84.0461 [M + H − HCOOH]+ | l-Pyroglutamic acid s | + | [10] | ||
| 56.0530 [M + H − H − NH3 − HCNOOH]+ | + | ||||||||
| 8 | 1.13 | C9H11NO3 | 182.0806 (−3.3) | 165.0547 [M + H − H − NH3]+ | Tyrosine s | + | [10] | ||
| 136.0761 [M + H − HCOOH]+ | |||||||||
| 119.0487 [M + H − HCOOH − H2O]+ | |||||||||
| 91.0548 | |||||||||
| 9 | 1.17 | C6H13NO2 | 132.1018 (−1.1) | 86.0964 [M + H − HCOOH]+, | Isoleucine s | + | [10] | ||
| 69.0711 [M + H − HCOOH − NH3]+ | |||||||||
| 10 | 1.21 | C9H8O3 | 165.0546 (0.02) | 123.0448 [M + H − H − C2H2O]+ | p-Coumaric acid s | + | [10] | ||
| 95.0500 [M + H − H − C2H2O − CO]+ | |||||||||
| 77.0397 [M + H − H − C2H2O − CO − H2O]+ | |||||||||
| 65.0414 | |||||||||
| 11 | 1.73 | C7H6O5 | 171.0285 (−1.7) | 169.0143 (0.4) | 153.0180 [M + H − H2O]+ | 125.0253 [M − H − CO2]− | Gallic acid s | − | [10] |
| 107.0131 [M + H − H2O − HCOOH]+ | 79.0217 [M − H − CO2 − HCOOH]− | ||||||||
| 12 | 2.04 | C9H11NO2 | 164.0716 (−0.9) | 147.0438 [M − H − NH3]− | Phenylalanine s | + | [10] | ||
| 103.0560 [M − H − NH3 − CO2]− | |||||||||
| 13 | 2.54 | C8H8O4 | 167.0350 (0.2) | 123.0449 [M − H − CO2]− | Vanillic acid | − | [12] | ||
| 93.0358 [M − H − CO2 − OCH3]− | |||||||||
| 97.0283, 69.0341, 65.0399 | |||||||||
| 14 | 2.92 | C7H6O4 | 153.01989 (3.6) | 109.0309 [M − H − CO2]− | Protocatechuic acid | − | [13] | ||
| 91.0208 [M − H − CO2 − H2O]− | |||||||||
| 15 | 3 | C5H7NOS | 130.0316 (−3.9) | 128.0442 (1.0) | 103.0536 [M + H − HCN]+ | Epigoitrin s | − | [14] | |
| 84.0819 [M + H − HCOOH]+ | |||||||||
| 70.0657, 60.9750 | |||||||||
| 16 | 3.08 | C11H12N2O2 | 203.0825 (−0.4) | 142.0664 [M − H − NH3 − CO2]− | Tryptophan s | + | [10] | ||
| 116.0513 [M − H − NH3 − 2OCH2]− | |||||||||
| 17 | 3.83 | C7H6O3 | 137.0248(2.8) | 119.0145 [M − H− H2O]− | Protocatechuic aldehyde s | − | [13] | ||
| 108.0288 [M − H − CO]− | |||||||||
| 92.0284 | |||||||||
| 18 | 3.87 | C14H20O7 | 299.1140 (1.3) | 137.0244 [M − H − Glc]− | Salidroside s | − | [15] | ||
| 119.0335 [M − H − Glc − H2O]− | |||||||||
| 93.0357, 59.0172 | |||||||||
| 19 | 4.31 | C8H8O5 | 183.0300 (0.7) | 168.0065 [M − H − CH3]− | Methyl gallate s | + | [13] | ||
| 124.0175, 78.0136 | |||||||||
| 20 | 4.49 | C15H18O9 | 341.0881 (0.8) | 179.0352 [M − H − Glc]− | Caffeoylglucose | + | [13] | ||
| 135.0454 [M − H − Glc − CO2]− | |||||||||
| 21 | 4.49 | C15H14O6 | 291.0860 (−0.9) | 207.0643, 147.0439 | Catechin s | [13] | |||
| 139.0392, 123.0440 | |||||||||
| 22 | 4.55 | C16H18O9 | 355.1023 (−0.1) | 353.0878 (3.0) | 163.0386 [M + H − H − Glc − OCH2]+ | 191.0564 [M − H − Glc]− | Chlorogenic acid s | − | [16] |
