Bifunctional Phosphorus Dendrimers and Their Properties
Abstract
:1. Introduction
2. Dendritic Structures with a Function at the Level of the Core
2.1. Functional Cores of Dendrimers (Type A)
2.2. Off-Center Functions Linked to the Core of Dendrimers (Type B)
2.3. Dendrons (Type C)
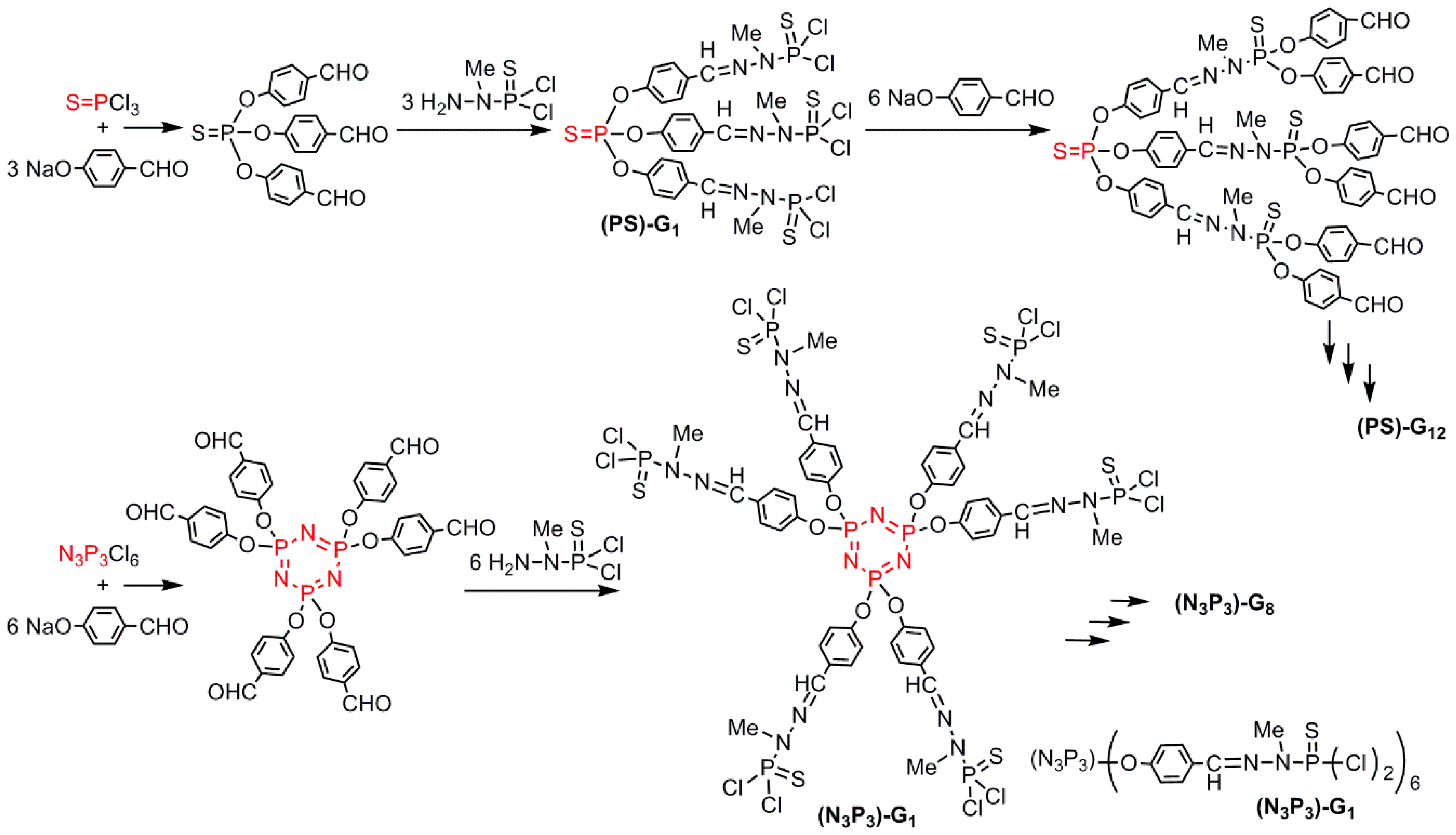
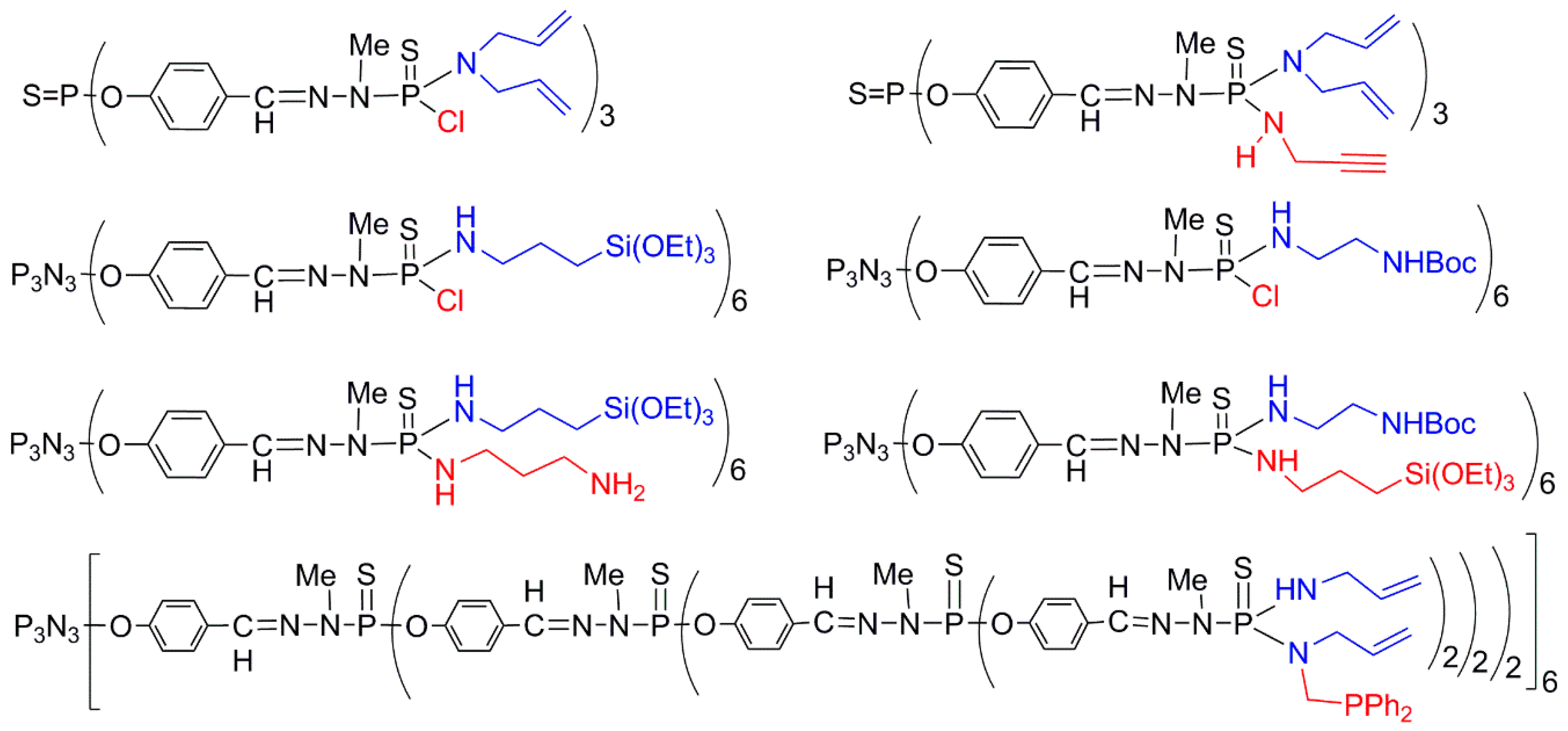
2.4. Dendrons for the Synthesis of Non-Symmetrical Dendrimers (Type D)
3. Dendritic Structures with Functional Internal Branches
3.1. Functions as Constituents of the Branches at One or Several Layers (Case E)
3.2. Functions as Intrinsic Constituents of the Branches of Layered Dendrimers (Case F)
3.3. Reactivity of the Internal Functions (Case G)
4. Dendritic Structures with Two Types of Terminal Functions in Close Proximity
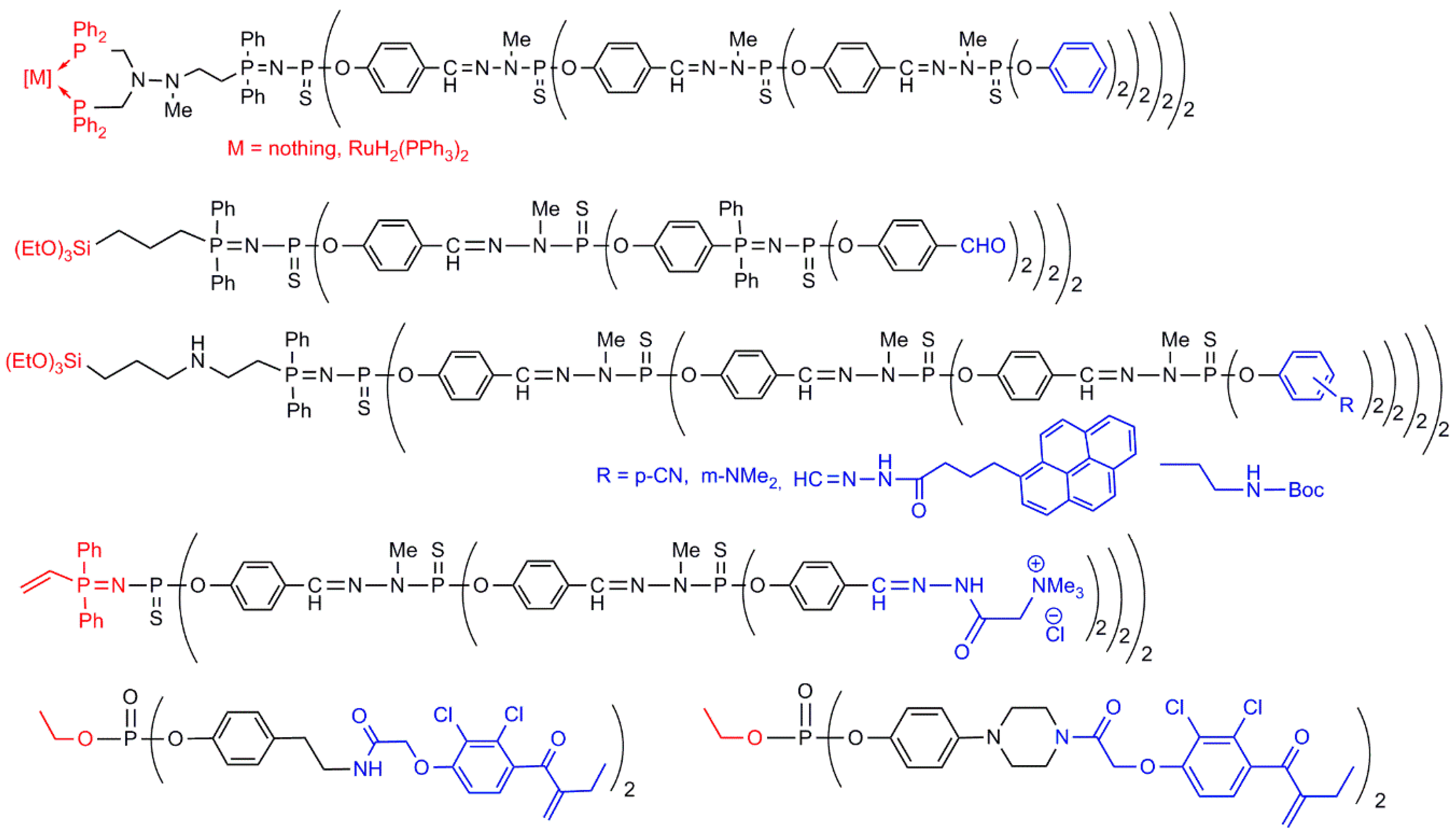
4.1. Dendritic Structures Having Sequentially Two Types of Terminal Functions (Case H)
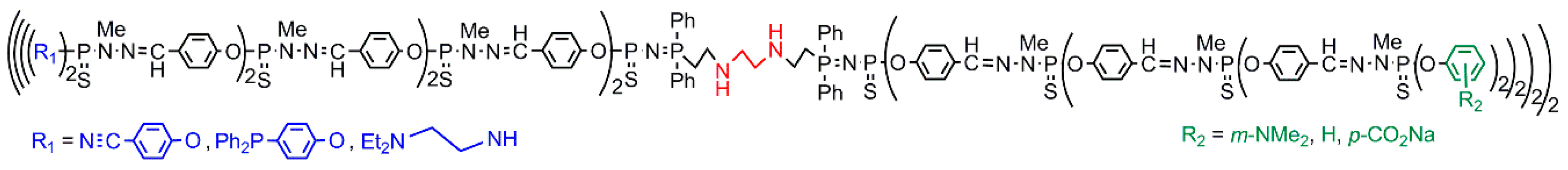
4.2. Dendritic Structures with Two Types of Functions on Each Terminal Branching Point (Case I)
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| A549 cells | Adenocarcinomic human alveolar basal epithelial cells |
| 3-APDMES | 3-AminoPropyl DiMethylEthoxySilane |
| B14 cells | Chinese hamster fibroblasts |
| Boc | tert-ButylOxyCarbonyl |
| CD | Circular Dichroism |
| Cp | CycloPentadienyl |
| Cp* | C5Me5 |
| Cyclam | 1,4,8,11-tetraazacyclotetradecane |
| dansyl | 5-(DimethylAmino)Naphthalene-1-SulfonYL |
| dba | DiBenzylidene Acetone |
| DFT | Density Functional Theory |
| DNA | DeoxyriboNucleic Acid |
| EGFP | Enhanced Green Fluorescent Protein |
| Felbinac | biphenylylacetic acid |
| GalCer | GalactosylCeramide |
| HEK 293 cells | Human Embryonic Kidney cells |
| HeLa cells | cervical cancer cells taken from the patient Henrietta Lacks |
| HIV | Human Immunodeficiency Virus |
| HSA | Human Serum Albumin |
| HUVEC cells | Human Umbilical Vein Endothelial Cells |
| IR | Infra-Red |
| LbL | Layer-by-Layer |
| MTT | diMethyl Thiazolyl diphenyl Tetrazolium salt |
| Mw | weight average Molecular Weight |
| N2a cells | fast-growing mouse Neuroblastoma cell line |
| NK cells | Natural Killer cells |
| NMR | Nuclear Magnetic Resonance |
| PAMAM | PolyAMidoAMine |
| PDI | PolyDispersity Index |
| PEG | PolyEthyleneGlycol |
| Rh | hydrodynamic Radius |
| ROS | Reactive Oxygen Species |
| RSC | Redox-Switchable Catalysis |
| TEOS | TetraEthOxySilane) |
| TFA | TriFluoroacetic Acid |
| THF | TetraHydroFuran |
| TPA | Two-Photon Absorption |
| Triflate | Trifluoromethanesulfonate |
| UV | Ultra-Violet |
References
- Nguyen, T.T.T.; Baumgarten, M.; Rouhanipour, A.; Rader, H.J.; Lieberwirth, I.; Mullen, K. Extending the Limits of Precision Polymer Synthesis: Giant Polyphenylene Dendrimers in the Megadalton Mass Range Approaching Structural Perfection. J. Am. Chem. Soc. 2013, 135, 4183–4186. [Google Scholar] [CrossRef] [PubMed]
- Caminati, G.; Turro, N.J.; Tomalia, D.A. Photophysical Investigation of Starburst Dendrimers and Their Interactions with Anionic and Cationic Surfactants. J. Am. Chem. Soc. 1990, 112, 8515–8522. [Google Scholar] [CrossRef]
- Denkewalter, R.G.; Kolc, J.; Tukasavage, W.J. Macromolecular Highly Branched Homogeneous Compound Based on Lysine units. U.S. Patent 4 289 872, 15 September 1981. [Google Scholar]
- Lartigue, M.L.; Donnadieu, B.; Galliot, C.; Caminade, A.M.; Majoral, J.P.; Fayet, J.P. Large dipole moments of phosphorus-containing dendrimers. Macromolecules 1997, 30, 7335–7337. [Google Scholar] [CrossRef]
- Lim, J.; Kostiainen, M.; Maly, J.; da Costa, V.C.P.; Annunziata, O.; Pavan, G.M.; Simanek, E.E. Synthesis of Large Dendrimers with the Dimensions of Small Viruses. J. Am. Chem. Soc. 2013, 135, 4660–4663. [Google Scholar] [CrossRef] [PubMed]
- Sowinska, M.; Urbanczyk-Lipkowska, Z. Advances in the chemistry of dendrimers. New J. Chem. 2014, 38, 2168–2203. [Google Scholar] [CrossRef]
- Majoral, J.P.; Caminade, A.M. Dendrimers containing heteroatoms (Si, P, B, Ge, or Bi). Chem. Rev. 1999, 99, 845–880. [Google Scholar] [CrossRef] [PubMed]
- Launay, N.; Caminade, A.M.; Lahana, R.; Majoral, J.P. A general synthetic strategy for neutral phosphorus-containing dendrimers. Angew. Chem. Int. Ed. 1994, 33, 1589–1592. [Google Scholar] [CrossRef]
- Launay, N.; Caminade, A.M.; Majoral, J.P. Synthesis of bowl-shaped dendrimers from generation 1 to generation 8. J. Organomet. Chem. 1997, 529, 51–58. [Google Scholar] [CrossRef]
