Neuroprotective and Cytotoxic Phthalides from Angelicae Sinensis Radix
Abstract
:1. Introduction
2. Results and Discussion
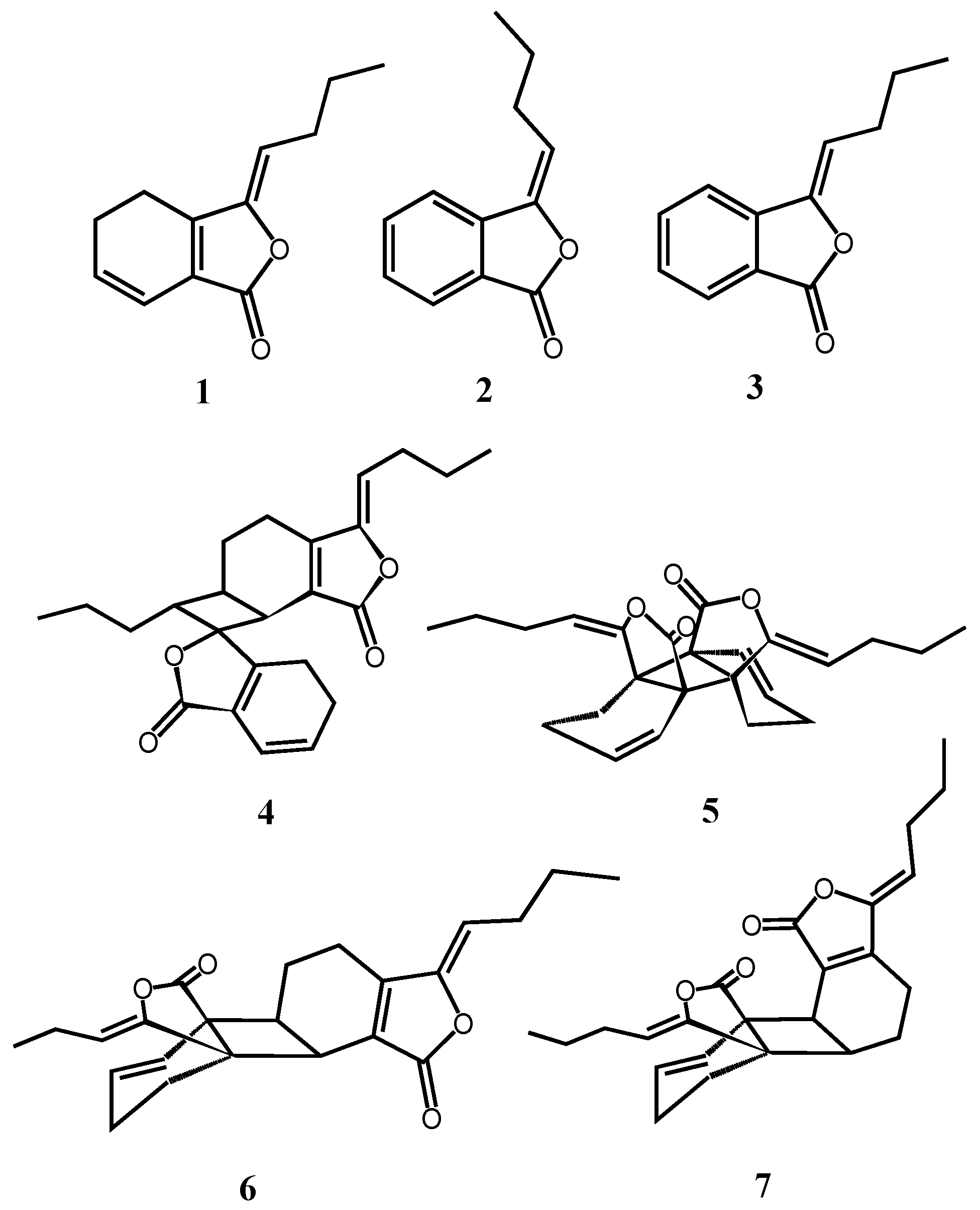
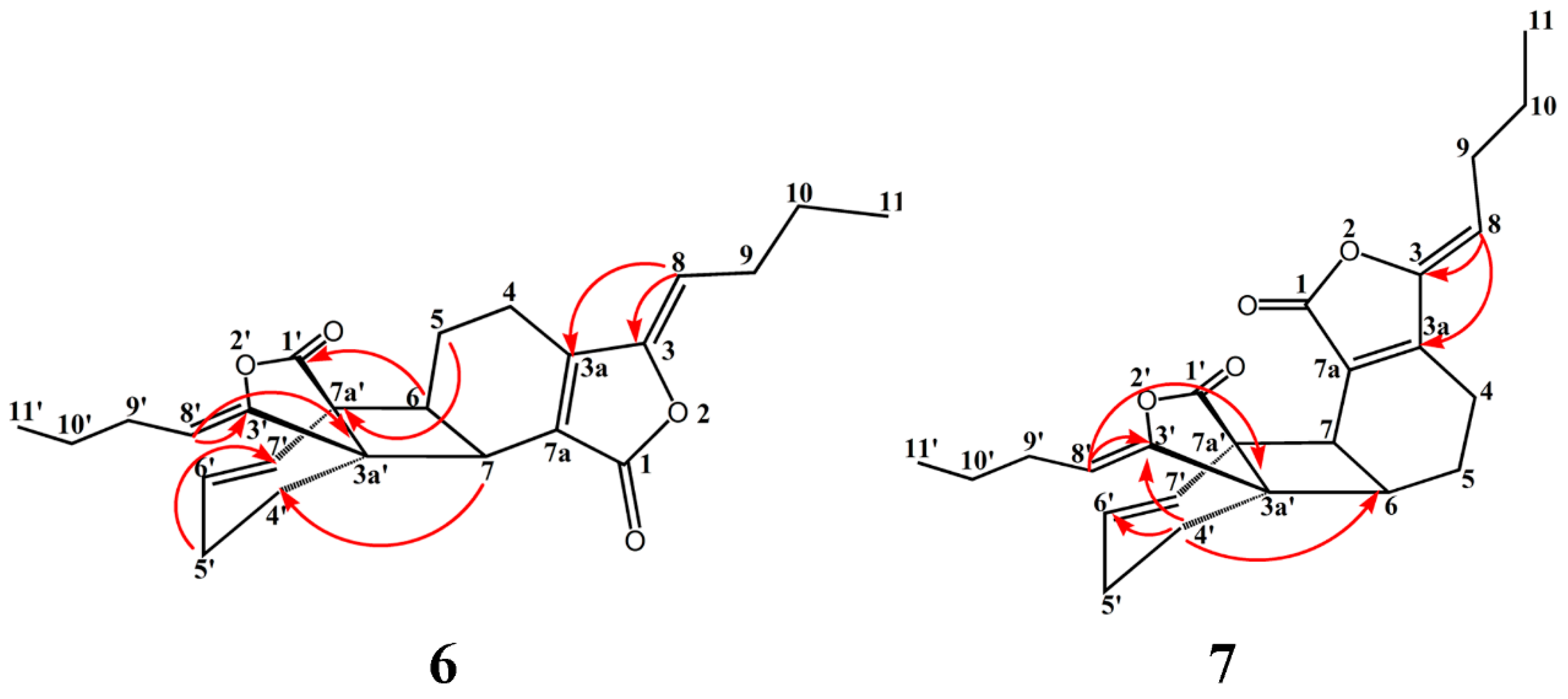
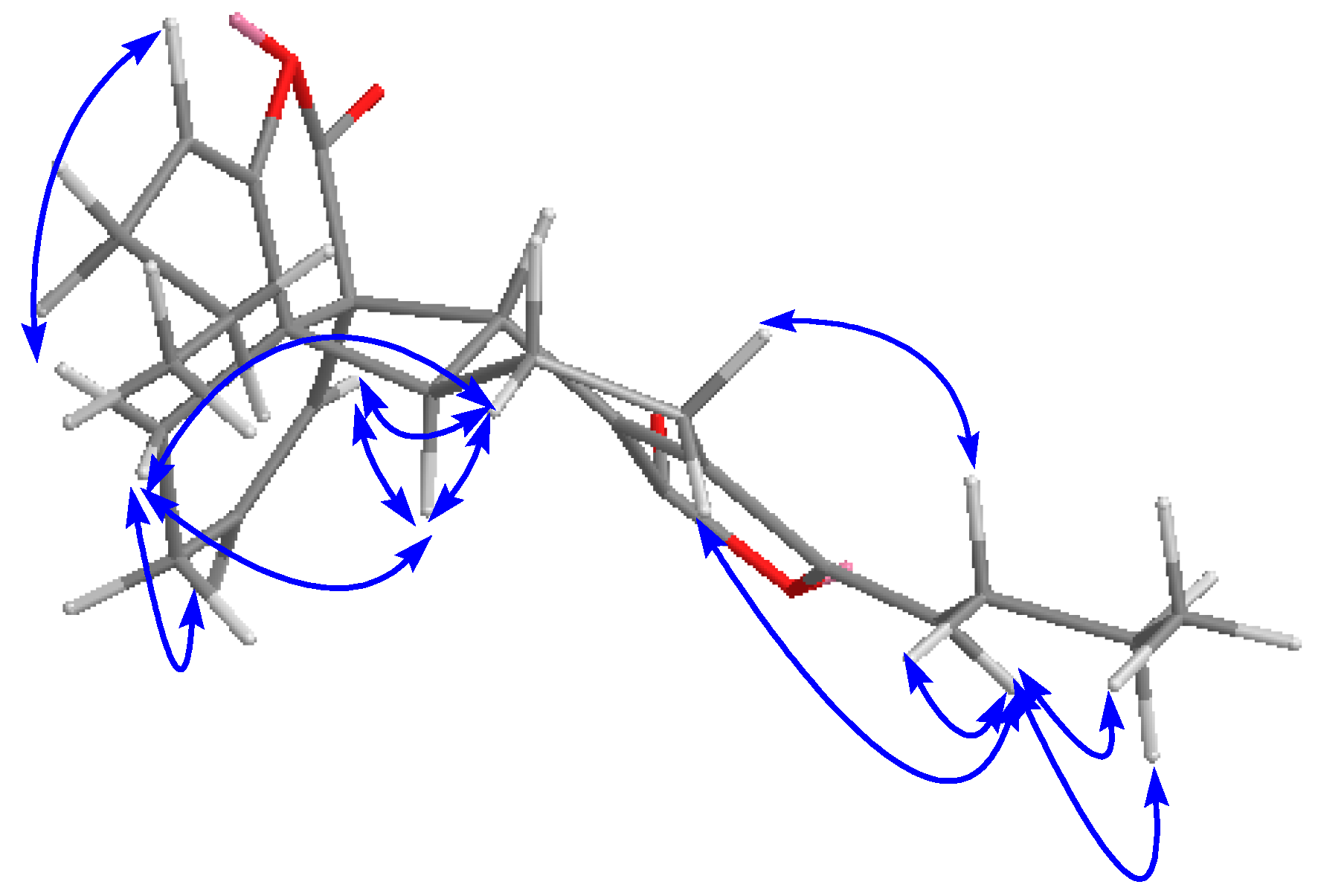
2.1. Isolation, Identification, and Structure Elucidation
2.2. Cytotoxicity Assay
2.2.1. Cytotoxic Activity of Compounds
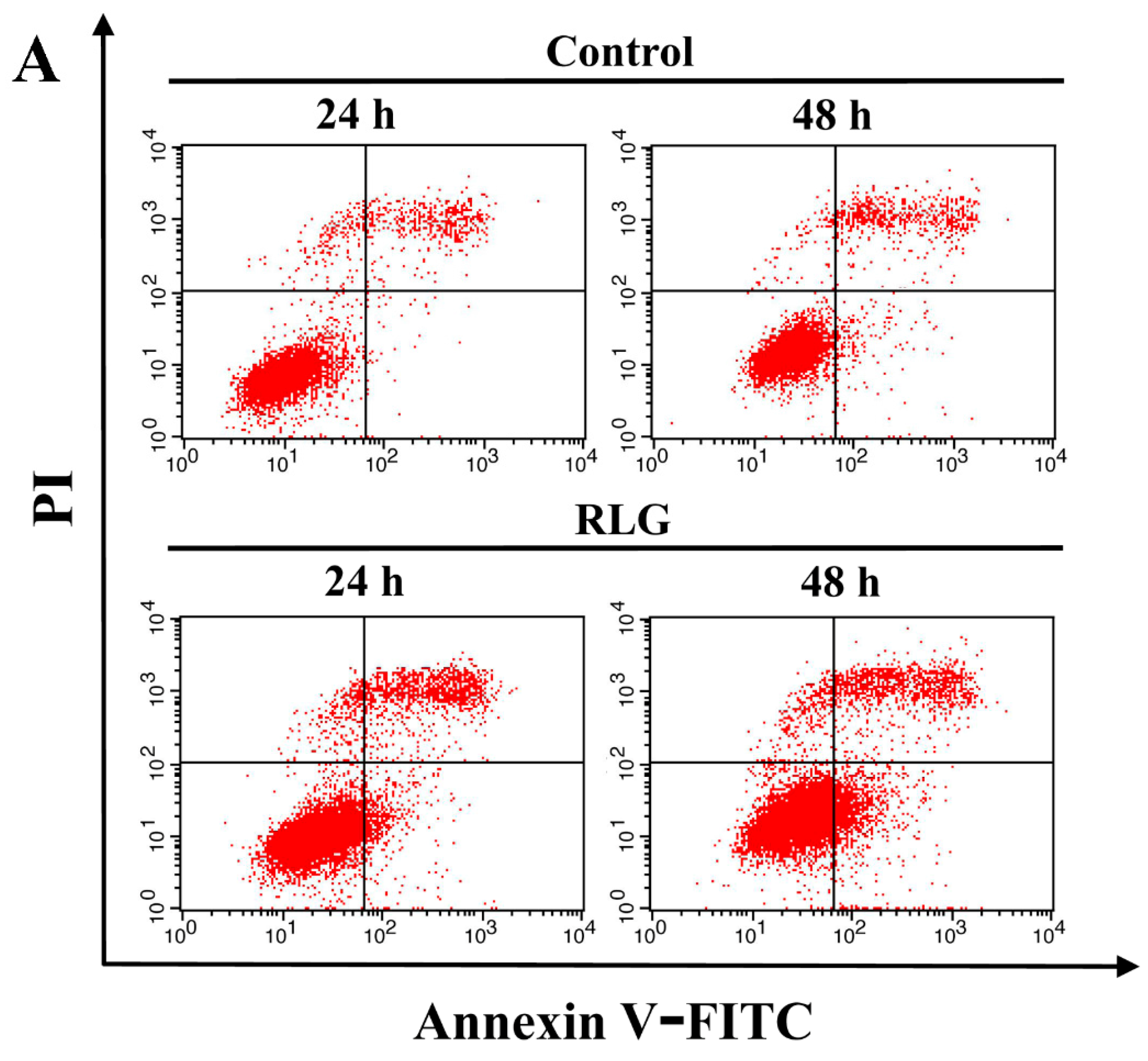
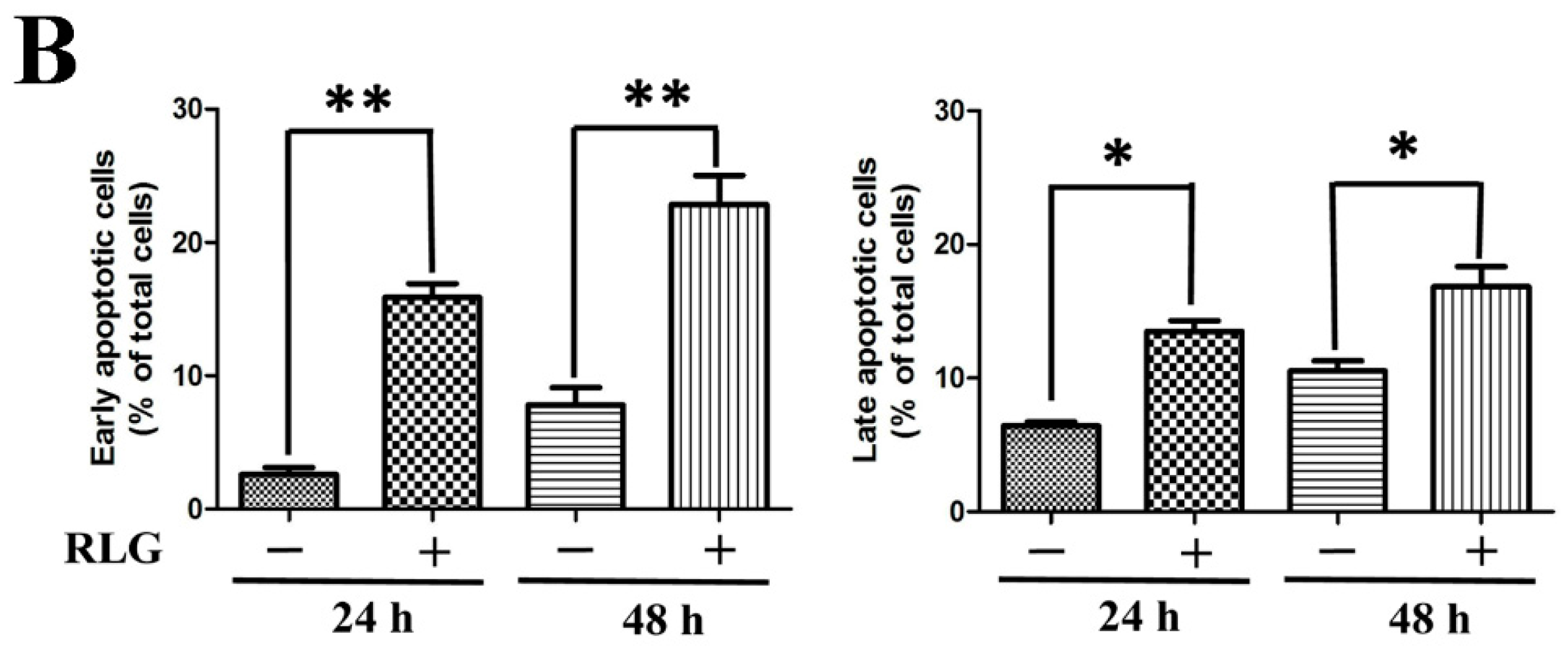
2.2.2. Apoptosis-Inducing Activity
2.2.3. Induction of Cell Cycle Arrest in G1 and S Phase
2.3. Neuroprotective Effect of Compounds
3. Experimental Section
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Product Characterization
3.5. Cytotoxicity Assay
3.5.1. Cell Line Cultures
3.5.2. Cell Viability Assay
3.5.3. Cell Apoptosis Assay
3.5.4. Cell Cycle Assay
3.6. Neuroprotective Effects Assay
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ma, J.P.; Guo, Z.B.; Jin, L.; Li, Y.D. Phytochemical progress made in investigations of Angelica sinensis (Oliv.) Diels. Chin. J. Nat. Med. 2015, 13, 241–249. [Google Scholar] [CrossRef]