| 145.0280, 117.0329 | |||||||||
| 23 | 4.77 | C17H24O9 | 373.1487 (−1.6) | Syringoside s | − | [17] | |||
| 24 | 4.96 | C9H8O4 | 179.035 4 (2.3) | 135.0459 [M − H − CO2]− | Caffeic acid s | + | [13] | ||
| 25 | 5.15 | C11H10O5 | 223.0595 (−2.5) | 221.0456 (0) | 162.0302 [M + H − H − CH3 − HCOOH]+ | 206.0213 [M − H − CH3]− | Isofraxidin s | − | [16] |
| 190.9989 [M − H − OCH3]− | |||||||||
| 177.0184 [M − H − CO2]− | |||||||||
| 163.0003 [M − H − OCH2 − CO]− | |||||||||
| 26 | 5.36 | C20H23O4N | 342.1695 (−1.3) | 340.1557 (0.8) | 297.1110 [M + H − HCN − H2O]+ | 310.1088 [M − H − OCH2]− | Magnoline | − | [14] |
| 282.0881 [M + H − HCN − H2O − CH3]+ | 252.0404 [M − H − CO2 − NH3 − HCNN]− | ||||||||
| 237.0902, 191.0848, 58.0680 | 224.0509 [M − H − CO2 − NH3 − HCNN − H2O]− | ||||||||
| 162.0180, 118.0288,79.9591 | |||||||||
| 27 | 5.51 | C19H18O11 | 423.0925 (0.7) | 421.0780 (0.8) | 405.0776 [M + H − H2O]+ | 331.0459 [M − H − HCOOH − CO2]− | Mangiferin | − | [18] |
| 339.0520, 327.0493, 303.0478 | 271.0253 [M − H − Xyl]− | ||||||||
| 285.0417 | 259.0230 [M − H − Glc]− | ||||||||
| 273.0395, | |||||||||
| 28 | 5.54 | C17H20N4O6 | 377.1450 (−1.6) | 359.1339, 243.0878, 198.0644 | Riboflavin | − | [19] | ||
| 29 | 5.97 | C17H20O9 | 367.1038 (0.9) | 191.0561 | 3-Feruloyquinic acid | − | [13] | ||
| 30 | 6.07 | C23H28O11 | 479.1569 (2) | 449.1433, 327.1081 | Paeoniflorin s | − | [20] | ||
| 255.0647, 165.0568, 121.0297 | |||||||||
| 31 | 6.08 | C9H10O5 | 197.0458 (1.4) | 169.0147, 124.0175, 78.0178 | Syringic acid s | + | |||
| 32 | 6.2 | C9H8O3 | 163.0400 (−0.4) | 119.0505 [M − H − CO2]− | trans-p-Coumaric acid s | + | |||
| 93.0352 [M − H − CO − C2H2O]− | |||||||||
| 33 | 6.48 | C34H46O18 | 743.2744 (−1.8) | 741.2628 (2.2) | 579.2111 [M − H − Glc]− | Eleutheroside E s | − | [13] | |
| 417.1579 [M − H − 2Glc]− | |||||||||
| 34 | 6.48 | C28H36O13 | 579.2100 (2.9) | Eleutheroside E1 | − | [13] | |||
| 35 | 6.82 | C10H10O4 | 193.0508 (0.8) | 178.0263 [M − H − CH3]− | Ferulic acid s | − | [13] | ||
| 134.0375 [M − H − CH3 − CO2]− | |||||||||
| 36 | 6.9 | C20H22O8 | 391.1372 (−3.9) | 389.1252 (2.7) | 229.0859 [M + H − H − Glc]+ | 227.0718 [M − H − Glc]− | Polydatin s | + | [21] |
| 185.0612 [M − H − Glc − C2H2O]− | |||||||||
| 37 | 6.91 | C14H12O3 | 229.0850 (3.9) | 227.0715 (0.7) | 183.0788 | 185.0601, 143.0508 | Resveratrol s | + | [22] |
| 165.0690 [M + H − H2O − HCOOH]+ | |||||||||
| 107.0489, 91.0540 | |||||||||
| 38 | 6.95 | C26H28O13 | 547.1479 (4) | 6-C-Arabinosyl-8-C-Glucosyl-Chrysin | + | [23] | |||
