Antitumor and Antibacterial Derivatives of Oridonin: A Main Composition of Dong-Ling-Cao
Abstract
:1. Introduction
2. Results and Discussions
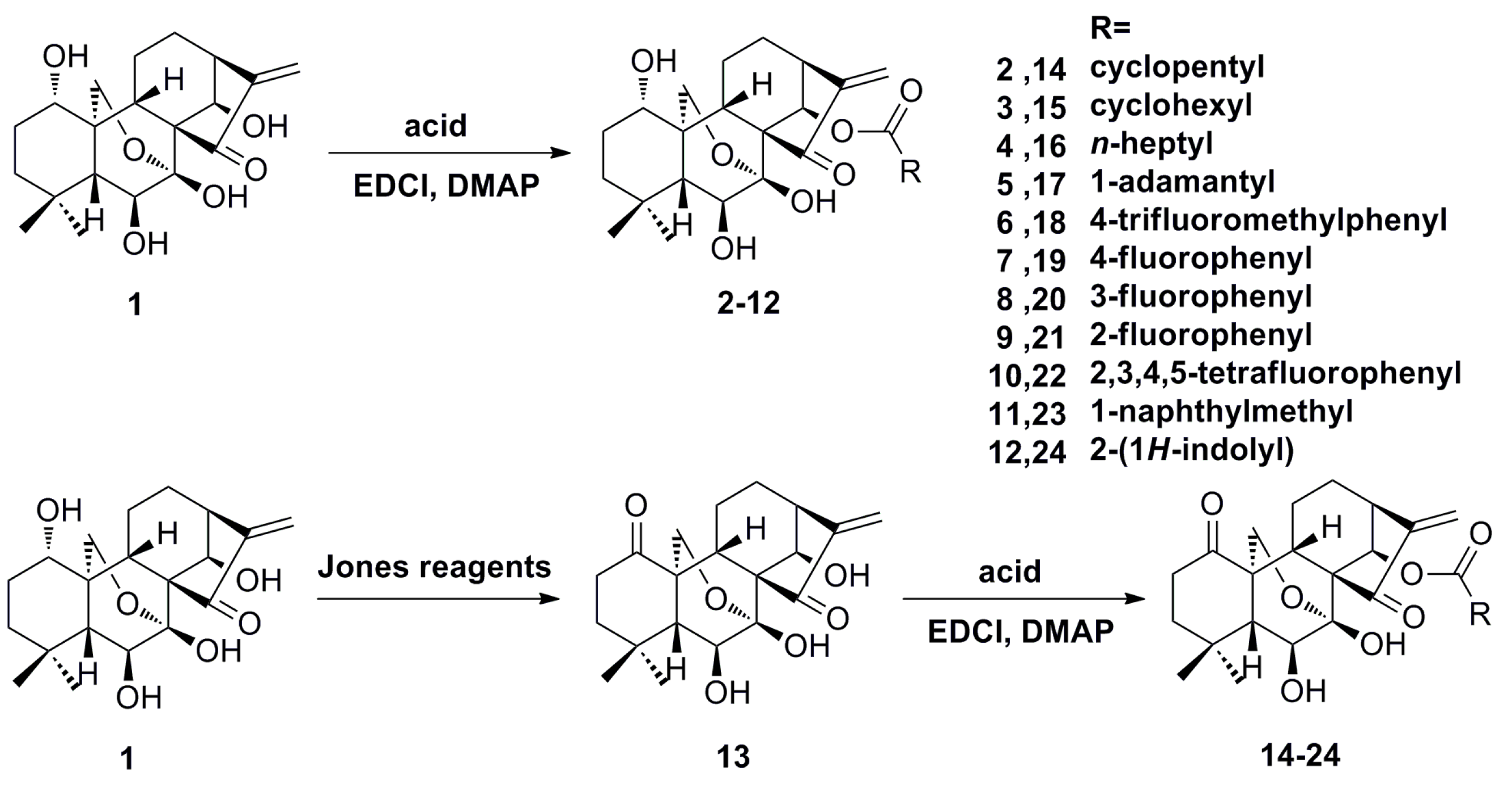
2.1. Synthesis of Compounds 2–12 and 14–24
2.2. Antimicrobial Activity
2.3. Antiproliferative Activity
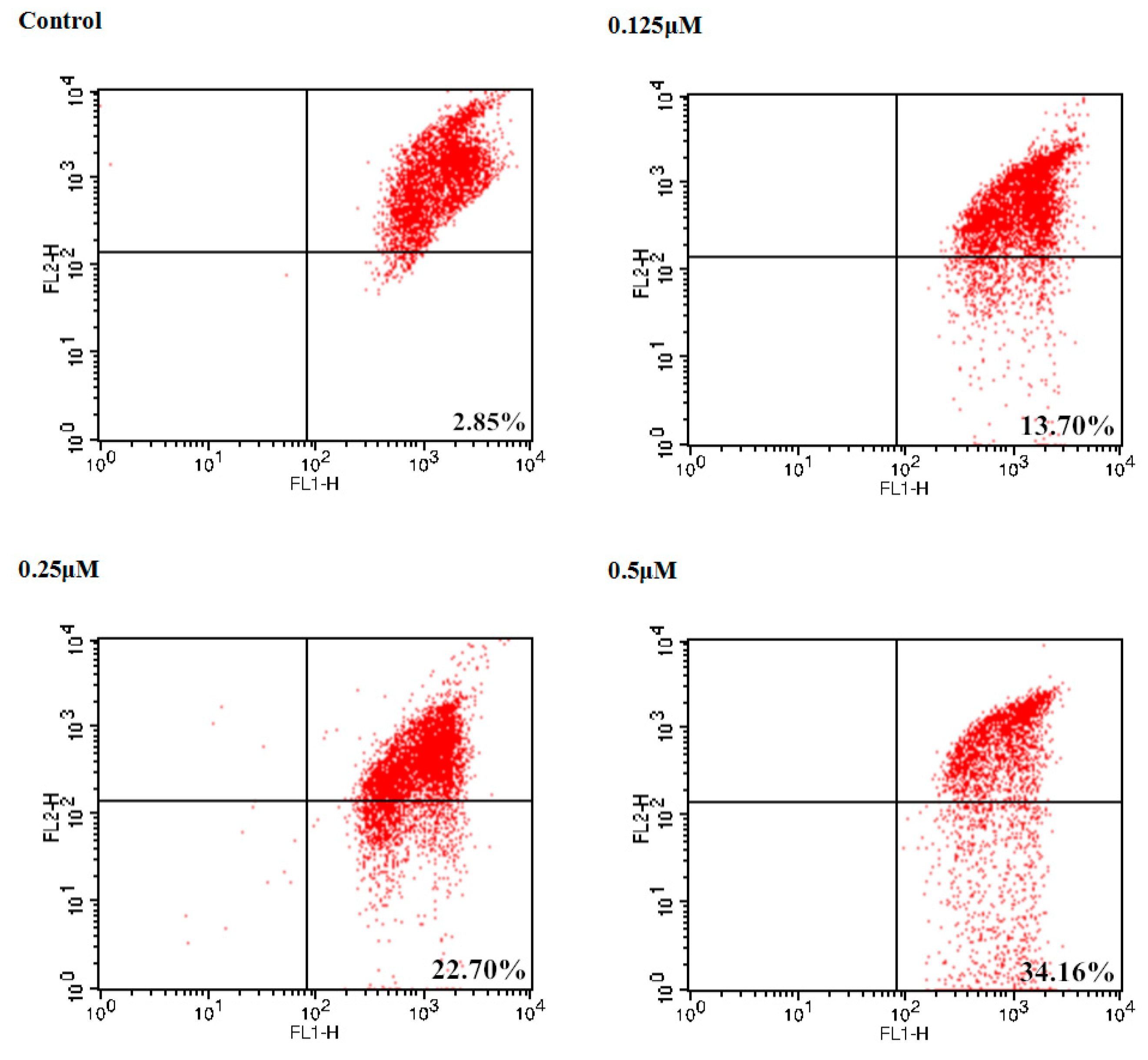
2.4. Apoptosis-Inducing Ability of 19 in CaEs-17 Cell Line
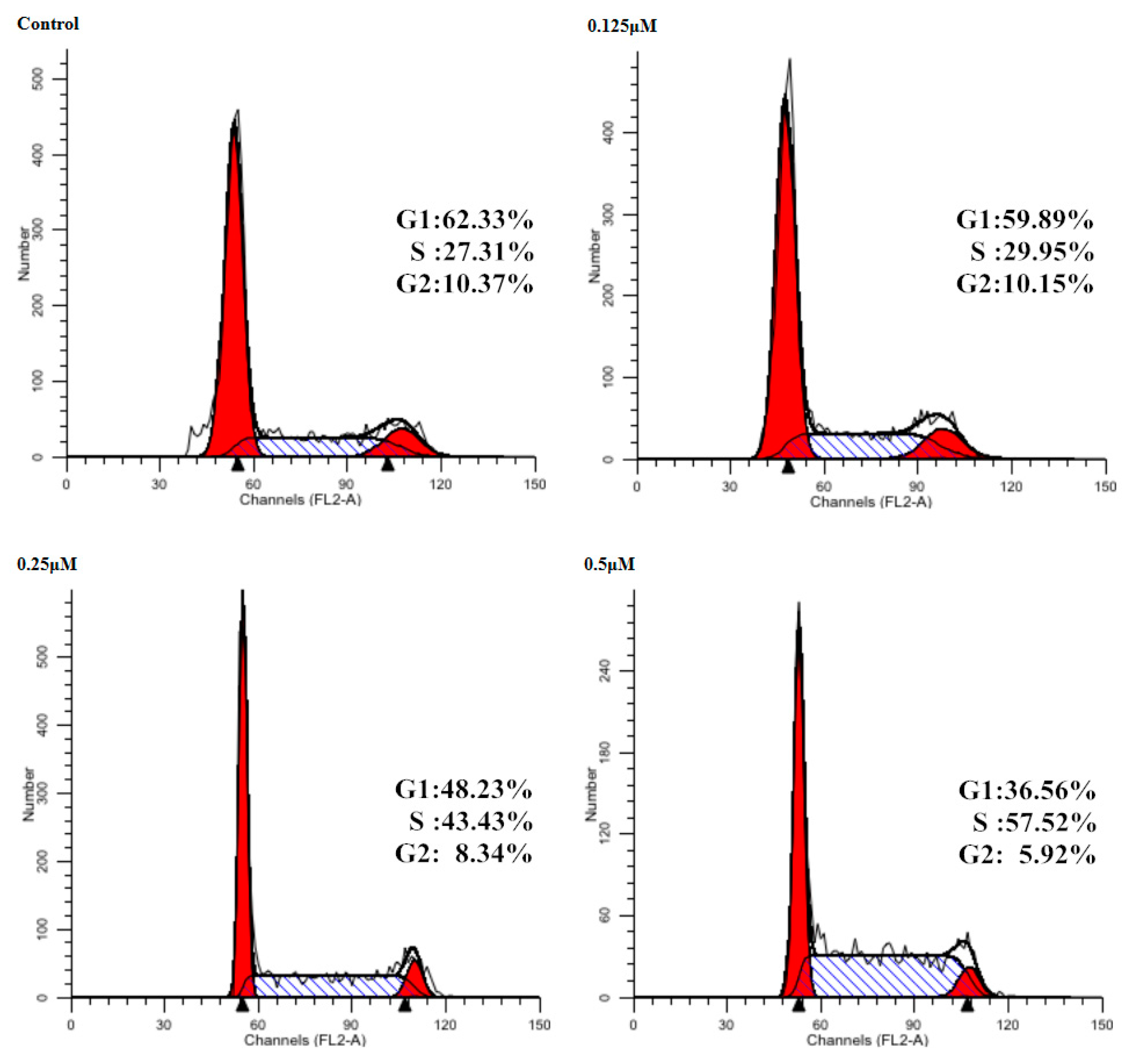
2.5. Cell Cycle Effect on CaEs-17 Cells by 19
2.6. Effect on Mitochondrial Membrane Potential
3. Materials and Methods
3.1. Chemistry
3.1.1. General
3.1.2. General Procedure to Synthesize 2–12 and 14–24
3.2. Biology
3.2.1. Antibacterial Assay
3.2.2. MTT Assay
3.2.3. Cell Apoptosis
3.2.4. Effect of Cell Cycle
3.2.5. Mitochondrial Membrane Potential
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SAR | Structure Activity Relationship |
| 1H-NMR | Proton Nuclear Magnetic Resonance |
| TMS | Tetramethlysilane |
| MS | Mass Spectrometry |
| ESI | Electrospray Ionization |
| DCM | Dichloromethane |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| FBS | Fetal Bovine Serum |
| MIC | Minimal Inhibitory Concentration |
| NT | Not Test |
References
- Liu, X.; Yang, J.; Wang, W.G.; Li, Y.; Wu, J.Z.; Pu, J.X.; Sun, H.D. Diterpene alkaloids with an aza-ent-kaurane skeleton from Isodon rubescens. J. Nat. Prod. 2015, 78, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhan, R.; Wang, W.G.; Du, X.; Li, X.N.; Yang, J.H.; Zhang, P.; Li, Y.; Pu, J.X.; Wu, J.Z.; et