- Chen, X.P.; Li, W.; Xiao, X.F.; Zhang, L.L.; Liu, C.X. Phytochemical and pharmacological studies on Radix Angelica Sinensis. Chin. J. Nat. Med. 2013, 11, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Winnie, L.T.; Chi, H.C.; John, A.R.; Ge, L. Study of the anti-proliferative effects and synergy of phthalides from Angelica sinensis on colon cancer cells. J. Ethnopharmacol. 2008, 120, 36–43. [Google Scholar]
- Lee, T.F.; Lin, Y.L.; Huang, Y.T. Studies on antiproliferative effects of phthalides from Ligusticum chuanxiong in hepatic stellate cells. Planta Med. 2007, 73, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.W.; Choi, R.J.; Seo, E.K.; Nam, J.W.; Dong, M.S.; Shin, E.M.; Guo, L.Y.; Kim, Y.S. Anti-inflammatory effects of (Z)-Ligustilide through suppression of mitogen-activated protein kinases and nuclear factor-κB activation pathways. Arch. Pharm. Res. 2012, 35, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.H.; Chen, I.S.; Hwang, T.L.; Wang, T.C.; Lee, T.H.; Cheng, L.Y.; Chang, Y.C.; Cho, J.Y.; Chen, J.J. Phthalides