| 487.1279 | |||||||||
| 457.1169 [M − H − C3H6O − OCH2]− | |||||||||
| 427.1052 | |||||||||
| 409.0946, 367.0839 | |||||||||
| 337.0734 [M − H − Glc − OCH2]− | |||||||||
| 39 | 7.04 | C27H30O16 | 611.1584 (−3.6) | 609.1482 (3.8) | 465.1016 [M + H − H − Rha]+ | 301.0354 [M − H − Rha − Glc]− | Rutin s | − | [18] |
| 303.0501 [M + H − H − Rha − Glc]+ | 271.0228 | ||||||||
| 40 | 7.10 | C21H18O12 | 461.0736 (2.2) | 285.0416 [M − H − Glc acid]− | Scutellarin s | + | [18] | ||
| 41 | 7.19 | C21H20O12 | 463.0891 (1.9) | 301.0360 [M − H − Glc]− | Hyperoside s | + | [24] | ||
| 271.0250 [M − H − Glc − OCH2]− | |||||||||
| 255.0310 [M − H − O − Glc − OCH2]− | |||||||||
| 178.9998, 151.0041 | |||||||||
| 42 | 7.21 | C23H24O13 | 509.1271 (−3.6) | 507.1156 (2.3) | 347.0750 [M + H − H − Glc]+, | 345.0632 [M − H − Glc]− | Viscidulin III- | − | [25] |
| 332.0523 [M + H − H − Glc − CH3]+ | 330.0383 [M − H − Glc − CH3]− | Glucopyranoside | |||||||
| 314.0409 | 315.0174 [M − H − Glc − 2CH3]− | ||||||||
| 43 | 7.32 | C26H28O13 | 547.1479 (4.0) | 487.1279 | 6-C-Glucosyl-8-C-Arabinosyl-Chrysin | [23] | |||
| 457.1169 [M − H − C3H6O − OCH2]− | |||||||||
| 427.1052 | |||||||||
| 409.0946, 367.0839 | |||||||||
| 337.0734 [M − H − Glc − OCH2]− | |||||||||
| 44 | 7.41 | C21H20O13 | 479.0831 (0.1) | 441.0761, 435.0957 | Isomyricitrin | − | [26] | ||
| 313.0570, 165.0548 | |||||||||
| 45 | 7.59 | C25H24O12 | 517.1326 (−2.8) | 515.1199 (0.7) | 499.1177 [M + H − H2O]+ | 353.0889 | Cynarin | − | [13] |
| 163.0382 | 191.0562 | ||||||||
| 179.0347 | |||||||||
| 46 | 7.63 | C33H40O18 | 723.2144 (0.2) | Ligustroflavone s | − | [27] | |||
| 47 | 7.63 | C31H42O17 | 687.24992 (0.6) | 685.2372 (3.4) | 523.1831 [M − H − Glc]− | Specnuezhenide | − | [15] | |
| 454.1404, 421.1508 | |||||||||
| 299.1137, 223.0603 | |||||||||
| 48 | 7.74 | C20H18O11 | 435.0913 (−2) | 433.0784 (1.7) | 303.0493 [M + H − H−Arab]+ | 301.0294 [M − H − Arab]− | Quercetin-3-arabinoside | − | [23] |
| 271.0257 [M − H − Arab − OCH2]− | |||||||||
| 255.0314, 178.9989, 151.0036 | |||||||||
| 49 | 7.93 | C21H20O9 | 417.1169 (−2.6) | 415.1044 (2.2) | 399.1071 [M + H − H2O]+ | 325.0692 [M − H − C3H6O3]− | Chrysin-8-C-glucoside | + | [23] |
| 297.0758 [M + H − H − C4H8O4]+ | 295.0624 [M − H − C4H8O4]− | ||||||||
| 279.0639 [M + H − H − C4H8O4−H2O]+ | 267.0676 [M − H − C4H8O4 − CO]− | ||||||||
| 267.0644 [M + H − H − C4H8O4 − 2H2O]+ | 149.0237 | ||||||||
| 50 | 7.97 | C21H20O11 | 449.1063 (−3.3) | 447.0947 (3.1) | 303.0488 [M + H − H − Rha]+ | 301.0379 [M − H − Rha]− | Quercetrin s | − | [18] |