al. A new diterpene glycoside from Rabdosia rubescens. Chem. Pharm. Bull. 2013, 61, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, W.G.; Du, X.; Li, X.N.; Kong, L.M.; Li, Y.; Pu, J.X.; Wu, J.Z.; Sun, H.D. Enmein-type diterpenoids from the aerial parts of Isodon rubescens and their cytotoxicity. Fitoterapia 2012, 83, 1451–1455. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Pan, L.; Li, Q.; Pu, J.; Yao, P.; Zhu, M.; Banas, J.A.; Zhang, H.; Sun, H. Rubesanolides C-E: abietane diterpenoids isolated from Isodon rubescens and evaluation of their anti-biofilm activity. Org. Biomol. Chem. 2012, 10, 5039–5044. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Pan, L.; Li, Q.; Zhao, J.; Pu, J.; Yao, P.; Gong, N.; Lu, Y.; Kondratyuk, T.P.; Pezzuto, J.M.; et al. Rubesanolides A and B: Diterpenoids from Isodon rubescens. Org. Lett. 2011, 13, 1406–1409. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.X.; Xiao, W.L.; Li, L.M.; Li, S.H.; Zhou, Y.; Ding, L.S.; Lou, L.G.; Sun, H.D. Bisrubescensins A–C: Three new dimeric ent-kauranoids isolated from Isodon rubescens. Org. Lett. 2006, 8, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.M.; Yan, X.; Kiuchi, F.; Liu, Z. A new diterpene glycoside from Rabdosia rubescens. Chem. Pharm. Bull. 2000, 48, 148–149. [Google Scholar] [CrossRef] [PubMed]