from Pittosporum illicioides var. illicioides with inhibitory activity on superoxide generation and elastase release by neutrophils. J. Nat. Prod. 2008, 71, 1692–1695. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ning, Z.Q.; Shan, S.; Zhang, K.; Deng, T.; Lu, X.P.; Cheng, Y.Y. Phthalide lactones from Ligusticum chuanxiong inhibit lipopolysaccharide-induced TNF-α production and TNF-α-mediated NF-κB activation. Planta Med. 2005, 71, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Du, J.R.; Wang, J.; Yu, D.K.; Chen, Y.S.; He, Y.; Wang, C.Y. Z-ligustilide extracted from Radix Angelica Sinensis decreased platelet aggregation induced by ADP ex vivo and arterio-venous shunt thrombosis in vivo in rats. Yakugaku Zasshi 2009, 129, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Yang, J.B.; Ren, J.; Wang, A.G.; Ji, T.F.; Su, Y.L. Bioactive phthalides from Ligusticum sinense Oliv cv. Chaxiong. Fitoterapia 2014, 93, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.Y.; Han, Y.F.; Rong, J.H. Potential roles of PI3K/Akt and Nrf2-Keap1 pathways in regulating hormesis of Z-ligustilide in PC12 cells against oxygen and glucose deprivation. Neuropharmacology 2012, 62, 1659–1670. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Zhao, P.; Lu, Y.P.; Chen, M.M.; Sun, H.; Wu, X.M.; Zhu, L. Z-ligustilide activates the Nrf2/HO-1 pathway and protects against cerebral ischemia-reperfusion injury in vivo and in vitro. Brain Res. 2013, 1520, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.Y.; Sheu, J.J.; Lin, P.C.; Lin, C.T.; Liu, Y.J.; Ho, L.I.; Chang, L.F.; Wu, W.C.; Chen, S.R.; Chen, J.; et al. Expression of Nur77 induced by an n-butylidenephthalide derivative promotes apoptosis and inhibits cell growth in oral squamous cell carcinoma. Investig. New Drugs 2012, 30, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.C.; Chen, S.P.; Huang, S.Y.; Wang, M.J.; Lin, S.Z.; Harn, H.J.; Pang, C.Y. Induction of apoptosis coupled to endoplasmic reticulum stress in human prostate cancer cells by N-butylidenephthalide. PLoS ONE 2012, 7, e33742. [Google Scholar] [CrossRef] [PubMed]