| 287.0549 [M + H − H − O − Rha]+ | 271.0265 [M − H − Rha − OCH2]− | ||||||||
| 129.0543, 85.0289 | 255.0315, 178.9983 | ||||||||
| 85.0286 | 151.0041 | ||||||||
| 51 | 8.22 | C32H38O16 | 679.2215 (−2.6) | 533.1669 [M + H − H − Rha]+ | hexandraside E | − | [14] | ||
| 371.1096 [M + H − H − Rha − Glc]+ | |||||||||
| 315.0457 [M + H − H − Rha − Glc − C4H8]+ | |||||||||
| 52 | 8.52 | C38H48O20 | 825.2794 (−2.1) | 663.2238 [M + H − H − Glc]+ | Diphylloside B | − | [14] | ||
| 517.1674 [M + H − H − Rha − Glc]+ | |||||||||
| 355.1199 [M + H − H − Rha−2Glc]+ | |||||||||
| 299.0562 [M + H − H − Rha−2Glc − C4H8]+ | |||||||||
| 53 | 8.53 | C26H28O11 | 517.1693 (−2.2) | 355.1169 [M + H − H − Glc]+ | Icarrin C | − | [14] | ||
| 299.0524 [M + H − H—Glc − C4H8]+ | |||||||||
| 54 | 8.58 | C21H18O11 | 447.0912 (−2.2) | 445.07858 (2.1) | 271.0595 [M + H − H − O − Glc]+ | 269.0459 [M − H − O − Glc]− | Baicalin s | + | [23] |
| 55 | 8.82 | C38H48O19 | 809.28357 (−3.3) | 807.2011 (0.33) | 663.2266 [M + H − H − Xyl]+ | Epimedin B | − | [14] | |
| 517.1687 [M + H − H − Xyl − Rha]+ | |||||||||
| 355.1182 [M + H − H − Xyl − Rha − Glc]+ | |||||||||
| 299.0542 [M + H − H − Xyl − Rha − Glc − C4H8]+ | |||||||||
| 56 | 8.86 | C22H22O9 | 431.1318 (−4.2) | 429.1101 (0.3) | 269.0802 [M + H − H − Glc]+ | Ononin | − | ||
| 57 | 8.87 | C21H22O9 | 417.1191 (0) | 255.0678 [M − H − Glc]− | Polygonimitin B | [18] | |||
| 211.0773 [M − H − Glc − CO2]− | |||||||||
| 58 | 8.89 | C21H20O11 | 449.1063 (−3.3) | 447.0946 (3.1) | 303.0488 [M + H − H − Rha]+ | 301.0379 [M − H − Rha]− | Isoquercetrin | − | |
| 287.0549 [M + H − H − O − Rha]+ | 271.0265 [M − H − Rha − CH2]− | ||||||||
| 129.0543,85.0289 | 255.0315,178.9983 | ||||||||
| 59 | 8.9 | C32H38O15 | 663.2265 (−2.7) | 661.2169 (4.7) | 517.1667 [M + H − H − Rha]+ | 515.1907 | Epimedoside A | − | |
| 355.1180 [M + H − H − Rha − Glc]+ | 353.1050 | ||||||||
| 299.0559 [M + H − H − Rha − Glc − C4H8]+ | |||||||||
| 60 | 9.0 | C17H14O8 | 347.0751 (−3.1) | 332.0523, 317.0288, 314.0414 | Viscidulin III | − | [23] | ||
| 289.0340, 169.0125 | |||||||||
| 61 | 9.04 | C39H50O20 | 839.2967 (−0.2) | 677.2346 [M + H − H − Glc]+ | Epimedin A | − | |||
| 531.1881 [M + H − H − Glc − Rha]+ | |||||||||
| 369.1342 [M + H − H − 2Glc − Rha]+ | |||||||||
| 313.0691 [M + H − H − 2Glc − Rha − C4H8]+ | |||||||||
| 62 | 9.15 | C33H40O16 | 693.2365 (−3.4) | 547.1791 [M + H − H − Rha]+ | anhydroicaritin | − | [14] | ||
| 531.1895 [M + H − H − Glc]+ | -3,7-di-O-glucoside | ||||||||
| 385.1284 [M + H − H− Rha − Glc]+ | |||||||||
| 369.1321 [M + H − H − O − Rha − Glc]+ | |||||||||