- Li, B.L.; Chen, S.N.; Shi, Z.X.; Chen, Y.Z. ent-Kaurene diterpenoids from Isodon rubescens. Phytochemistry 2000, 53, 855–859. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, Y. Oridonin, a promising antitumor natural product in the chemotherapy of hematological malignancies. Curr. Pharm. Biotechnol. 2014, 15, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ouyang, L.; Peng, H.; Zhang, W.Z. Oridonin: Targeting programmed cell death pathways as an anti-tumour agent. Cell Prolif. 2012, 45, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Wang, E.Q.; Cheng, Y.; Bao, J.K. Oridonin: An active diterpenoid targeting cell cycle arrest, apoptotic and autophagic pathways for cancer therapeutics. Int. J. Biochem. Cell B. 2011, 43, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Pei, L.; Li, D.; Yao, H.; Cai, H.; Yao, H.; Wu, X.; Xu, J. Synthesis and antimycobacterial evaluation of natural oridonin and its enmein-type derivatives. Fitoterapia 2014, 99, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, D.; Pei, L.; Yao, H.; Wang, C.; Cai, H.; Yao, H.; Wu, X.; Xu, J. Design, synthesis and antimycobacterial activity evaluation of natural oridonin derivatives. Bioorg. Med. Chem. Lett. 2014, 24, 2811–2814. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, D.; Xu, S.; Cai, H.; Yao, H.; Zhang, Y.; Jiang, J.; Xu, J. The conversion of oridonin to spirolactone-type or enmein-type diterpenoid: synthesis and biological evaluation of ent-6,7-seco-oridonin derivatives as novel potential anticancer agents. Eur. J. Med. Chem. 2012, 52, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cai, H.; Jiang, B.; Liu, G.; Wang, Y.; Wang, L.; Yao, H.; Wu, X.; Sun, Y.; Xu, J. Synthesis