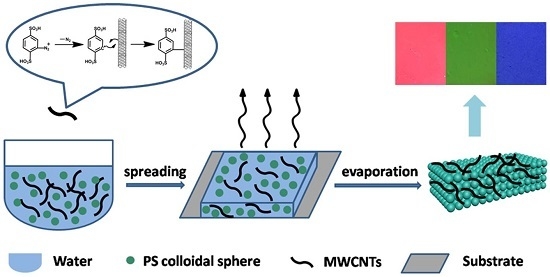
Hydrophilic Modification of Multi-Walled Carbon Nanotube for Building Photonic Crystals with Enhanced Color Visibility and Mechanical Strength
Abstract
:1. Introduction
2. Results and Discussion
2.1. Dispersion Behavior of Modified MWCNTs
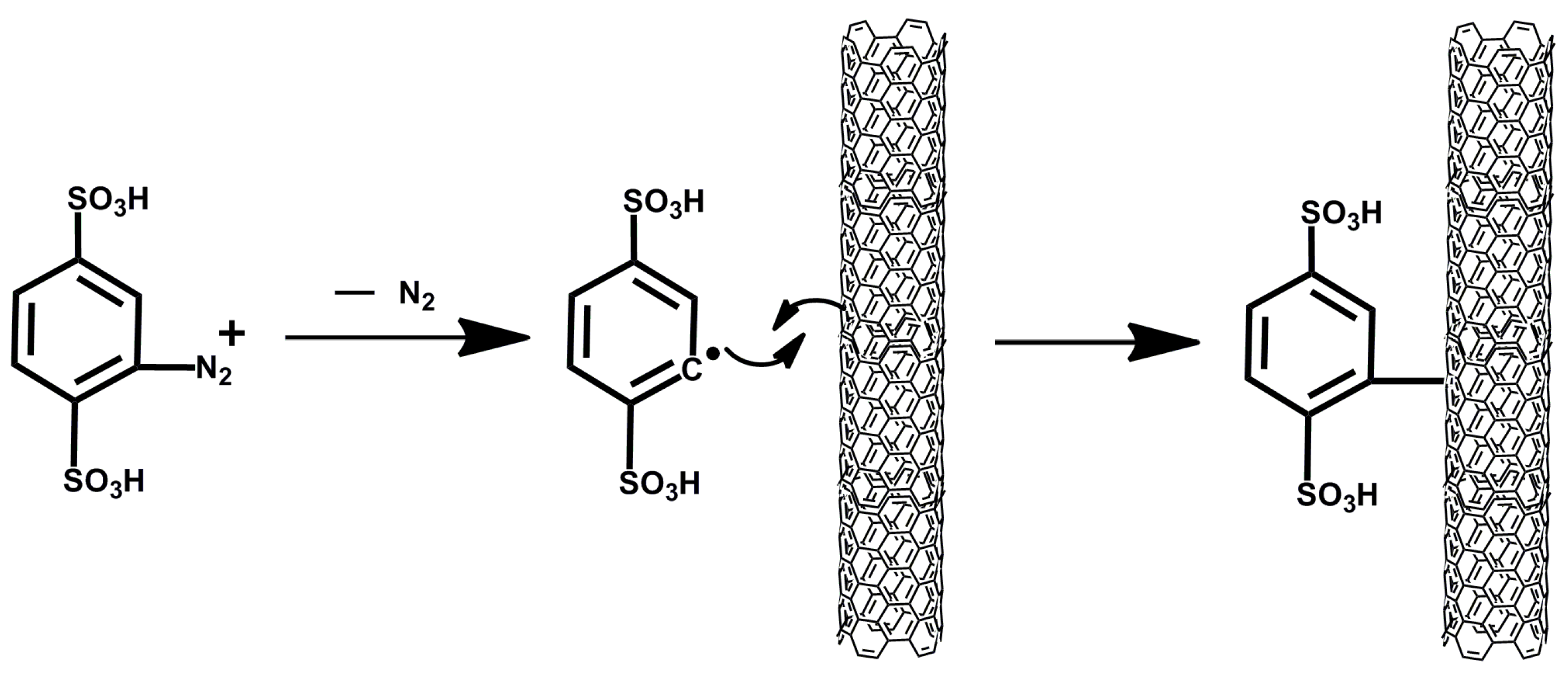
2.2. Characterization of the Modified MWCNTs
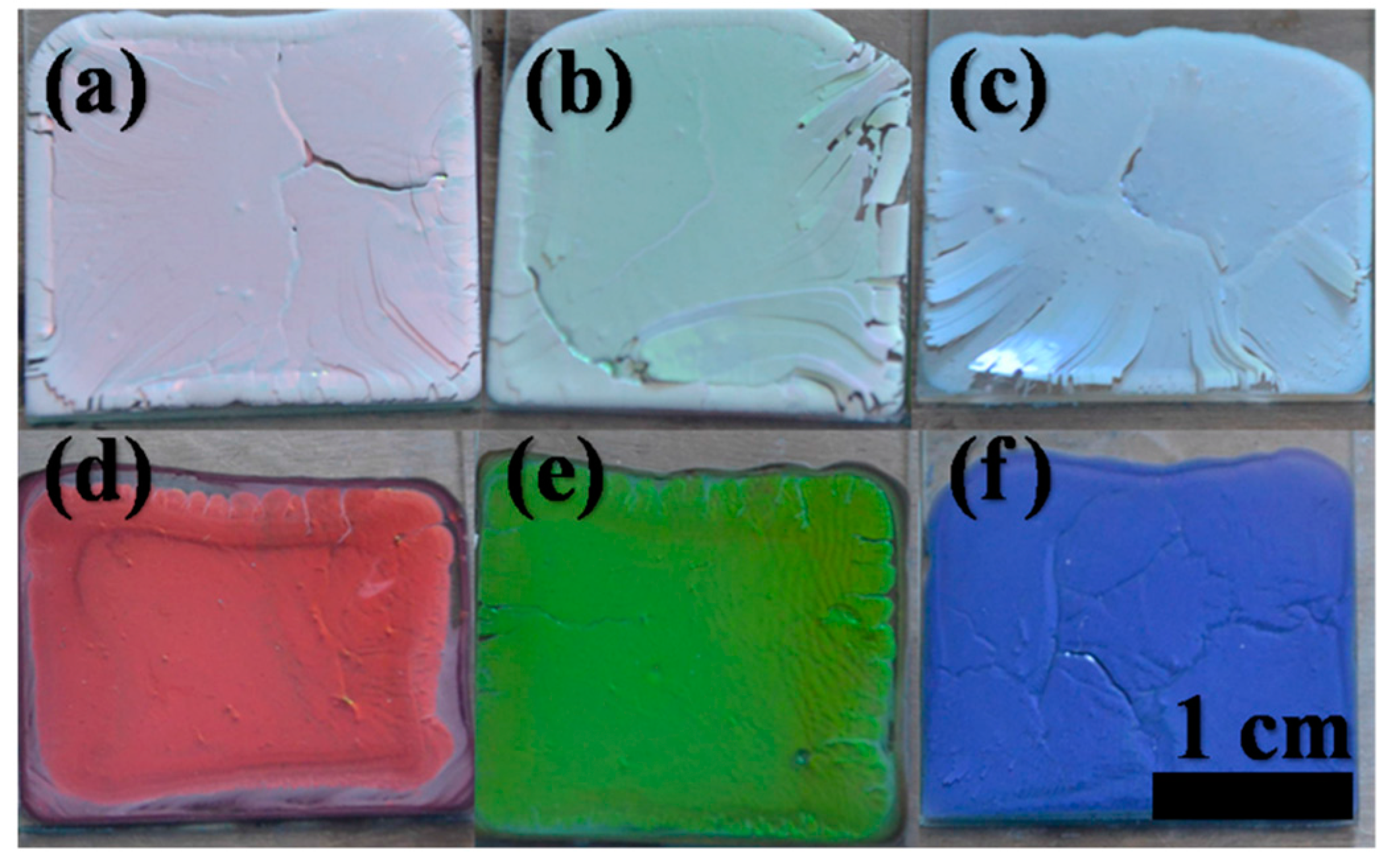
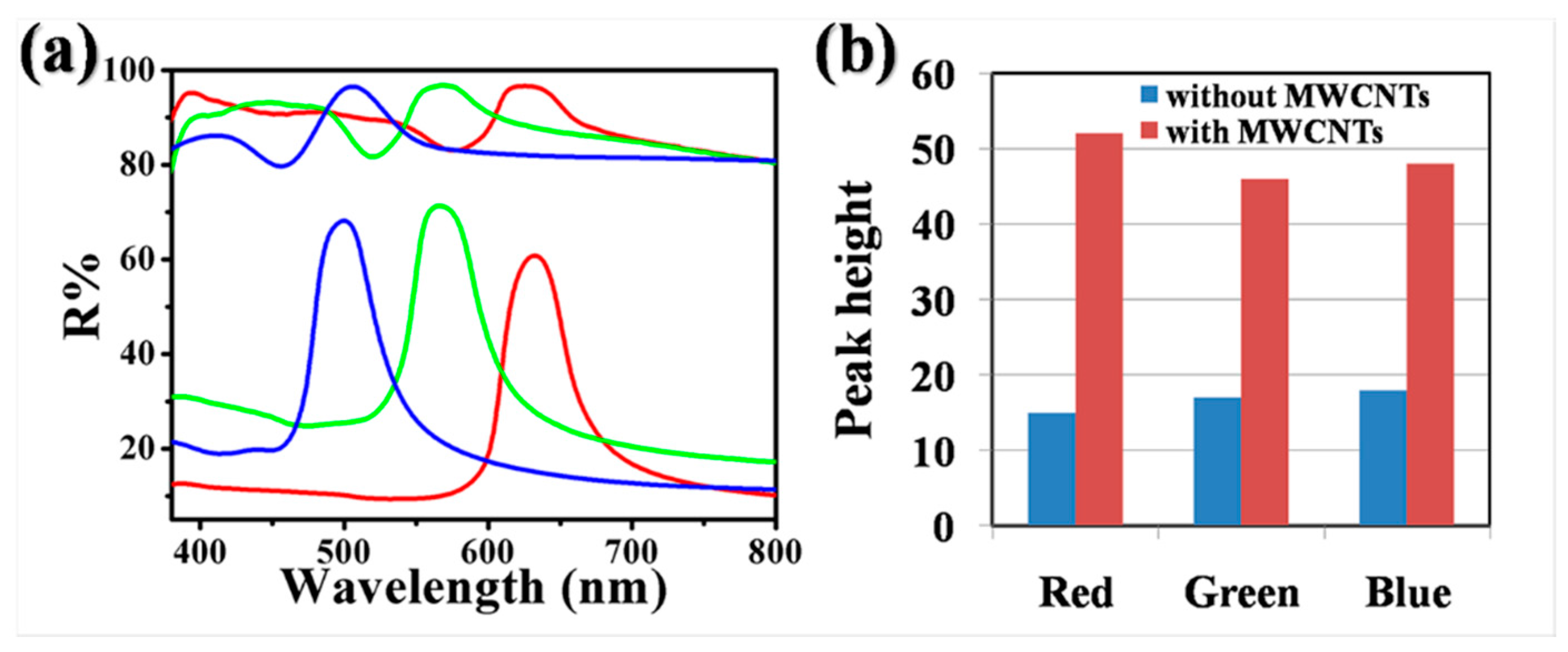
2.3. Effect of MWCNTs on the Color of Photonic Crystal Films
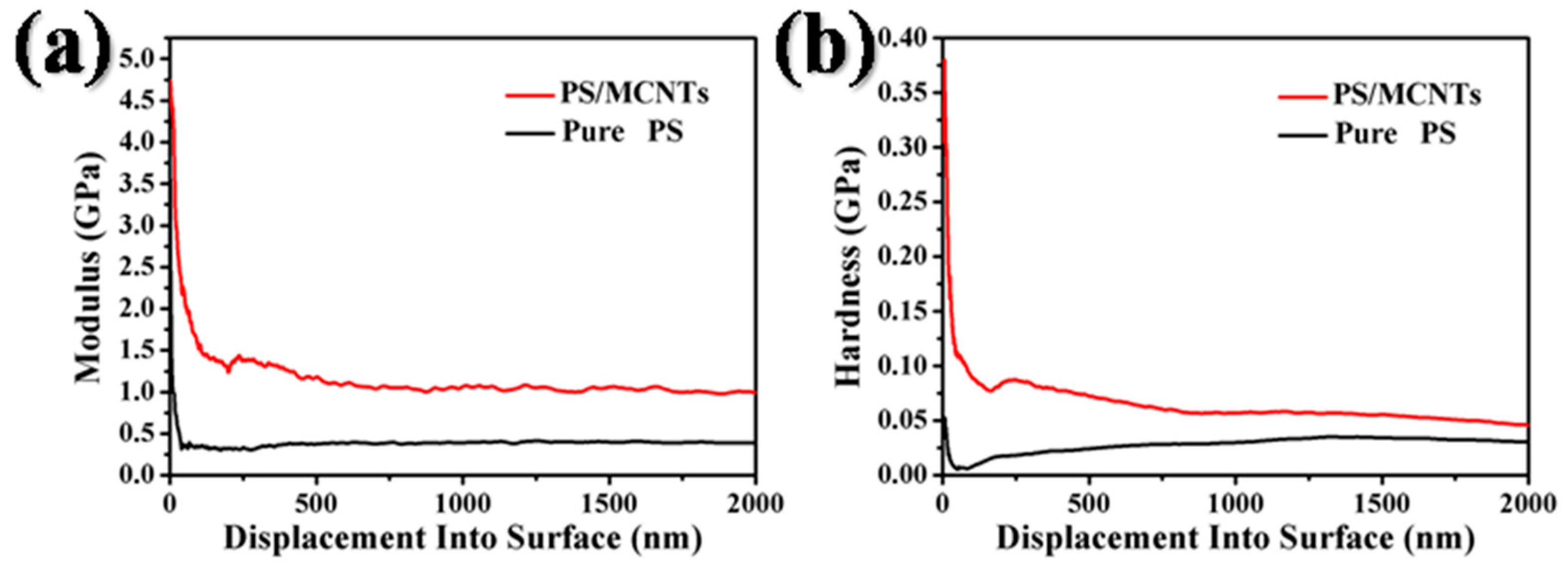
2.4. Effect of MWCNTs on the Mechanical Strength of Photonic Crystal Films
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. Synthesis of Aniline 2,5-Double Sulfonic Acid Diazonium Salt
3.4. Surface-Modification of MWCNTs
3.5. Preparation of Monodisperse PS Emulsion
3.6. Fabrication of Photonic Crystal Films
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of carbon nanotubes. Chem. Rev. 2006, 106, 1105–1136. [Google Scholar] [CrossRef] [PubMed]
- De Volder, M.F.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Shuai, Y.; Guo, Z.; Feng, Y. Functionalization of multi-walled carbon nanotubes with thermo-responsive azide-terminated poly(n-isopropylacrylamide) via click reactions. Molecules 2013, 18, 4599–4612. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Afzali, A.; Han, S.-J.; Tulevski, G.S.; Franklin, A.D.; Tersoff, J.; Hannon, J.B.; Haensch, W. High-density integration of carbon nanotubes via chemical self-assembly. Nat. Nanotechnol. 