| 313.0687 [M + H − H − O − Rha − Glc − C4H8]+ | |||||||||
| 63 | 9.15 | C30H32O13 | 599.1787 (2.8) | 581.1699 [M − H − H2O]− | Benzoyloxypaeoniflorin | − | [20] | ||
| 477.1593 [M − H − C7H6O2]− | |||||||||
| 449.7983 [M − H − C7H6O2 − CO]− | |||||||||
| 431.1372 [M − H − H2O − Xyl]− | |||||||||
| 281.0676 [M − H − H2O − Xyl − C5H10O5]− | |||||||||
| 64 | 9.45 | C22H22O10 | 447.1269 (−3.8) | 445.1143 (0.6) | 285.0757 [M + H − H − Glc]+, | 430.0906 [M − H − CH3]− | Wogonin-7-O-glucoside | − | [23] |
| 270.0526 [M + H − H − Glc − CH3]+ | 268.0456 [M − H − CH3 − Glc]− | ||||||||
| 65 | 9.51 | C22H20O12 | 477.1026 (−2.3) | 475.0892 (2) | 301.0706 [M + H − H − Glucuronic acid]+ | 299.0573 [M − H − Glucuronic acid]− | 5,4′-Dihydroxy-8-methoxy-flavone-7-O-glucuronide | + | [23] |
| 286.0470 [M + H − H − Glucuronic acid − CH3]+ | 284.0324 [M − H − Glucuronic acid − CH3]− | ||||||||
| 66 | 9.8 | C15H10O7 | 301.0356 (0.9) | 273.0396 [M − H − CO]− | Quercetin | − | [18] | ||
| 245.0407 [M − H − CO − H2O]− | |||||||||
| 178.9978 | |||||||||
| 151.0035, 121.0292 | |||||||||
| 107.0144 | |||||||||
| 67 | 9.9 | C21H18O11 | 447.0919 (−0.7) | 271.0595 | Baicalein-6-O-glucuronide | + | [23] | ||
| 68 | 9.96 | C11H12O4 | 207.0667 (2.1) | 179.0345, 135.0469 | Ethyl Caffeate s | [23] | |||
| 69 | 9.99 | C22H22O11 | 461.1088 (−0.4) | 459.0949 (3.4) | 285.0759, 270.0522 | 283.0624, 268.0387, 175.0256, 113.0261 | Wogonoside s | − | [23] |
| 70 | 10.03 | C22H20O11 | 461.1069 (−2) | 459.0949 (3.4) | 285.0759 [M + H − H − Glucuronic acid]+ | 283.0624 [M − H − Glucuronic acid]− | Oroxyloside s | + | [23] |
| 270.0522 [M + H − H − Glucuronic acid − CH3]+ | 268.0387 [M − H − Glucuronic acid − CH3]− | ||||||||
| 71 | 10.33 | C39H50O19 | 823.30066 (−1.5) | 677.2421 [M + H − H − Rha]+ | Epmedin C | − | [14] | ||
| 531.1840 [M + H − H−2Rha]+ | |||||||||
| 369.1326 [M + H − H−2Rha − Glc]+ | |||||||||
| 313.0708 [M + H − H−2Rha − Glc − C4H8]+ | |||||||||
| 72 | 10.34 | C27H30O11 | 531.1846 (−2.8) | 369.1326 [M + H − H − Glc]+ | Icariside I s | − | [14] | ||
| 313.0695 [M + H − H − Glc − C4H8]+ | |||||||||
| 73 | 10.55 | C33H40O15 | 677.24256 (−2.1) | 675.2326 (4.7) | 531.1847 [M + H − H − Rha]+ | 367.1099 | Icariin s | + | [14] |
| 369.1332 [M + H − H − Rha − Glc]+ | 269.0461 [M − H − Rha − Glc]− | ||||||||
| 313.0712 [M + H − H − Rha − Glc − C4H8]+ | |||||||||
| 74 | 11.3 | C9H10O3 | 165.0557 (0) | 150.0333 [M − H − CH3]− | Paeonol s | + | [28] | ||
| 135.0094 [M − H − OCH2]− | |||||||||
| 122.0385, 91.0208 | |||||||||