of spirolactone-type diterpenoid derivatives from kaurene-type oridonin with improved antiproliferative effects and their apoptosis-inducing activity in human hepatoma Bel-7402 cells. Eur. J. Med. Chem. 2013, 59, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xu, S.; Cai, H.; Pei, L.; Wang, L.; Wu, X.; Yao, H.; Jiang, J.; Sun, Y.; Xu, J. Library construction and biological evaluation of enmein-type diterpenoid analogues as potential anticancer agents. Chem. Med. Chem. 2013, 8, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xu, S.; Cai, H.; Pei, L.; Zhang, H.; Wang, L.; Yao, H.; Wu, X.; Jiang, J.; Sun, Y.; et al. Enmein-type diterpenoid analogs from natural kaurene-type oridonin: Synthesis and their antitumor biological evaluation. Eur. J. Med. Chem. 2013, 64, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Fujita, E.; Nagao, Y.; Kohno, T.; Matsuda, M.; Ozaki, M. Antitumor activity of acylated oridonin. Chem. Pharm. Bull. 1981, 29, 3208–3213. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, M.B.; Valizadeh, H.; Asghari, B.; Dinparast, L.; Farimani, M.M.; Bahadori, S. Chemical composition and antimicrobial, cytotoxicity, antioxidant and enzyme inhibitory activities of Salvia spinosa L. J. Funct. Foods 2015, 18, 727–736. [Google Scholar] [CrossRef]