- Leclaire, J.; Dagiral, R.; Pla-Quintana, A.; Caminade, A.M.; Majoral, J.P. Metallated phthalocyanines as the core of dendrimers—Synthesis and spectroscopic studies. Eur. J. Inorg. Chem. 2007, 2007, 2890–2896. [Google Scholar] [CrossRef]
- Leclaire, J.; Dagiral, R.; Fery-Forgues, S.; Coppel, Y.; Donnadieu, B.; Caminade, A.M.; Majoral, J.P. Octasubstituted metal-free phthalocyanine as core of phosphorus dendrimers: A probe for the properties of the internal structure. J. Am. Chem. Soc. 2005, 127, 15762–15770. [Google Scholar] [CrossRef] [PubMed]
- Leclaire, J.; Coppel, Y.; Caminade, A.M.; Majoral, J.P. Nanometric sponges made of water-soluble hydrophobic dendrimers. J. Am. Chem. Soc. 2004, 126, 2304–2305. [Google Scholar] [CrossRef] [PubMed]
- Furer, V.L.; Vandyukova, I.I.; Vandyukov, A.E.; Majoral, J.P.; Caminade, A.M.; Kovalenko, V.I. DFT study of structure, IR and Raman spectra of P0′ and P4′ dendrimers built from octasubstituted metal-free phthalocyanine core. Chem. Phys. 2009, 358, 177–183. [Google Scholar] [CrossRef]
- Furer, V.L.; Vandyukova, I.I.; Pla-Quintana, A.; Majoral, J.R.; Caminade, A.M.; Kovalenko, V.I. IR spectroscopy investigation using DFF analysis on the structure of P-1 phosphorus dendrimer built from octasubstituted metal-free phthalocyanine core. Spectroch. Acta A Mol. Biomol. Spectrosc. 2009, 72, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Maszewska, M.; Leclaire, J.; Cieslak, M.; Nawrot, B.; Okruszek, A.; Caminade, A.M.; Majoral, J.P. Water-soluble polycationic dendrimers with a phosphoramidothioate backbone: Preliminary studies of cytotoxicity and oligonucleotide/plasmid delivery in human cell culture. Oligonucleotides 2003, 13, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Loup, C.; Zanta, M.A.; Caminade, A.M.; Majoral, J.P.; Meunier, B. Preparation of water-soluble cationic phosphorus-containing dendrimers as DNA transfecting agents. Chem. Eur. J. 1999, 5, 3644–3650. [Google Scholar] [CrossRef]
- Briz, V.; Serramia, M.J.; Madrid, R.; Hameau, A.; Caminade, A.M.; Majoral, J.P.; Munoz-Fernandez, M.A. Validation of a Generation 4 Phosphorus-Containing Polycationic Dendrimer for Gene Delivery Against HIV-1. Curr. Med. Chem. 2012, 19, 5044–5051. [Google Scholar] [CrossRef] [PubMed]
- Badetti, E.; Franc, G.; Majoral, J.P.; Caminade, A.M.; Sebastian, R.M. 15-Membered Azamacrocycles as Core and End Groups of Phosphorus Dendrimers. Synthesis 2011, 21, 1427–1434. [Google Scholar]
- Badetti, E.; Franc, G.; Majoral, J.P.; Caminade, A.M.; Sebastian, R.M.; Moreno-Manas, M. Macrocyclic Core Phosphorus Dendrimers Covered on the Surface by N,P Ligands. Eur. J. Org. Chem. 2011, 2011, 1256–1265. [Google Scholar] [CrossRef]
- Badetti, E.; Caminade, A.M.; Majoral, J.P.; Moreno-Manas, M.; Sebastian, R.M. Palladium(0) nanoparticles stabilized by phosphorus dendrimers containing coordinating 15-membered triolefinic macrocycles in periphery. Langmuir 2008, 24, 2090–2101. [Google Scholar] [CrossRef] [PubMed]
- Franc, G.; Badetti, E.; Colliere, V.; Majoral, J.P.; Sebastian, R.M.; Caminade, A.M. Dendritic structures within dendritic structures: Dendrimer-induced formation and self-assembly of nanoparticle networks. Nanoscale 2009, 1, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Mongin, O.; Pla-Quintana, A.; Terenziani, F.; Drouin, D.; le Droumaguet, C.; Caminade, A.M.; Majoral, J.P.; Blanchard-Desce, M. Organic nanodots for multiphotonics: synthesis and photophysical studies. New J. Chem. 2007, 31, 1354–1367. [Google Scholar] [CrossRef]
- Rouxel, C.; Charlot, M.; Mongin, O.; Krishna, T.R.; Caminade, A.M.; Majoral, J.P.; Blanchard-Desce, M. From Graftable Biphotonic Chromophores to Water-Soluble Organic Nanodots for Biophotonics: The Importance of Environmental Effects. Chem. Eur. J. 2012, 18, 16450–16462. [Google Scholar] [CrossRef] [PubMed]
- Krishna, T.R.; Parent, M.; Werts, M.H.V.; Moreaux, L.; Gmouh, S.; Charpak, S.; Caminade, A.M.; Majoral, J.P.; Blanchard-Desce, M. Water-soluble dendrimeric two-photon tracers for in vivo imaging. Angew. Chem. Int. Ed. 2006, 45, 4645–4648. [Google Scholar] [CrossRef] [PubMed]