| 75 | 11.44 | C15H10O6 | 285.0407 (0.8) | 241.0517 [M − H − CO2]− | Kaempferol s | + | [18] | ||
| 211.0408 [M − H − CO2−OCH2]− | |||||||||
| 195.0463,167.0509 | |||||||||
| 76 | 11.56 | C16H12O6 | 299.0563 (0.7) | 284.0327 [M − H − CH3]− | 5,7,4’-Trihydroxy-8- | − | [23] | ||
| 240.0424 [M − H − CH3 − CO2]− | methoxyflavone | ||||||||
| 171.0452,153.9909 | |||||||||
| 125.9968 | |||||||||
| 77 | 11.59 | C16H12O7 | 315.0510 (0) | 300.0279 [M − H − CH3]− | Isorhamnetin s | + | [29] | ||
| 282.0178 [M − H − CH3 − H2O]− | |||||||||
| 255.0240, 151.0016 | |||||||||
| 78 | 11.71 | C32H38O15 | 663.3011 (3.1) | 661.2163 (−3.5) | 517.1667 [M + H − HCOOH]+ | 353.0978 [M − H − Rha − Glc]− | Ikarisoside B | + | [26] |
| 355.1165 [M + H − H − Rha − Glc]+ | |||||||||
| 299.0599 [M + H − H − Rha − Glc − C4H8]+ | |||||||||
| 79 | 11.73 | C15H10O5 | 271.0597 (−1.6) | 269.0456(1.9) | 253.0500 [M + H − H2O]+ | 251.036 [M − H − H2O]− | Baicalein s | + | [23] |
| 123.0080 | 223.0407, 169.0664 | ||||||||
| 80 | 12.15 | C31H36O14 | 631.2050 (2.8) | 481.1616 [M − H − Xyl]− | Ikarisoside F | + | [26] | ||
| 353.1076, 352.0928, 341.0528 | |||||||||
| 81 | 12.37 | C16H12O4 | 269.0805 (−1.2) | 267.0664 (0.4) | 254.0576, 237.0532 | 252.0430, 223.0396, 195.0445 | Formononetin s | − | [10] |
| 197.0599 | 132.0221 | ||||||||
| 181.0644, 118.0416 | |||||||||
| 82 | 12.65 | C26H28O10 | 501.1744 (−2.3) | 499.1618 (1.6) | 355.1169 [M + H − H − Rha]+ | 353.1043 [M − H − Rha]− | Baohuoside II s | + | [14] |
| 299.0559 [M + H − H − Rha − C4H8]+ | 309.0445 [M − H − Rha − CO2]− | ||||||||
| 147.0645,129.0533 | |||||||||
| 83 | 13.34 | C33H40O15 | 677.2417 (−3.4) | 675.2326 (4.7) | 531.1847 [M + H − H − Rha]+ | 367.1099 [M − H − Rha − Glc]− | Baohuoside VII | + | [26] |
| 369.1324 [M + H − H − Rha − Glc]+ | 352.0997 | ||||||||
| 313.0712 [M + H − H − Rha − Glc − C4H8]+ | |||||||||
| 84 | 13.49 | C43H70O15 | 827.4768 (−2.3) | 455.3660, 437.335 | Astragaloside II | − | [10] | ||
| 175.0610,157.0474,143.1087 | |||||||||
| 85 | 13.74 | C42H68O13 | 781.4736 (0.4) | 745.4447 | Saikosaponin a | − | [30] | ||
| 619.4203, 583.3921 | |||||||||
| 455.3486, 437.3374 | |||||||||
| 419.3231 | |||||||||
| 86 | 13.79 | C16H12O5 | 285.0754 (−1.3) | 283.0615 (0.9) | 270.0514 [M + H − H − CH3]+ | 268.0380 [M − H − CH3]− | Wogonin s | + | [23] |
| 179.0488 | 163.0036 | ||||||||
| 87 | 13.80 | C32H38O14 | 647.2323 (−1.7) | 501.1726 [M + H − H − Rha]+ | Sagittatoside B | + | [26] | ||
| 465.1557 [M + H − H − Rha − HCOOH]+ | |||||||||
| 409.0917, 355.1171 | |||||||||
| 299.0594 | |||||||||