- Dumont, R.J.; Okonkwo, D.O.; Verma, S.; Hurlbert, R.J.; Boulos, P.T.; Ellegala, D.B.; Dumont, A.S. Acute spinal cord injury, part I: Pathophysiologic mechanisms. Clin. Neuropharmacol. 2001, 24, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Lankiewicz, S.; Marc, L.C.; Truc, B.N.; Krohn, A.J.; Poppe, M.; Cole, G.M.; Saido, T.C.; Prehn, J.H. Activation of calpain I converts excitotoxic neuron death into a caspase-independent cell death. J. Biol. Chem. 2000, 275, 17064–17071. [Google Scholar] [CrossRef] [PubMed]
- León, A.; Chávez, M.I.; Delgado, G. 1H and DOSY NMR spectroscopy analysis of Ligusticum porteri rhizome extracts. Magn. Reson. Chem. 2011, 49, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Roscini, C.; Davies, D.M.; Berry, M.; Orr-Ewing, A.J.; Booker-Milburn, K.I. Product selection through photon flux: Laser-specific lactone synthesis. Angew. Chem. Int. Ed. Engl. 2008, 47, 2283–2286. [Google Scholar] [CrossRef] [PubMed]
- Kaouadji, M.; Pachtere, F.D.; Pouget, C.; Chulia, A.J.; Lavaitte, S. Three additional phthalide derivatives, an epoxymonomer and two dimers, from Ligusticum wallichii rhizomes. J. Nat. Prod. 1986, 49, 872–877. [Google Scholar] [CrossRef]
- Li, W.; Wu, Y.; Liu, X.; Yan, C.; Liu, D.; Pan, Y.; Yang, G.; Yin, F.; Weng, Z.; Zhao, D.; et al. Antioxidant properties of cis-Z,Z′-3a.7a′,7a.3a′-dihydroxy ligustilide on human umbilical vein endothelial cells in vitro. Molecules 2013, 18, 520–534. [Google Scholar] [CrossRef] [PubMed]
- Takashi, T.; Masaru, K.; Ko, K.; Hiroshi, M. Studies on the constituents of Umbelliferae plants. XVI. Isolation and structures of three new ligustilide derivatives from Angelica acutiloba. Chem. Pharm. Bull. 1987, 35, 4460–4464. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Nampoothiri, M.; Reddy, N.D.; John, J.; Kumar, N.; Kutty, N.G.; Rao, C.M. Insulin blocks glutamate-induced neurotoxicity in differentiated SH-SY5Y neuronal cells. Behav. Neurol. 2014, 2014, 674164. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1–7 are available from the authors.