2012, 7, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.-K.; Je, J.; Baldwin, J.W.; Kooi, S.; Pehrsson, P.E.; Buckley, L.J. Solubilization of single-wall carbon nanotubes by supramolecular encapsulation of helical amylose. J. Am. Chem. Soc. 2003, 125, 4426–4427. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, X. Recent progress on carbon-based support materials for electrocatalysts of direct methanol fuel cells. J. Mater. Chem. A 2014, 2, 6266–6291. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, B.; Zhang, S. Single-walled carbon nanotube/phase change material composites: Sunlight-driven, reversible, form-stable phase transitions for solar thermal energy storage. Adv. Funct. Mater. 2013, 23, 4354–4360. [Google Scholar] [CrossRef]
- Tang, B.; Wang, Y.; Qiu, M.; Zhang, S. A full-band sunlight-driven carbon nanotube/PEG/SiO2 composites for solar energy storage. Sol. Energy Mater. Sol. Cells 2014, 123, 7–12. [Google Scholar] [CrossRef]
- Manchado, M.L.; Valentini, L.; Biagiotti, J.; Kenny, J. Thermal and mechanical properties of single-walled carbon nanotubes–polypropylene composites prepared by melt processing. Carbon 2005, 43, 1499–1505. [Google Scholar] [CrossRef]
- Coleman, J.N.; Khan, U.; Gun’ko, Y.K. Mechanical reinforcement of polymers using carbon nanotubes. Adv. Mater. 2006, 18, 689–706. [Google Scholar] [CrossRef]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Dai, H. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Sinani, V.A.; Gheith, M.K.; Yaroslavov, A.A.; Rakhnyanskaya, A.A.; Sun, K.; Mamedov, A.A.; Wicksted, J.P.; Kotov, N.A. Aqueous dispersions of single-wall and multiwall carbon nanotubes with designed amphiphilic polycations. J. Am. Chem. Soc. 2005, 127, 3463–3472. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Gao, C.; Yan, D. Controlled functionalization of multiwalled carbon nanotubes by in situ atom transfer radical polymerization. J. Am. Chem. Soc. 2004, 126, 412–413. [Google Scholar] [CrossRef] [PubMed]
- Assresahegn, B.D.; Brousse, T.; Bélanger, D. Advances on the use of diazonium chemistry for functionalization of materials used in energy storage systems. Carbon 2015, 92, 362–381. [Google Scholar] [CrossRef]
- Toupin, M.; Bélanger, D. Spontaneous functionalization of carbon black by reaction with 4-nitrophenyldiazonium cations. Langmuir 2008, 24, 1910–1917. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Taylor, S.; Li, H.; Fernando, K.S.; Qu, L.; Wang, W.; Gu, L.; Zhou, B.; Sun, Y.-P. Advances toward bioapplications of carbon nanotubes. J. Mater. Chem. 2004, 14, 527–541. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, L.; Liu, B.; Dong, S. Covalent modification of a glassy carbon surface by 4-aminobenzoic acid and its application in fabrication of a polyoxometalates-consisting monolayer and multilayer films. Langmuir 2000, 16, 7471–7476. [Google Scholar] [CrossRef]