| 88 | 13.81 | C16H12O5 | 285.0754 (−1.3) | 283.0615 (0.9) | 270.0514 [M + H − H − CH3]+ | 268.0380 [M − H − CH3]− | Oroxylin A s | + | [23] |
| 179.0488 | 163.0036 | ||||||||
| 89 | 13.82 | C32H38O14 | 647.2323 (−1.7) | 629.2323, 501.1726, | 2′′-O–Rhamnosyl-ikarisoside A | [26] | |||
| 465.1557, 355.1171, 299.0059 | |||||||||
| 90 | 13.9 | C33H40O14 | 661.2470 (−3.1) | 659.2374 (4.4) | 515.1897 [M + H − H − C6H10O4]+ | 366.1127 | 2′′-O-Rhamnosyl-ikariside II | + | [14] |
| 369.1333, 355.0809, 313.0703 | |||||||||
| 91 | 13.96 | C15H10O4 | 255.0642 (−3.9) | 253.0708 (0.7) | 153.0177 [M + H − H − C5H8O − H2O]+ | 209.0609 [M − H − CO2]− | Chrysin s | + | [23] |
| 103.0544 | 143.0505, 63.0276 | ||||||||
| 92 | 14.01 | C17H14O6 | 315.0856 (−2.3) | 313.0718 (0.2) | 300.0618, 285.0396 | 298.0485, 283.0252, 211.0396 | Kumatakenin | [29] | |
| 257.0440, 182.9919, 154.9964 | 155.0506 | ||||||||
| 93 | 14.5 | C24H32O7 | 433.2215 (−1.3) | 415.2106 [M + H − H2O]+ | Schisandrin s | + | [31] | ||
| 400.1869 [M + H − H2O − CH3]+ | |||||||||
| 384.1923 [M + H − H − OCH3 − H2O]+ | |||||||||
| 359.14907 [M + H − H2O − C4H8]+ | |||||||||
| 315.1223 | |||||||||
| 94 | 14.63 | C27H30O10 | 515.1901 (−2) | 513.1780 (2.7) | 369.1321 [M + H − H − Rha]+ | 367.1134 [M − H − Rha]− | Icarisid II s | + | [14] |
| 313.0700 [M + H − H − Rha − C4H8]+ | 351.0882 [M − H − O − Rha]−, 323.0912 | ||||||||
| 95 | 15.2 | C45H72O16 | 869.4881 (−1.4) | 689.4234, 437.3396 | Astragaloside I s | − | [10] | ||
| 217.0720, 157.0506, 143.1059 | |||||||||
| 96 | 15.5 | C23H28O7 | 417.1890 (−4.2) | 399.1815 [M + H − H2O]+ | Schisandrol B | − | [31] | ||
| 384.1501 [M + H − H2O − CH3]+ | |||||||||
| 368.1618 [M + H − H2O−OCH3]+ | |||||||||
| 357.1394 [M + H − H2O − C3H6]+ | |||||||||
| 343.1187 [M + H − H2O − C4H8]+ | |||||||||
| 97 | 16.02 | C30H48O5 | 489.3566 (−1.8) | 487.3434 (1) | 453.3324 [M + H − H − 2H2O]+ | 469.3361 [M − H − H2O]− | Tormentic acid | + | [15] |
| 407.3254 [M + H − HCOOH−2H2O]+ | 407.1533 | ||||||||
| 201.1702, 127.0767 | |||||||||
| 98 | 16.07 | C21H20O6 | 369.1323 (−2.6) | 313.0693 | Icaritin | − | [14] | ||
| 99 | 16.08 | C15H10O5 | 271.0596 (−2) | 269.0460 (1.5) | 225.0560, 241.0514, 197.0608, 182.0375 | Emodin s | + | [18] | |
| 100 | 17.88 | C23H30O6 | 403.2112 (−0.8) | 388.1848 [M + H − H − CH3]+, | Schisanhenol | − | [31] | ||
| 372.1919 [M + H − H − OCH3]+ | |||||||||
| 371.1859 [M + H − H − CH3OH]+ | |||||||||
| 333.1326, 302.1149 | |||||||||
| 101 | 19.09 | C28H34O9 | 515.2264 (−2.3) | 415.2009 [M + H − H − C4H6COOH]+ | Schisantherin B | − | [31] | ||
| 385.1637 [M + H − H − C4H6COOH − OCH2]+ | |||||||||