- Liao, J.; Yang, F.; Zhang, L.; Chai, X.; Zhao, Q.; Yu, S.; Zou, Y.; Meng, Q.; Wu, Q. Synthesis and biological evaluation of novel fluconazole analogues bearing 1,3,4-oxadiazole moiety as potent antifungal agents. Arch. Pharm. Res. 2015, 38, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Qiao, A.; Wang, Y.; Xiang, L.; Zhang, Z.; He, X. Novel triterpenoids isolated from hawthorn berries functioned as antioxidant and antiproliferative activities. J. Funct. Foods 2015, 13, 308–313. [Google Scholar] [CrossRef]
- Gurkan-Alp, A.S.; Göker, H.; Alp, M.; Ozkan, T.; Sunguroglu, A. Synthesis and anticancer effects of some novel 2-(4-phenoxyphenyl)-1H-benzimidazole derivatives on K562 cell line. Arch. Pharm. Res. 2015, 38, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yu, Y.; Ji, P.; He, H.; Qiao, C. Antitumor activity of endoperoxide-iron chelator conjugates-design, synthesis and biological evaluation. Eur. J. Med. Chem. 2015, 102, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.L.; Kuo, Y.M.; Lee, Y.R.; Yang, S.F.; Chen, W.R.; Lee, H.J. Apple polyphenol induces cell apoptosis, cell cycle arrest at G2/M phase, and mitotic catastrophe in human bladder transitional carcinoma cells. J. Funct. Foods 2015, 14, 384–394. [Google Scholar] [CrossRef]
- Lepiarczyk, M.; Kałuża, Z.; Bielawska, A.; Czarnomysy, R.; Gornowicz, A.; Bielawski, K. Cytotoxic activity of octahydropyrazin[2,1-a:5,4-a′]diisoquinoline derivatives in human breast cancer cells. Arch. Pharm. Res. 2015, 38, 628–641. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the target compounds are available from the authors.