- Mongin, O.; Rouxel, C.; Robin, A.C.; Pla-Quintana, A.; Krishna, T.R.; Recher, G.; Tiaho, F.; Caminade, A.M.; Majoral, J.P.; Blanchard-Desce, M. Brilliant organic nanodots: Novel nano-objects for bionanophotonics. In Nanobiosystems: Processing, Characterization, and Applications; Heckman, E.M., Singh, T.B., Yoshida, J., Eds.; Spie-Int Soc Optical Engineering: Bellingham, WA, USA, 2008; Volume 7040. [Google Scholar]
- Spataro, G.; Malecaze, F.; Turrin, C.O.; Soler, V.; Duhayon, C.; Elena, P.P.; Majoral, J.P.; Caminade, A.M. Designing dendrimers for ocular drug delivery. Eur. J. Med. Chem. 2010, 45, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.M.; Hameau, A.; Majoral, J.P. The specific functionalization of cyclotriphosphazene for the synthesis of smart dendrimers. Dalton Trans. 2016, 45, 1810–1822. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S.; Pla-Quintana, A.; Mazeres, S.; Caminade, A.M.; Majoral, J.P. Cationic and Fluorescent “Janus” Dendrimers. Org. Lett. 2008, 10, 4751–4754. [Google Scholar] [CrossRef] [PubMed]
- Hameau, A.; Fuchs, S.; Laurent, R.; Majoral, J.P.; Caminade, A.M. Synthesis of dye/fluorescent functionalized dendrons based on cyclotriphosphazene. Beilstein J. Org. Chem. 2011, 7, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- Tristany, M.; Laurent, R.; Dib, H.; Gonsalvi, L.; Peruzzini, M.; Majoral, J.P.; Caminade, A.M. Bifunctional metallodendrimers based on AB(5) derivatives of cyclotriphosphazene as core and P,N ligands as terminal functions. Inorg. Chim. Acta 2014, 409, 121–126. [Google Scholar] [CrossRef]
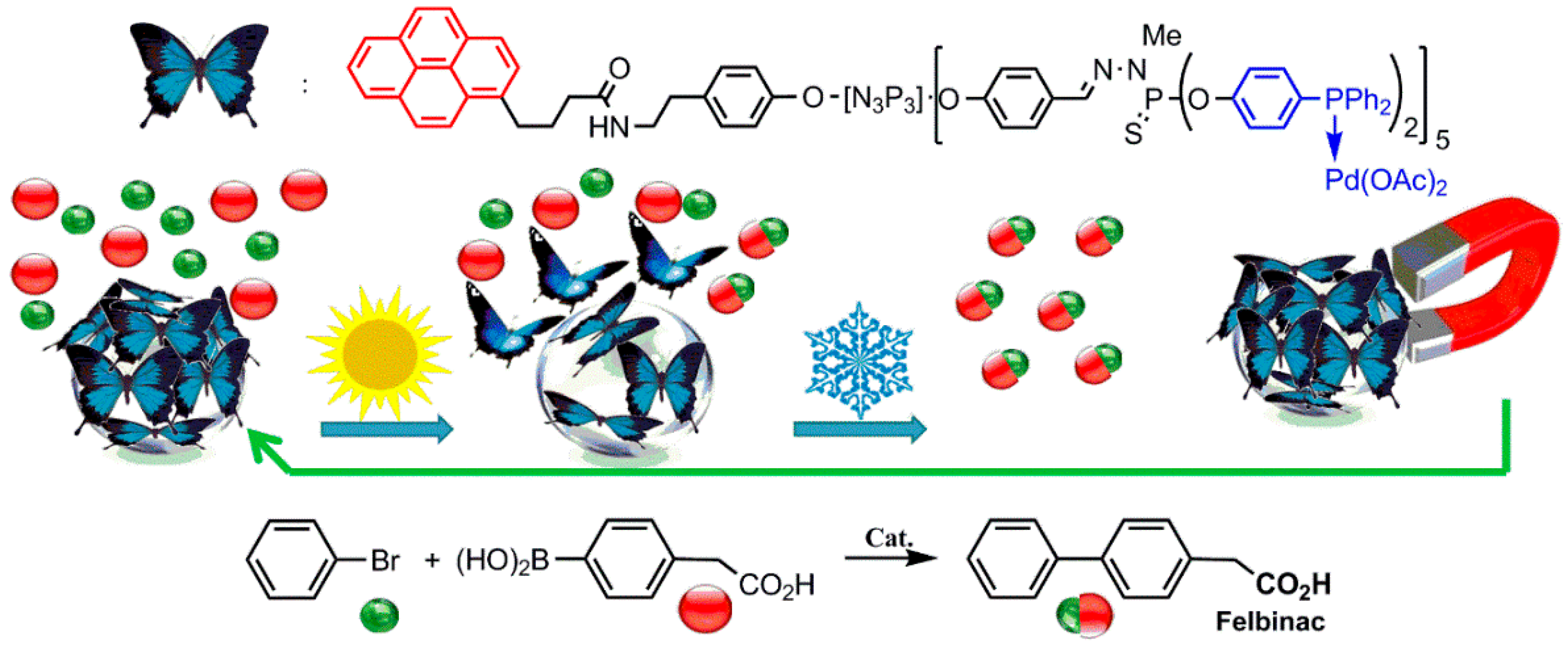
- Keller, M.; Colliere, V.; Reiser, O.; Caminade, A.M.; Majoral, J.P.; Ouali, A. Pyrene-Tagged Dendritic Catalysts Noncovalently Grafted onto Magnetic Co/C Nanoparticles: An Efficient and Recyclable System for Drug Synthesis. Angew. Chem. Int. Ed. 2013, 52, 3626–3629. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.M.; Ouali, A.; Laurent, R.; Turrin, C.O.; Majoral, J.P. Coordination chemistry with phosphorus dendrimers. Applications as catalysts, for materials, and in biology. Coord. Chem. Rev. 2016, 308, 478–497. [Google Scholar] [CrossRef]
- Riegert, D.; Pla-Quintana, A.; Fuchs, S.; Laurent, R.; Turrin, C.O.; Duhayon, C.; Majoral, J.P.; Chaumonnot, A.; Caminade, A.M. Diversified Strategies for the Synthesis of Bifunctional Dendrimeric Structures. Eur. J. Org. Chem. 2013, 2013, 5414–5422. [Google Scholar] [CrossRef]
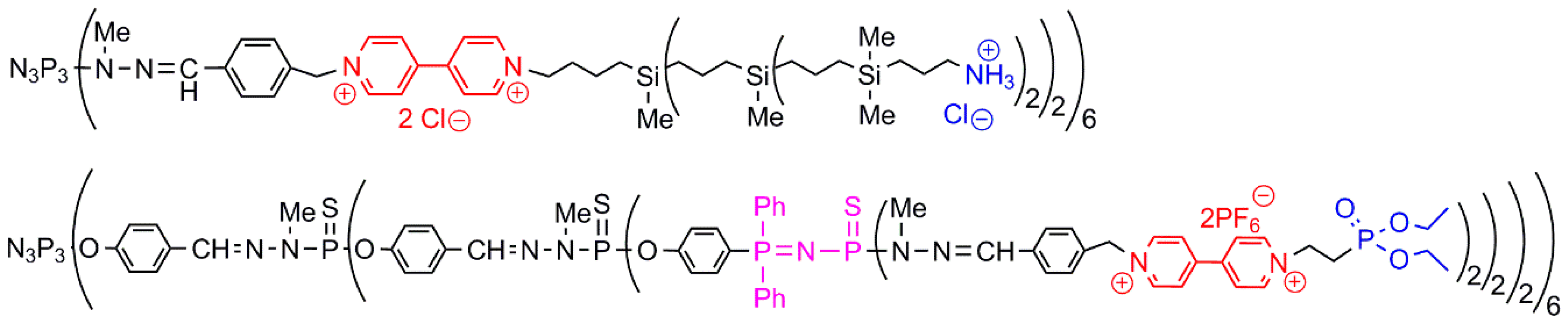
- De Jong, E.R.; Deloch, N.; Knoll, W.; Turrin, C.O.; Majoral, J.P.; Caminade, A.M.; Koper, I. Synthesis and characterization of bifunctional dendrimers: Preliminary use for the coating of gold surfaces and the proliferation of human osteoblasts (HOB). New J. Chem. 2015, 39, 7194–7205. [Google Scholar] [CrossRef]
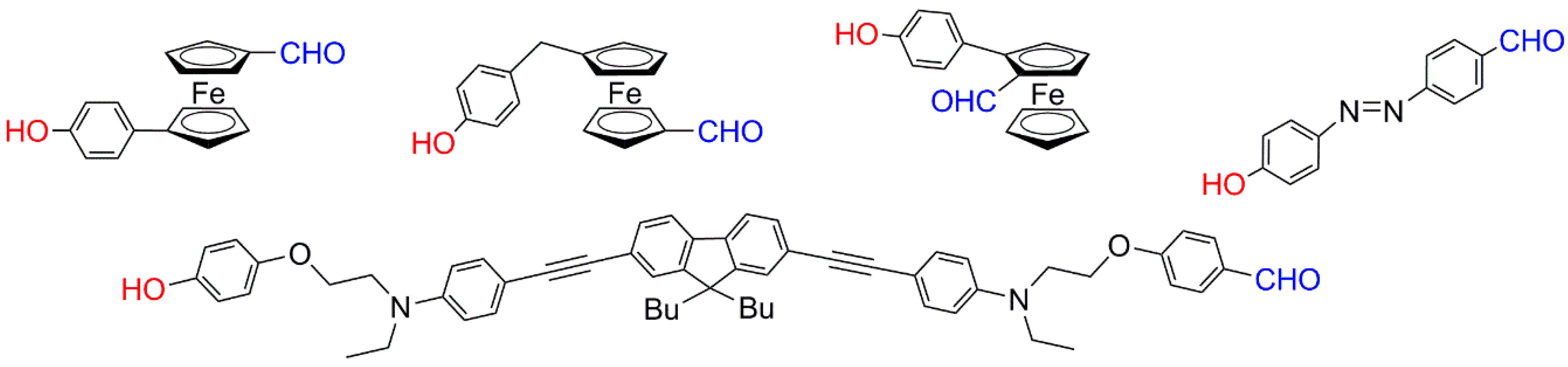
- Franc, G.; Mazeres, S.; Turrin, C.O.; Vendier, L.; Duhayon, C.; Caminade, A.M.; Majoral, J.P. Synthesis and properties of dendrimers possessing the same fluorophore(s) located either peripherally or off-center. J. Org. Chem. 2007, 72, 8707–8715. [Google Scholar] [CrossRef] [PubMed]
- Kazmierczak-Baranska, J.; Pietkiewicz, A.; Janicka, M.; Wei, Y.Q.; Turrin, C.O.; Majoral, J.P.; Nawrot, B.; Caminade, A.M. Synthesis of a Fluorescent Cationic Phosphorus Dendrimer and Preliminary Biological Studies of Its Interaction with DNA. Nucleosides Nucleotides Nucleic Acids 2010, 29, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Rolland, O.; Griffe, L.; Poupot, M.; Maraval, A.; Ouali, A.; Coppel, Y.; Fournie, J.J.; Bacquet, G.; Turrin, C.O.; Caminade, A.M.; et al. Tailored control and optimisation of the number of phosphonic acid termini on phosphorus-containing dendrimers for the ex vivo activation of human monocytes. Chem. Eur. J. 2008, 14, 4836–4850. [Google Scholar] [CrossRef] [PubMed]
- Poupot, M.; Griffe, L.; Marchand, P.; Maraval, A.; Rolland, O.; Martinet, L.; L’Faqihi-Olive, F.E.; Turrin, C.O.; Caminade, A.M.; Fournie, J.J.; et al. Design of phosphorylated dendritic architectures to promote human monocyte activation. FASEB J. 2006, 20, 2339–2351. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.M.; Fruchon, S.; Turrin, C.O.; Poupot, M.; Ouali, A.; Maraval, A.; Garzoni, M.; Maly, M.; Furer, V.; Kovalenko, V.; et al. The key role of the scaffold on the efficiency of dendrimer nanodrugs. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Griffe, L.; Poupot, M.; Marchand, P.; Maraval, A.; Turrin, C.O.; Rolland, O.; Metivier, P.; Bacquet, G.; Fournie, J.J.; Caminade, A.M.; et al. Multiplication of human natural killer cells by nanosized phosphonate-capped dendrimers. Angew. Chem. Int. Ed. 2007, 46, 2523–2526. [Google Scholar] [CrossRef] [PubMed]