- Pinson, J.; Podvorica, F. Attachment of organic layers to conductive or semiconductive surfaces by reduction of diazonium salts. Chem. Soc. Rev. 2005, 34, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.T.; Gooding, J.J. Modification of carbon electrode surfaces. In Electrochemistry of Carbon Electrodes; John Wiley and Sons: New York, NY, USA, 2015. [Google Scholar]
- Zhang, Y.; Dong, B.; Chen, A.; Liu, X.; Shi, L.; Zi, J. Using cuttlefish ink as an additive to produce non-iridescent structural colors of high color visibility. Adv. Mater. 2015, 27, 4719–4724. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, X.; Lin, Y.; Wang, L.; Zhu, J. Structural coloration pigments based on carbon modified ZnS@SiO2 nanospheres with low-angle dependence, high color saturation, and enhanced stability. ACS Appl. Mater. Interfaces 2016, 8, 5009–5016. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Xu, Y.; Lin, T.; Zhang, S. Polymer opal with brilliant structural color under natural light and white environment. J. Mater. Res. 2015, 30, 3134–3141. [Google Scholar] [CrossRef]
- Wang, W.; Tang, B.; Ma, W.; Zhang, J.; Ju, B.; Zhang, S. Easy approach to assembling a biomimetic color film with tunable structural colors. J. Opt. Soc. Am. A 2015, 32, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, C.I.; Reguera, E.; Stein, A. Colloidal photonic crystal pigments with low angle dependence. ACS Appl. Mater. Interfaces 2010, 2, 3257–3262. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gou, Z.; Ge, Y.; An, K. Preparation of tunable structural colour film by coating ps with titania on glass. Micro Nano Lett. 2016, 11, 50–53. [Google Scholar] [CrossRef]