| Compound | E. coli | S. aureus | B. subtilis | M. albicans | Clog p 2 |
|---|---|---|---|---|---|
| 1 | >100 | 31.2 | 31.2 | >100 | −1.70 |
| 2 | >100 | 62.5 | 31.2 | >100 | 0.65 |
| 3 | >100 | 31.2 | 15.6 | >100 | 1.21 |
| 4 | >100 | 15.6 | 3.9 | >100 | 2.35 |
| 5 | >100 | 15.6 | 7.8 | >100 | 1.84 |
| 6 | >100 | 31.2 | 15.6 | >100 | 1.65 |
| 7 | >100 | 31.2 | 15.6 | >100 | 0.91 |
| 8 | >100 | 31.2 | 15.6 | >100 | 0.91 |
| 9 | >100 | 15.6 | 15.6 | >100 | 0.47 |
| 10 | >100 | 62.5 | 31.2 | >100 | 0.76 |
| 11 | >100 | 15.6 | 7.8 | >100 | 1.96 |
| 12 | >100 | 3.9 | 2.0 | >100 | 1.17 |
| 13 | >100 | 62.5 | 31.2 | >100 | −0.06 |
| 14 | >100 | >100 | >100 | >100 | 1.70 |
| 15 | >100 | 62.5 | 31.2 | >100 | 2.26 |
| 16 | >100 | 15.6 | 7.8 | >100 | 3.40 |
| 17 | >100 | 15.6 | 15.6 | >100 | 2.89 |
| 18 | >100 | 62.5 | 15.6 | >100 | 2.70 |
| 19 | >100 | 31.2 | 15.6 | >100 | 1.96 |
| 20 | >100 | 31.2 | 15.6 | >100 | 1.96 |
| 21 | >100 | 31.2 | 15.6 | >100 | 1.52 |
| 22 | >100 | >100 | 62.5 | >100 | 1.81 |
| 23 | >100 | 31.2 | 7.8 | >100 | 3.01 |
| 24 | >100 | 3.9 | 3.9 | >100 | 2.22 |
| chloromycetin | 3.9 | 3.9 | 7.8 | NT 1 | NT |
| fluconazole | NT | NT | NT | 3.91 | NT |
| Compound | Bel-7402 | K562 | MGC-803 | CaEs-17 |
|---|---|---|---|---|
| 1 | 7.48 ± 0.53 | 4.76 ± 0.32 | 5.69 ± 0.39 | 11.03 ± 1.02 |
| 2 | 1.18 ± 0.03 | 2.03 ± 0.17 | 1.18 ± 0.21 | 3.36 ± 0.22 |
| 3 | 1.01 ± 0.04 | 1.97 ± 0.18 | 1.12 ± 0.10 | 3.25 ± 0.31 |
| 4 | 0.96 ± 0.06 | 1.83 ± 0.23 | 1.08 ± 0.06 | 3.20 ± 0.29 |
| 5 | 0.97 ± 0.11 | 1.84 ± 0.36 | 1.14 ± 0.23 | 3.16 ± 0.37 |
| 6 | 1.63 ± 0.81 | 0.25 ± 0.02 | 0.81 ± 0.10 | 0.61 ± 0.13 |
| 7 | 1.07 ± 0.52 | 0.31 ± 0.04 | 0.37 ± 0.04 | 0.43 ± 0.10 |
| 8 | 1.13 ± 0.14 | 0.37 ± 0.07 | 0.61 ± 0.01 | 0.28 ± 0.22 |
| 9 | 1.39 ± 0.72 | 0.59 ± 0.19 | 1.03 ± 0.55 | 0.29 ± 0.04 |
| 10 | 3.80 ± 0.92 | 2.66 ± 0.13 | 4.02 ± 0.75 | 7.23 ± 1.03 |
| 11 | 0.90 ± 0.02 | 1.87 ± 0.07 | 1.37 ± 0.09 | 3.92 ± 0.36 |