- Hayder, M.; Poupot, M.; Baron, M.; Nigon, D.; Turrin, C.O.; Caminade, A.M.; Majoral, J.P.; Eisenberg, R.A.; Fournie, J.J.; Cantagrel, A.; et al. A Phosphorus-Based Dendrimer Targets Inflammation and Osteoclastogenesis in Experimental Arthritis. Science Transl. Med. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Hayder, M.; Varilh, M.; Turrin, C.O.; Saoudi, A.; Caminade, A.M.; Poupot, R.; Liblau, R.S. Phosphorus-Based Dendrimer ABP Treats Neuroinflammation by Promoting IL-10-Producing CD4(+) T Cells. Biomacromolecules 2015, 16, 3425–3433. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.M.; Hameau, A.; Majoral, J.P. Multicharged and/or Water-Soluble Fluorescent Dendrimers: Properties and Uses. Chem. Eur. J. 2009, 15, 9270–9285. [Google Scholar] [CrossRef] [PubMed]
- Hawker, C.J.; Frechet, J.M.J. Preparation of polymers with controlled molecular architecture—A new convergent approach to dendritic macromolecules. J. Am. Chem. Soc. 1990, 112, 7638–7647. [Google Scholar] [CrossRef]
- Maraval, V.R.; Prevote-Pinet, D.; Laurent, R.; Caminade, A.M.; Majoral, J.P. Choice of strategies for the divergent synthesis of phosphorus-containing dendrons, depending on the function located at the core. New J. Chem. 2000, 24, 561–566. [Google Scholar] [CrossRef]
- Sebastian, R.M.; Griffe, L.; Turrin, C.O.; Donnadieu, B.; Caminade, A.M.; Majoral, J.P. Synthesis and core and surface reactivity of phosphorus-based dendrons. Eur. J. Inorg. Chem. 2004, 2459–2466. [Google Scholar] [CrossRef]
- Barriere, C.; Latour, V.; Fau, P.; Caminade, A.M.; Turrin, C.O. Low generation PEGylated phosphorus-containing dendrons with phosphonate anchors. Tetrahedron Lett. 2012, 53, 1908–1911. [Google Scholar] [CrossRef]
- Brauge, L.; Maraval, V.; Laurent, R.; Caminade, A.M.; Majoral, J.P. Synthesis and reactivity of small phosphorus-containing dendritic wedges (dendrons). Arkivoc 2002, 2002, 151–160. [Google Scholar]
- Maraval, V.; Laurent, R.; Donnadieu, B.; Caminade, A.M.; Majoral, J.P. 1,2,3,4-heterohexatrienes as new tools for Michael-type additions usable for the synthesis of phosphorus dendrimers. Synthesis 2003, 2003, 389–396. [Google Scholar]
- Angurell, I.; Turrin, C.O.; Laurent, R.; Maraval, V.; Servin, P.; Rossell, O.; Seco, M.; Caminade, A.M.; Majoral, J.P. Decorating step-by-step and independently the surface and the core of dendrons. J. Organomet. Chem. 2007, 692, 1928–1939. [Google Scholar] [CrossRef]
- Turrin, C.O.; Maraval, V.; Leclaire, J.; Dantras, E.; Lacabanne, C.; Caminade, A.M.; Majoral, J.P. Surface, core, and structure modifications of phosphorus-containing dendrimers. Influence on the thermal stability. Tetrahedron 2003, 59, 3965–3973. [Google Scholar] [CrossRef]
- Maraval, V.; Laurent, R.; Caminade, A.M.; Majoral, J.P. Phosphorus-containing dendrimers and their transition metal complexes as efficient recoverable multicenter homogeneous catalysts in organic synthesis. Organometallics 2000, 19, 4025–4029. [Google Scholar] [CrossRef]
- Turrin, C.O.; Maraval, V.; Caminade, A.M.; Majoral, J.P.; Mehdi, A.; Reye, C. Organic-inorganic hybrid materials incorporating phosphorus-containing dendrimers. Chem. Mater. 2000, 12, 3848–3856. [Google Scholar] [CrossRef]
- Larpent, C.; Genies, C.; Delgado, A.P.D.; Caminade, A.M.; Majoral, J.P.; Sassic, J.F.; Leising, F. Giant dendrimer-like particles from nanolatexes. Chem. Commun. 2004, 1816–1817. [Google Scholar] [CrossRef] [PubMed]
- Marmillon, C.; Gauffre, F.; Gulik-Krzywicki, T.; Loup, C.; Caminade, A.M.; Majoral, J.P.; Vors, J.P.; Rump, E. Organophosphorus dendrimers as new gelators for hydrogels. Angew. Chem. Int. Ed. 2001, 40, 2626–2629. [Google Scholar] [CrossRef]
- El Brahmi, N.; Mignani, S.M.; Caron, J.; El Kazzouli, S.; Bousmina, M.M.; Caminade, A.M.; Cresteil, T.; Majoral, J.P. Investigations on dendrimer space reveal solid and liquid tumor growth-inhibition by original phosphorus-based dendrimers and the corresponding monomers and dendrons with ethacrynic acid motifs. Nanoscale 2015, 7, 3915–3922. [Google Scholar] [CrossRef] [PubMed]
- Magro, G.; Donnadieu, B.; Caminade, A.M.; Majoral, J.P. The first linear multiphosphazene having five different types of side groups and its use as the core of a dendrimeric species. Chem. Eur. J. 2003, 9, 2151–2159. [Google Scholar] [CrossRef] [PubMed]