| Position | δc, Type | δH, (J in Hz) | HMBC | Position | δc, Type | δH, (J in Hz) | HMBC |
|---|---|---|---|---|---|---|---|
| 1 | 169.5, qC | 1′ | 175.4, qC | ||||
| 3 | 149.0, qC | 3′ | 151.4, qC | ||||
| 3a | 151.4, qC | 3a′ | 48.2, qC | ||||
| 4 | 17.8, CH2 | 2.56 m, 2.40 m | 5, 3a, 7a | 4′ | 29.7, CH2 | 1.94 m, 2.03 m | 5′, 7, 3a′, 7a′ |
| 5 | 20.1, CH2 | 1.70 m, 2.12 m | 4, 6, 7, 7a′ | 5′ | 21.0, CH2 | 2.10 m | 7′, 3a′, 4′, 6′ |
| 6 | 40.2, CH | 2.90 m | 4, 5, 7, 1′,7a′ | 6′ | 131.1, CH | 6.11 m | 4′, 5′, 7a′ |
| 7 | 34.1, CH | 3.23 d (9.4) | 4′, 6, 3a, 7a, 3a′ | 7′ | 124.6, CH | 6.00 d (9.8) | 5′, 3a′, 7a′ |
| 7a | 125.3, qC | 7a′ | 46.4, qC | ||||
| 8 | 112.1, CH | 5.21 t (7.9) | 3, 3a, 10 | 8′ | 106.9, CH | 4.66 dd (7.1, 8.3) | 3′,3a′, 10′ |
| 9 | 27.9, CH2 | 2.36 m | 3, 8, 10, 11 | 9′ | 27.2, CH2 | 2.08 m, 1.90 m | 3′, 8′, 10′, 11′ |
| 10 | 22.4, CH2 | 1.49 m | 8, 9, 11 | 10′ | 22.5, CH2 | 1.25 m | 8′, 9′,11′ |
| 11 | 13.7, CH3 | 0.95 t (7.4) | 9, 10 | 11′ | 13.5, CH3 | 0.80, (7.4) | 9′,10′ |
| Position | δc, Type | δH, (J in Hz) | HMBC (H → C) | NOESY |
|---|---|---|---|---|
| 1 | 168.7, qC | |||
| 3 | 148.9, qC | |||
| 3a | 153.5, qC | |||
| 4 | 19.0, CH2 | 2.21 m, 2.57 m | 3, 5, 6, 3a, 7a | 8, 9 |
| 5 | 21.9, CH2 | 1.98 m | 6, 3a | 6, 4′ |
| 6 | 37.1, CH | 2.99 dt (10.4, 8.5) | 5, 7, 3a′ | 5, 4′, 7′ |
| 7 | 37.0, CH | 3.39 d (8.5) | 6, 3a, 7a, 7a′ | |
| 7a | 124.5, qC | |||
| 8 | 112.2, CH | 5.24 t (7.9) | 3, 3a, 10 | 4, 9, 10, 11 |
| 9 | 27.2, CH2 | 2.37 m | 3, 8, 10, 11 | 4, 8, 10, 11 |
| 10 | 22.34, CH2 | 1.51 m | 8, 9, 11 | 8, 9, 11 |
| 11 | 13.56, CH3 | 0.96 t (7.4) | 9, 10 | 8, 9, 10 |
| 1′ | 176.9, qC | |||
| 3′ | 153.4, qC | |||
| 3a′ | 48.5, qC | |||
| 4′ | 24.1, CH2 | 1.77 m, 1.88 m | 6, 3′, 6′, 7a′ | 6′, 7′, 8′, 6, 5 |
| 5′ | 20.6, CH2 | 1.86 m | 3a′, 4′, 6′ | 8′ |
| 6′ | 133.8, CH | 6.11 ddd (9.9) | 4′, 5′, 7a′ | 4′, 7′ |
| 7′ | 121.0, CH | 5.77 dd (1.7, 9.9) | 4′, 3a′ | 4′, 6, 5 |
| 7a′ | 48.3, qC | |||