- Shen, Z.; Shi, L.; You, B.; Wu, L.; Zhao, D. Large-scale fabrication of three-dimensional ordered polymer films with strong structure colors and robust mechanical properties. J. Mater. Chem. 2012, 22, 8069–8075. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Zhang, L.; Cao, M.; Lin, Y.; Zhu, J. Rapid fabrication of angle-independent structurally colored films with a superhydrophobic property. Dyes Pigment. 2016, 130, 202–208. [Google Scholar] [CrossRef]
- Ge, J.; Yin, Y. Responsive photonic crystals. Angew. Chem. Int. Ed. 2011, 50, 1492–1522. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Shim, T.S.; Hwang, H.; Yang, S.-M.; Kim, S.-H. Colloidal photonic crystals toward structural color palettes for security materials. Chem. Mater. 2013, 25, 2684–2690. [Google Scholar] [CrossRef]
- Zhao, Y.; Xie, Z.; Gu, H.; Zhu, C.; Gu, Z. Bio-inspired variable structural color materials. Chem. Soc. Rev. 2012, 41, 3297–3317. [Google Scholar] [CrossRef] [PubMed]
- Kohri, M.; Nannichi, Y.; Taniguchi, T.; Kishikawa, K. Biomimetic non-iridescent structural color materials from polydopamine black particles that mimic melanin granules. J. Mater. Chem. C 2015, 3, 720–724. [Google Scholar] [CrossRef]
- Arsenault, A.C.; Puzzo, D.P.; Manners, I.; Ozin, G.A. Photonic-crystal full-colour displays. Nat. Photonics 2007, 1, 468–472. [Google Scholar] [CrossRef]