- Turrin, C.O.; Maraval, A.; Magro, G.; Maraval, V.; Caminade, A.M.; Majoral, J.P. First example of dendrons as topological amplifiers. Eur. J. Inorg. Chem. 2006, 2006, 2556–2560. [Google Scholar] [CrossRef]
- Caminade, A.M.; Maraval, A.; Majoral, J.P. Phosphorus-containing dendrons: Synthesis, reactivity, properties, and use as building blocks for various dendritic architectures. Eur. J. Inorg. Chem. 2006, 2006, 887–901. [Google Scholar] [CrossRef]
- Caminade, A.M.; Laurent, R.; Delavaux-Nicot, B.; Majoral, J.P. “Janus” dendrimers: Syntheses and properties. New J. Chem. 2012, 36, 217–226. [Google Scholar] [CrossRef]
- Maraval, V.; Laurent, R.; Donnadieu, B.; Mauzac, M.; Caminade, A.M.; Majoral, J.P. Rapid synthesis of phosphorus-containing dendrimers with controlled molecular architectures: First example of surface-block, layer-block, and segment-block dendrimers issued from the same dendron. J. Am. Chem. Soc. 2000, 122, 2499–2511. [Google Scholar] [CrossRef]
- Maraval, V.; Laurent, R.; Merino, S.; Caminade, A.M.; Majoral, J.P. Michael-type addition of amines to the vinyl core of dendrons—Application to the synthesis of multidendritic systems. Eur. J. Org. Chem. 2000, 2000, 3555–3568. [Google Scholar] [CrossRef]
- Maraval, V.; Maraval, A.; Spataro, G.; Caminade, A.M.; Majoral, J.P.; Kim, D.H.; Knoll, W. Design of tailored multi-charged phosphorus surface-block dendrimers. New J. Chem. 2006, 30, 1731–1736. [Google Scholar] [CrossRef]
- Knoll, W.; Caminade, A.M.; Char, K.; Duran, H.; Feng, C.L.; Gitsas, A.; Kim, D.H.; Lau, A.; Lazzara, T.D.; Majoral, J.P.; et al. Nanostructuring Polymeric Materials by Templating Strategies. Small 2011, 7, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Ahl, S.; Caminade, A.M.; Majoral, J.P.; Knoll, W.; Erlebacher, J. Simultaneous excitation of propagating and localized surface plasmon resonance in nanoporous gold membranes. Anal. Chem. 2006, 78, 7346–7350. [Google Scholar] [CrossRef] [PubMed]
- Lazzara, T.D.; Lau, K.H.A.; Abou-Kandil, A.I.; Caminade, A.M.; Majoral, J.P.; Knoll, W. Polyelectrolyte Layer-by-Layer Deposition in Cylindrical Nanopores. ACS Nano 2010, 4, 3909–3920. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Karan, P.; Goring, P.; Leclaire, J.; Caminade, A.M.; Majoral, J.P.; Gosele, U.; Steinhart, M.; Knoll, W. Formation of dendrimer nanotubes by layer-by-layer deposition. Small 2005, 1, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.L.; Zhong, X.H.; Steinhart, M.; Caminade, A.M.; Majoral, J.P.; Knoll, W. Graded-bandgap quantum-dot-modified nanotubes: A sensitive biosensor for enhanced detection of DNA hybridization. Adv. Mater. 2007, 19, 1933–1936. [Google Scholar] [CrossRef]
- Feng, C.L.; Zhong, X.H.; Steinhart, M.; Caminade, A.M.; Majoral, J.P.; Knoll, W. Functional quantum-dot/dendrimer nanotubes for sensitive detection of DNA hybridization. Small 2008, 4, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Lebedeva, O.V.; Kim, D.H.; Caminade, A.M.; Majoral, J.P.; Knoll, W.; Vinogradova, O.I. Assembly and mechanical properties of phosphorus dendrimer/polyelectrolyte multilayer microcapsules. Langmuir 2005, 21, 7200–7206. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Lopez, J.L.; Khor, H.L.; Caminade, A.M.; Majoral, J.P.; Mittler, S.; Knoll, W.; Kim, D.H. Bioactive multilayer thin films of charged N,N-disubstituted hydrazine phosphorus dendrimers fabricated by layer-by-layer self-assembly. Thin Solid Films 2008, 516, 1256–1264. [Google Scholar] [CrossRef]
- Yu, Y.M.; Feng, C.L.; Caminade, A.M.; Majoral, J.P.; Knoll, W. The Detection of DNA Hybridization on Phosphorus Dendrimer Multilayer Films by Surface Plasmon Field Enhanced-Fluorescence Spectroscopy. Langmuir 2009, 25, 13680–13684. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.M.; Majoral, J.P. Dendrimers and nanotubes: A fruitful association. Chem. Soc. Rev. 2010, 39, 2034–2047. [Google Scholar] [CrossRef] [PubMed]
- Gottis, S.; Rodriguez, L.I.; Laurent, R.; Angurell, I.; Seco, M.; Rossell, O.; Majoral, J.P.; Caminade, A.M. Janus carbosilane/phosphorhydrazone dendrimers synthesized by the ‘click’ Staudinger reaction. Tetrahedron Lett. 2013, 54, 6864–6867. [Google Scholar] [CrossRef]