| 12 | 0.82 ± 0.22 | 1.74 ± 0.23 | 1.12 ± 0.17 | 3.63 ± 0.29 |
| 13 | 2.98 ± 0.14 | 4.34 ± 0.04 | 3.98 ± 0.66 | 7.23 ± 0.73 |
| 14 | 0.98 ± 0.06 | 1.77 ± 0.13 | 1.31 ± 0.14 | 2.72 ± 0.30 |
| 15 | 0.93 ± 0.11 | 1.76 ± 0.24 | 1.08 ± 0.09 | 2.56 ± 0.25 |
| 16 | 0.95 ± 0.07 | 1.81 ± 0.24 | 1.09 ± 0.13 | 3.13 ± 0.22 |
| 17 | 0.99 ± 0.10 | 1.91 ± 0.45 | 1.17 ± 0.15 | 3.46 ± 0.44 |
| 18 | 2.81 ± 0.41 | 0.86 ± 0.03 | 1.02 ± 0.38 | 0.89 ± 0.30 |
| 19 | 0.98 ± 0.05 | 0.29 ± 0.05 | 0.60 ± 0.40 | 0.22 ± 0.09 |
| 20 | 1.66 ± 0.39 | 0.35 ± 0.06 | 0.87 ± 0.05 | 0.64 ± 0.11 |
| 21 | 1.45 ± 0.42 | 0.47 ± 0.01 | 0.90 ± 0.22 | 0.51 ± 0.03 |
| 22 | 3.17 ± 0.65 | 2.16 ± 0.37 | 3.94 ± 0.71 | 8.55 ± 0.80 |
| 23 | 1.07 ± 0.13 | 1.72 ± 0.24 | 1.25 ± 0.16 | 3.62 ± 0.18 |
| 24 | 0.81 ± 0.08 | 1.66 ± 0.26 | 1.09 ± 0.24 | 3.57 ± 0.16 |
| Taxol 1 | 1.89 ± 0.09 | 0.41 ± 0.02 | 0.85 ± 0.06 | 0.43 ± 0.03 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Han, T.; Xu, S.; Zhou, T.; Tian, K.; Hu, X.; Cheng, K.; Li, Z.; Hua, H.; Xu, J. Antitumor and Antibacterial Derivatives of Oridonin: A Main Composition of Dong-Ling-Cao. Molecules 2016, 21, 575. https://doi.org/10.3390/molecules21050575
Li D, Han T, Xu S, Zhou T, Tian K, Hu X, Cheng K, Li Z, Hua H, Xu J. Antitumor and Antibacterial Derivatives of Oridonin: A Main Composition of Dong-Ling-Cao. Molecules. 2016; 21(5):575. https://doi.org/10.3390/molecules21050575
Chicago/Turabian StyleLi, Dahong, Tong Han, Shengtao Xu, Tingting Zhou, Kangtao Tian, Xu Hu, Keguang Cheng, Zhanlin Li, Huiming Hua, and Jinyi Xu. 2016. "Antitumor and Antibacterial Derivatives of Oridonin: A Main Composition of Dong-Ling-Cao" Molecules 21, no. 5: 575. https://doi.org/10.3390/molecules21050575
APA StyleLi, D., Han, T., Xu, S., Zhou, T., Tian, K., Hu, X., Cheng, K., Li, Z., Hua, H., & Xu, J. (2016). Antitumor and Antibacterial Derivatives of Oridonin: A Main Composition of Dong-Ling-Cao. Molecules, 21(5), 575. https://doi.org/10.3390/molecules21050575