- Meng, Y.; Tang, B.; Xiu, J.; Zheng, X.; Ma, W.; Ju, B.; Zhang, S. Simple fabrication of colloidal crystal structural color films with good mechanical stability and high hydrophobicity. Dyes Pigment. 2015, 123, 420–426. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the PS emulsion with different particle sizes are available from the authors.




| Sample | SDS (g) | Size (nm) | PDI 3 | Zeta Potential (mV) | |
|---|---|---|---|---|---|
| Da 1 Dh 2 | |||||
| (a) | 0.050 | 278 | 317 | 0.009 | −35.1 |
| (b) | 0.075 | 239 | 261 | 0.017 | −31.7 |
| (c) | 0.100 | 198 | 235 | 0.013 | −38.3 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Tang, B.; Xiu, J.; Zhang, S. Hydrophilic Modification of Multi-Walled Carbon Nanotube for Building Photonic Crystals with Enhanced Color Visibility and Mechanical Strength. Molecules 2016, 21, 547. https://doi.org/10.3390/molecules21050547
Li F, Tang B, Xiu J, Zhang S. Hydrophilic Modification of Multi-Walled Carbon Nanotube for Building Photonic Crystals with Enhanced Color Visibility and Mechanical Strength. Molecules. 2016; 21(5):547. https://doi.org/10.3390/molecules21050547
Chicago/Turabian StyleLi, Feihu, Bingtao Tang, Jinghai Xiu, and Shufen Zhang. 2016. "Hydrophilic Modification of Multi-Walled Carbon Nanotube for Building Photonic Crystals with Enhanced Color Visibility and Mechanical Strength" Molecules 21, no. 5: 547. https://doi.org/10.3390/molecules21050547
APA StyleLi, F., Tang, B., Xiu, J., & Zhang, S. (2016). Hydrophilic Modification of Multi-Walled Carbon Nanotube for Building Photonic Crystals with Enhanced Color Visibility and Mechanical Strength. Molecules, 21(5), 547. https://doi.org/10.3390/molecules21050547