- Maraval, V.; Sebastian, R.M.; Ben, F.; Laurent, R.; Caminade, A.M.; Majoral, J.P. Varying topology of dendrimers—A new approach toward the synthesis of di-block dendrimers. Eur. J. Inorg. Chem. 2001, 2001, 1681–1691. [Google Scholar] [CrossRef]
- Turrin, C.O.; Chiffre, J.; de Montauzon, D.; Daran, J.C.; Caminade, A.M.; Manoury, E.; Balavoine, G.; Majoral, J.P. Phosphorus-containing dendrimers with ferrocenyl units at the core, within the branches, and on the periphery. Macromolecules 2000, 33, 7328–7336. [Google Scholar] [CrossRef]
- De Jong, E.R.; Manoury, E.; Daran, J.C.; Turrin, C.O.; Chiffre, J.; Sournia-Saquet, A.; Knoll, W.; Majoral, J.P.; Caminade, A.M. Synthesis and characterization of water-soluble ferrocene-dendrimers. J. Organomet. Chem. 2012, 718, 22–30. [Google Scholar] [CrossRef]
- Turrin, C.O.; Chiffre, J.; de Montauzon, D.; Balavoine, G.; Manoury, E.; Caminade, A.M.; Majoral, J.P. Behavior of an optically active ferrocene chiral shell located within phosphorus-containing dendrimers. Organometallics 2002, 21, 1891–1897. [Google Scholar] [CrossRef]
- Sebastian, R.M.; Blais, J.C.; Caminade, A.M.; Majoral, J.P. Synthesis and photochemical behavior of phosphorus dendrimers containing azobenzene units within the branches and/or on the surface. Chem. Eur. J. 2002, 8, 2172–2183. [Google Scholar] [CrossRef]
- Deloncle, R.; Coppel, Y.; Rebout, C.; Majoral, J.P.; Caminade, A.M. Characterization of two series of nitrogen-containing dendrimers by natural abundance N-15 NMR. Magn. Reson. Chem. 2008, 46, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Mongin, O.; Rouxel, C.; Vabre, J.M.; Mir, Y.; Pla-Quintana, A.; Wei, Y.Q.; Caminade, A.M.; Majoral, J.P.; Blanchard-Desce, M. Customized multiphotonics nanotools for bioapplications: Soft organic nanodots as an eco-friendly alternative to quantum dots. In Nanobiosystems: Processing, Characterization, and Applications Ii; Kobayashi, N., Ouchen, F., Rau, I., Eds.; Spie-Int Soc Optical Engineering: Bellingham, WA, USA, 2009; Volume 7403. [Google Scholar]
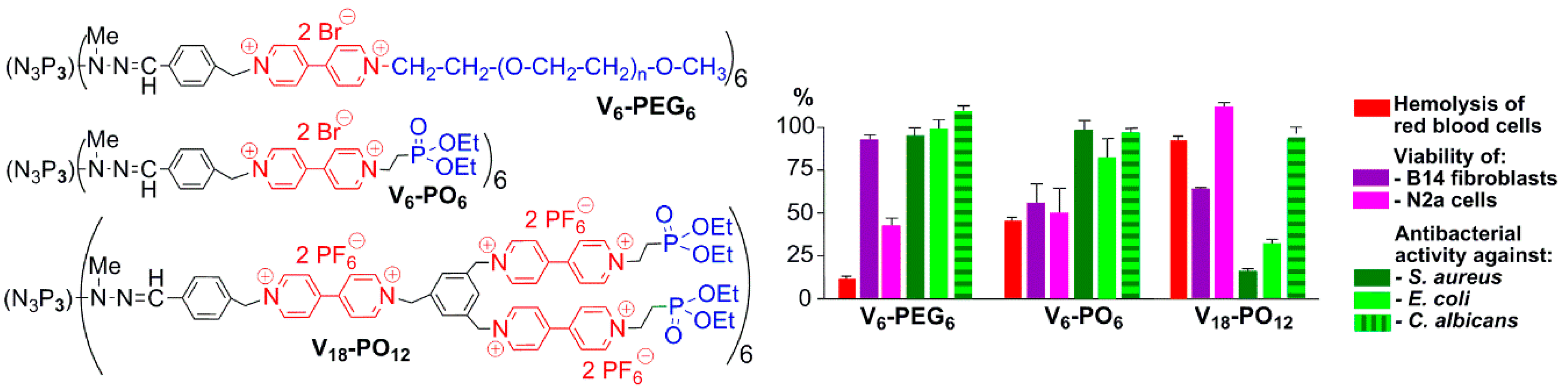
- Katir, N.; Majoral, J.P.; El Kadib, A.; Caminade, A.M.; Bousmina, M. Molecular and Macromolecular Engineering with Viologens as Building Blocks: Rational Design of Phosphorus-Viologen Dendritic Structures. Eur. J. Org. Chem. 2012, 2012, 269–273. [Google Scholar] [CrossRef]
- Ciepluch, K.; Katir, N.; El Kadib, A.; Weber, M.; Caminade, A.M.; Bousmina, M.; Majoral, J.P.; Bryszewska, M. Photo-physical and structural interactions between viologen phosphorus-based dendrimers and human serum albumin. J. Lumin. 2012, 132, 1553–1563. [Google Scholar] [CrossRef]
- Ciepluch, K.; Katir, N.; El Kadib, A.; Felczak, A.; Zawadzka, K.; Weber, M.; Klajnert, B.; Lisowska, K.; Caminade, A.M.; Bousmina, M.; et al. Biological Properties of New Viologen-Phosphorus Dendrimers. Mol. Pharm. 2012, 9, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Ciepluch, K.; Weber, M.; Katir, N.; Caminade, A.M.; El Kadib, A.; Klajnert, B.; Majoral, J.P.; Bryszewska, M. Effect of viologen-phosphorus dendrimers on acetylcholinesterase and butyrylcholinesterase activities. Int. J. Biol. Macromol. 2013, 54, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Naha, P.C.; Davoren, M.; Lyng, F.M.; Byrne, H.J. Reactive oxygen species (ROS) induced cytokine production and cytotoxicity of PAMAM dendrimers in J774A.1 cells. Toxicol. Appl. Pharmacol. 2010, 246, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Naha, P.C.; Byrne, H.J. Generation of intracellular reactive oxygen species and genotoxicity effect to exposure of nanosized polyamidoamine (PAMAM) dendrimers in PLHC-1 cells in vitro. Aquat. Toxicol. 2013, 132, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Lazniewska, J.; Janaszewska, A.; Milowska, K.; Caminade, A.M.; Mignani, S.; Katir, N.; El Kadib, A.; Bryszewska, M.; Majoral, J.P.; Gabryelak, T.; et al. Promising Low-Toxicity of Viologen-Phosphorus Dendrimers against Embryonic Mouse Hippocampal Cells. Molecules 2013, 18, 12222–12240. [Google Scholar] [CrossRef] [PubMed]