| 8′ | 103.9, CH | 4.88 t (7.9) | 3′, 3a′, 10′ | 4′, 9′, 10′, 11′ |
| 9′ | 28.0, CH2 | 2.18 m | 3′, 8′, 10′,11′ | 8′, 10′, 11′ |
| 10′ | 22.6, CH2 | 1.44 m | 8′, 9′, 11′ | 8′, 9′, 11′ |
| 11′ | 13.7, CH3 | 0.92 t (7.4) | 9′, 10′ | 8′, 9′, 10′ |
| Compound | IC50 (μM) a | ||
|---|---|---|---|
| A549 | HCT-8 | HepG2 | |
| 1 | >80 | >80 | >80 |
| 2 | >80 | >80 | >80 |
| 3 | >80 | >80 | >80 |
| 4 | 13.82 ± 2.23 | 6.79 ± 1.14 | 7.92 ± 1.38 |
| 5 | >80 | >80 | >80 |
| 6 | 47.63 ± 4.51 | 55.84 ± 5.99 | 30.92 ± 2.36 |
| 7 | 34.34 ± 3.80 | 27.79 ± 3.42 | 32.54 ± 2.69 |
| Doxorubicin b | 0.28 ± 0.05 | 1.55 ± 0.45 | 0.65 ± 0.11 |
| Group | Cell Survival Rate (% of Control) | Inhibition (% of Model) |
|---|---|---|
| Control | 100 ± 9.4 | |
| Model | 60.2 ± 2.3 ## | |
| MK-801 b | 72.8 ± 5.9 ** | 31.7 |
| 1 | 71.0 ± 4.1 ** | 27.2 |
| 2 | 63.6 ± 4.9 | 8.3 |
| 3 | 67.0 ± 2.1 * | 17.0 |
| 4 | 50.7 ± 7.0 | −24.0 |
| 5 | 59.3 ± 4.2 | −2.4 |
| 6 | 69.0 ± 7.6 * | 22.2 |
| 7 | 62.5 ± 2.0 | 5.6 |
| Group | Cell Survival Rate (% of Control) | Group | Cell Survival Rate (% of Control) |
|---|---|---|---|
| Control | 100.0 ± 10.5 | 4 | 57.4 ± 4.9 ** |
| 1 | 99.6 ± 5.9 | 5 | 97.9 ± 5.2 |
| 2 | 96.6 ± 2.3 | 6 | 90.9 ± 14.8 |
| 3 | 100.8 ± 13.8 | 7 | 93.0 ± 5.4 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, W.; Zhou, Y.; Li, X.; Gao, X.; Tian, J.; Qin, X.; Du, G. Neuroprotective and Cytotoxic Phthalides from Angelicae Sinensis Radix. Molecules 2016, 21, 549. https://doi.org/10.3390/molecules21050549
Gong W, Zhou Y, Li X, Gao X, Tian J, Qin X, Du G. Neuroprotective and Cytotoxic Phthalides from Angelicae Sinensis Radix. Molecules. 2016; 21(5):549. https://doi.org/10.3390/molecules21050549
Chicago/Turabian StyleGong, Wenxia, Yuzhi Zhou, Xiao Li, Xiaoxia Gao, Junsheng Tian, Xuemei Qin, and Guanhua Du. 2016. "Neuroprotective and Cytotoxic Phthalides from Angelicae Sinensis Radix" Molecules 21, no. 5: 549. https://doi.org/10.3390/molecules21050549