| 355.1534, 343.1160, 316.0939 | |||||||||
| 102 | 20.31 | C24H32O6 | 417.2259 (−3) | 402.1999 [M + H − H − CH3]+ | Schizandrin A s | − | [31] | ||
| 386.2059 [M + H − H − OCH3]+ | |||||||||
| 347.1480 [M + H − H − C5H10]+ | |||||||||
| 316.1289 [M + H − H − C5H10 − OCH3]+ | |||||||||
| 301.1058, 285.1104, 273.1091, 242.0911 | |||||||||
| 103 | 21.3 | C23H28O6 | 401.1948 (−2.6) | 386.1728 [M + H − H − CH3]+ | γ-Schizandrin B s | − | [31] | ||
| 331.1168 [M + H − H − C5H10]+ | |||||||||
| 300.0987, 242.0929 | |||||||||
| 104 | 21.87 | C22H24O6 | 385.1638 (−1.9) | 355.1561 [M + H − H − OCH2]+ | Schizandrin C | − | [31] | ||
| 315.0895 [M + H − H − C5H10]+ | |||||||||
| 285.0757 [M + H − H − C5H10−OCH2]+ | |||||||||
| 257.0809, 242.0588, 227.0703, 153.0663 | |||||||||
| 105 | 22.98 | C32H50O5 | 515.3723 (−1.6) | 513.3594 (1.7) | 409.3466, 191.1793 | 495.3496 [M − H − H2O]− | 19α−Hydroxyl−3−acetyl ursolic acid | − | [15] |
| 469.3720 [M − H − CO2]− | |||||||||
| 106 | 23.56 | C30H48O3 | 455.3537 (1.3) | Oleanic acid s | − | [15] | |||
| 107 | 23.73 | C30H48O3 | 455.3537 (1.3) | Ursolic acid | − | [15] | |||
| Batch | Baicalein | Paeonol | Wogonin | Emodin |
|---|---|---|---|---|
| 1 | 1893 ± 32 | 160 ± 2.2 | 542 ± 6.7 | 590 ± 7.2 |
| 2 | 17557.2 | 14557.2 | 48357.2 | 55757.2 |
| 3 | 19247.2 | 17547.2 | 56747.2 | 60247.2 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, F.; Chen, M.; Luo, C.; Chen, F.; Shen, Q.; Mo, Z. Developing an Absorption–Based Quality Control Method for Hu–Gan–Kang–Yuan Capsules by UFLC–QTOF–MS/MS Screening and HPLC–DAD Quantitative Determination. Molecules 2016, 21, 592. https://doi.org/10.3390/molecules21050592
Wei F, Chen M, Luo C, Chen F, Shen Q, Mo Z. Developing an Absorption–Based Quality Control Method for Hu–Gan–Kang–Yuan Capsules by UFLC–QTOF–MS/MS Screening and HPLC–DAD Quantitative Determination. Molecules. 2016; 21(5):592. https://doi.org/10.3390/molecules21050592
Chicago/Turabian StyleWei, Fenghuan, Minting Chen, Chaohua Luo, Feilong Chen, Qun Shen, and Zhixian Mo. 2016. "Developing an Absorption–Based Quality Control Method for Hu–Gan–Kang–Yuan Capsules by UFLC–QTOF–MS/MS Screening and HPLC–DAD Quantitative Determination" Molecules 21, no. 5: 592. https://doi.org/10.3390/molecules21050592
APA StyleWei, F., Chen, M., Luo, C., Chen, F., Shen, Q., & Mo, Z. (2016). Developing an Absorption–Based Quality Control Method for Hu–Gan–Kang–Yuan Capsules by UFLC–QTOF–MS/MS Screening and HPLC–DAD Quantitative Determination. Molecules, 21(5), 592. https://doi.org/10.3390/molecules21050592