- Milowska, K.; Szwed, A.; Zablocka, M.; Caminade, A.M.; Majoral, J.P.; Mignani, S.; Gabryelak, T.; Bryszewska, M. In vitro PAMAM, phosphorus and viologen-phosphorus dendrimers prevent rotenone-induced cell damage. Int. J. Pharm. 2014, 474, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kottari, N.; Chabre, Y.M.; Abbassi, L.; Shiao, V.; Roy, R. A highly versatile convergent/divergent “onion peel” synthetic strategy toward potent multivalent glycodendrimers. Chem. Commun. 2014, 50, 13300–13303. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.; Szwed, A.; El Brahmi, N.; Milowska, K.; Kurowska, J.; Fuentes-Paniagua, E.; Pedziwiatr-Werbicka, E.; Gabryelak, T.; Katir, N.; de la Mata, F.J.; et al. Synthesis, characterization and biological properties of new hybrid carbosilane-viologen-phosphorus dendrimers. RSC Adv. 2015, 5, 25942–25958. [Google Scholar] [CrossRef]
- Katir, N.; El Brahmi, N.; El Kadib, A.; Mignani, S.; Caminade, A.M.; Bousmina, M.; Majoral, J.P. Synthesis of Onion-Peel Nanodendritic Structures with Sequential Functional Phosphorus Diversity. Chem. Eur. J. 2015, 21, 6400–6408. [Google Scholar] [CrossRef] [PubMed]
- Hawker, C.J.; Frechet, J.M.J. Unusual macromolecular architectures—The convergent growth approach to dendritic polyesters and novel block copolymers. J. Am. Chem. Soc. 1992, 114, 8405–8413. [Google Scholar] [CrossRef]
- Brauge, L.; Magro, G.; Caminade, A.M.; Majoral, J.P. First divergent strategy using two AB(2) unprotected monomers for the rapid synthesis of dendrimers. J. Am. Chem. Soc. 2001, 123, 6698–6699. [Google Scholar] Correction: Brauge, L.; Magro, G.; Caminade, A.M.; Majoral, J.P. First divergent strategy using two AB(2) unprotected monomers for the rapid synthesis of dendrimers (vol 123, pg 6698, 2001). J. Am. Chem. Soc. 2001, 123, 8446–8446. [Google Scholar]
- Maraval, V.; Pyzowski, J.; Caminade, A.M.; Majoral, J.P. “Lego” chemistry for the straightforward synthesis of dendrimers. J. Org. Chem. 2003, 68, 6043–6046. [Google Scholar] [CrossRef] [PubMed]
- Maraval, V.; Caminade, A.M.; Majoral, J.P.; Blais, J.C. Dendrimer design: How to circumvent the dilemma of a reduction of steps or an increase of function multiplicity? Angew. Chem. Int. Ed. 2003, 42, 1822–1826. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Brauge, L.; Caminade, A.M.; Majoral, J.P.; Taton, D.; Gnanou, Y. Synthesis and characterization of linear, hyperbranched, and dendrimer-like polymers constituted of the same repeating unit. Chem. Eur. J. 2001, 7, 3095–3105. [Google Scholar] [CrossRef]
- Majoral, J.P.; Caminade, A.M.; Merino, S.; Brauge, L.; Taton, D.; Gnanou, Y. Linear, hyperbranched, and dendrimer-like polymers containing phosphorus: Synthesis and properties. Macromol. Symp. 2001, 174, 301–306. [Google Scholar] [CrossRef]
- Balueva, A.; Merino, S.; Caminade, A.M.; Majoral, J.P. Synthesis of dendrimers with phosphine end groups at each generation. J. Organomet. Chem. 2002, 643, 112–124. [Google Scholar] [CrossRef]
- Furer, V.L.; Vandyukov, A.E.; Majoral, J.P.; Caminade, A.M.; Gottis, S.; Laurent, R.; Kovalenko, V.I. DFT study of structure, IR and Raman spectra of dendrimer with P=N-P=S linkages and its complexation with gold. J. Mol. Struct. 2015, 1084, 103–113. [Google Scholar] [CrossRef]
- Majoral, J.P.; Larre, C.; Laurent, R.; Caminade, A.M. Chemistry in the internal voids of dendrimers. Coord. Chem. Rev. 1999, 192, 3–18. [Google Scholar] [CrossRef]
- Larre, C.; Caminade, A.M.; Majoral, J.P. Chemoselective polyalkylations of phosphorus-containing dendrimers. Angew. Chem. Int. Ed. 1997, 36, 596–599. [Google Scholar] [CrossRef]
- Larre, C.; Donnadieu, B.; Caminade, A.M.; Majoral, J.P. Phosphorus-containing dendrimers: Chemoselective functionalization of internal layers. J. Am. Chem. Soc. 1998, 120, 4029–4030. [Google Scholar] [CrossRef]
- Larre, C.; Bressolles, D.; Turrin, C.; Donnadieu, B.; Caminade, A.M.; Majoral, J.P. Chemistry within megamolecules: Regiospecific functionalization after construction of phosphorus dendrimers. J. Am. Chem. Soc. 1998, 120, 13070–13082. [Google Scholar] [CrossRef]
- Galliot, C.; Larre, C.; Caminade, A.M.; Majoral, J.P. Regioselective stepwise growth of dendrimer units in the internal voids of a main dendrimer. Science 1997, 277, 1981–1984. [Google Scholar] [CrossRef]
- Cadierno, V.; Igau, A.; Donnadieu, B.; Caminade, A.M.; Majoral, J.P. Dendrimers containing zwitterionic phosphonium anionic zirconocene(IV) complexes. Organometallics 1999, 18, 1580–1582. [Google Scholar] [CrossRef]
- Brauge, L.; Caminade, A.M.; Majoral, J.P.; Slomkowski, S.; Wolszczak, M. Segmental mobility in phosphorus-containing dendrimers. Studies by fluorescent spectroscopy. Macromolecules 2001, 34, 5599–5606. [Google Scholar] [CrossRef]
- Severac, M.; Leclaire, J.; Sutra, P.; Caminade, A.M.; Majoral, J.P. A new way for the internal functionalization of dendrimers. Tetrahedron Lett. 2004, 45, 3019–3022. [Google Scholar] [CrossRef]
- Launay, N.; Slany, M.; Caminade, A.M.; Majoral, J.P. Phosphorus-containing dendrimers. Easy access to new multi-difunctionalized macromolecules. J. Org. Chem. 1996, 61, 3799–3805. [Google Scholar] [CrossRef] [PubMed]
- Turrin, C.O.; Chiffre, J.; Daran, J.C.; de Montauzon, D.; Balavoine, G.; Manoury, E.; Caminade, A.M.; Majoral, J.P. New phosphorus-containing dendrimers with ferrocenyl units in each layer. C. R. Chim. 2002, 5, 309–318. [Google Scholar] [CrossRef]
- Neumann, P.; Dib, H.; Sournia-Saquet, A.; Grell, T.; Handke, M.; Caminade, A.M.; Hey-Hawkins, E. Ruthenium Complexes with Dendritic Ferrocenyl Phosphanes: Synthesis, Characterization, and Application in the Catalytic Redox Isomerization of Allylic Alcohols. Chem. Eur. J. 2015, 21, 6590–6604. [Google Scholar] [CrossRef] [PubMed]
- Allgeier, A.M.; Mirkin, C.A. Ligand Design for Electrochemically Controlling Stoichiometric and Catalytic Reactivity of Transition Metals. Angew. Chem. Int. Ed. 1998, 37, 894–908. [Google Scholar] [CrossRef]
- Neumann, P.; Dib, H.; Caminade, A.M.; Hey-Hawkins, E. Redox Control of a Dendritic Ferrocenyl-Based Homogeneous Catalyst. Angew. Chem. Int. Ed. 2015, 54, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Mullen, D.G.; Fang, M.; Desai, A.; Baker, J.R.; Orr, B.G.; Holl, M.M.B. A Quantitative Assessment of Nanoparticle-Ligand Distributions: Implications for Targeted Drug and Imaging Delivery in Dendrimer Conjugates. ACS Nano 2010, 4, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.P.; Huang, B.H.; Choi, S.K.; Silpe, J.E.; Kotlyar, A.; Desai, A.M.; Zong, H.; Gam, J.; Joice, M.; Baker, J.R. Polyvalent Dendrimer-Methotrexate as a Folate Receptor-Targeted Cancer Therapeutic. Mol. Pharm. 2012, 9, 2669–2676. [Google Scholar] [CrossRef] [PubMed]
- Servin, P.; Laurent, R.; Romerosa, A.; Peruzzini, M.; Majoral, J.P.; Caminade, A.M. Synthesis of dendrimers terminated by bis(diphenylphosphinomethyl)amino ligands and use of their palladium complexes for catalyzing C-C cross-coupling reactions. Organometallics 2008, 27, 2066–2073. [Google Scholar] [CrossRef]
- Marchand, P.; Griffe, L.; Poupot, M.; Turrin, C.O.; Bacquet, G.; Fournie, J.J.; Majoral, J.P.; Poupot, R.; Caminade, A.M. Dendrimers ended by non-symmetrical azadiphosphonate groups: Synthesis and immunological properties. Bioorg. Med. Chem. Lett. 2009, 19, 3963–3966. [Google Scholar] [CrossRef] [PubMed]
- Lartigue, M.L.; Slany, M.; Caminade, A.M.; Majoral, J.P. Phosphorus-containing dendrimers: Synthesis of macromolecules with multiple tri- and tetrafunctionalization. Chem. Eur. J. 1996, 2, 1417–1426. [Google Scholar] [CrossRef]
- Riegert, D.; Bareille, L.; Laurent, R.; Majoral, J.P.; Caminade, A.M.; Chaumonnot, A. Silica functionalized by bifunctional dendrimers: Hybrid nanomaterials for trapping CO2. Eur. J. Inorg. Chem. Accepted for publication.
- Slany, M.; Caminade, A.M.; Majoral, J.P. Specific functionalization on the surface of dendrimers. Tetrahedron Lett. 1996, 37, 9053–9056. [Google Scholar] [CrossRef]
- Perez-Anes, A.; Stefaniu, C.; Moog, C.; Majoral, J.P.; Blanzat, M.; Turrin, C.O.; Caminade, A.M.; Rico-Lattes, I. Multivalent catanionic GalCer analogs derived from first generation dendrimeric phosphonic acids. Bioorg. Med. Chem. 2010, 18, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Blanzat, M.; Turrin, C.O.; Aubertin, A.M.; Couturier-Vidal, C.; Caminade, A.M.; Majoral, J.P.; Rico-Lattes, I.; Lattes, A. Dendritic catanionic assemblies: In vitro anti-HIV activity of phosphorus-containing dendrimers bearing Gal beta(1)cer analogues. ChemBioChem 2005, 6, 2207–2213. [Google Scholar] [CrossRef] [PubMed]








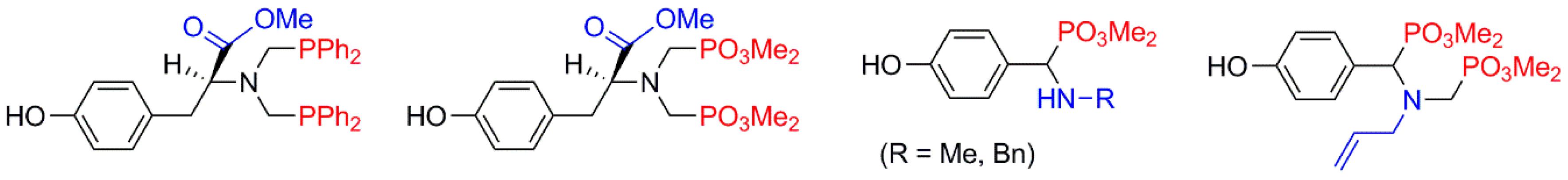

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caminade, A.-M.; Majoral, J.-P. Bifunctional Phosphorus Dendrimers and Their Properties. Molecules 2016, 21, 538. https://doi.org/10.3390/molecules21040538
Caminade A-M, Majoral J-P. Bifunctional Phosphorus Dendrimers and Their Properties. Molecules. 2016; 21(4):538. https://doi.org/10.3390/molecules21040538
Chicago/Turabian StyleCaminade, Anne-Marie, and Jean-Pierre Majoral. 2016. "Bifunctional Phosphorus Dendrimers and Their Properties" Molecules 21, no. 4: 538. https://doi.org/10.3390/molecules21040538






