Repellent Activity of the Essential Oil from the Heartwood of Pilgerodendron uviferum (D. Don) Florin against Aegorhinus superciliosus (Coleoptera: Curculionidae)
Abstract
:1. Introduction
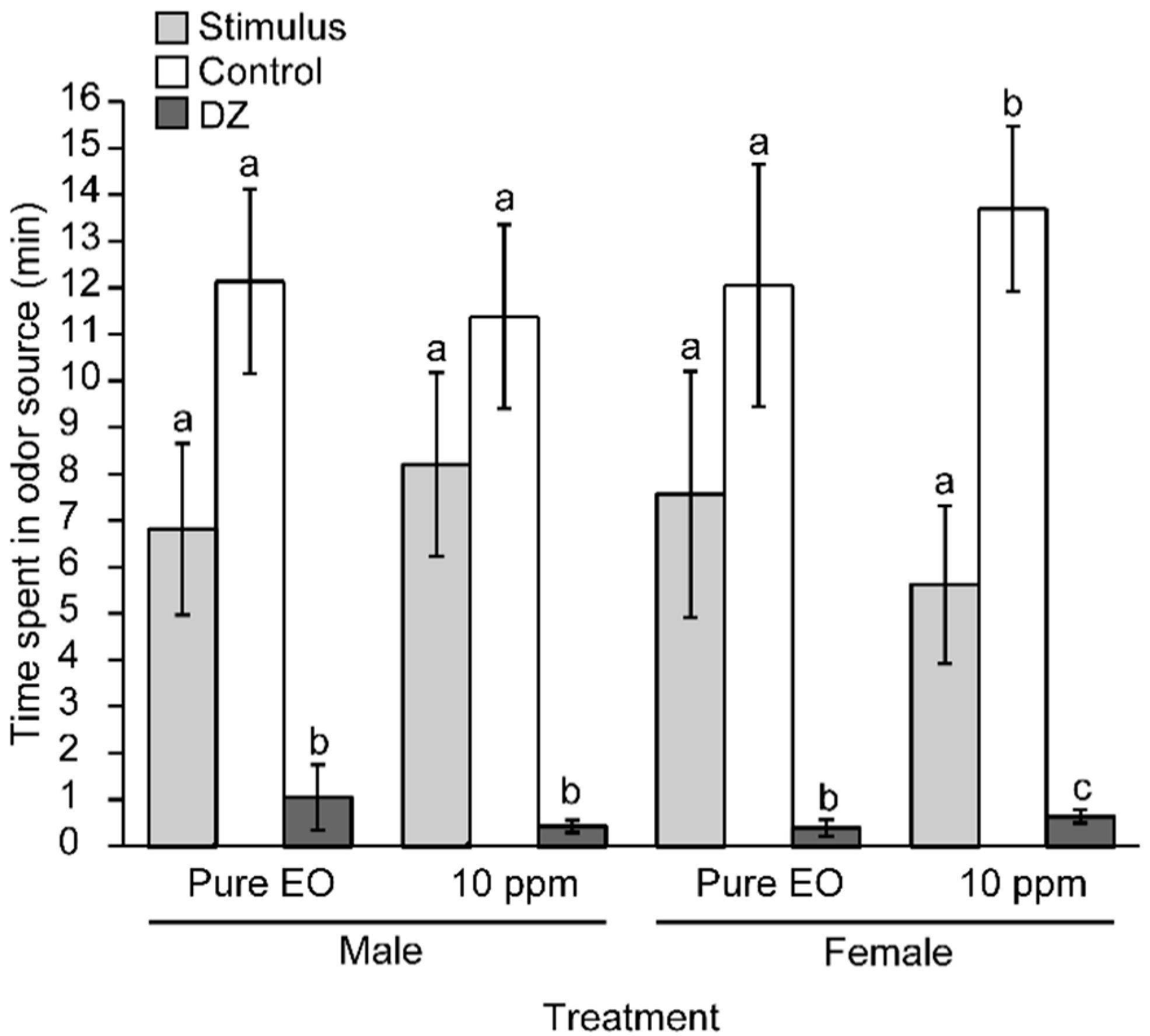
2. Results and Discussion
3. Experimental Section
3.1. Insects
3.2. Plant Material
3.3. Essential Oil Extraction and Analysis by GC/MS
3.4. Olfactometer Bioassays
3.5. Data Analyses
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zavala, A.; Elgueta, M.; Abarzúa, J.; Aguilera, A.; Quiroz, A.; Rebolledo, R. Diversity and distribution of the Aegorhinus genus in the La Araucanía Region of chile, with special reference to A. superciliosus and A. nodipennis. Cienc. Investig. Agrar. 2011, 38, 367–377. [Google Scholar] [CrossRef]
- Medel, V.; Palma, R.; Mercado, D.; Rebolledo, R.; Quiroz, A.; Mutis, A. The effect of protease inhibitors on digestive proteolytic activity in the Raspberry Weevil, Aegorhinus superciliosus (Guerin) (coleoptera: Curculionidae). Neotrop. Entomol. 2015, 44, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, P.A.; Zampezzi, V.M.; Araneda, D.X.; Klein, K.C.; Rebolledo, R.R. Efectividad de la azadirachtina en la inhibición de la embriogénesis de Aegorhinus superciliosus (Guérin) (Coleoptera: Curculionidae). IDESIA 2009, 27, 47–55. [Google Scholar] [CrossRef]
- Parra, L.; Mutis, A.; Aguilera, A.; Rebolledo, A.; Quiroz, A. Knowledge of the “Cabrito del frambueso” weevil (CF) Aegorhinus superciliosus (Guérin) (Coleoptera: Curculionidae). IDESIA 2009, 27, 57–65. [Google Scholar] [CrossRef]
- Mutis, A.; Parra, L.; Manosalva, L.; Palma, R.; Candia, O.; Lizama, M.; Pardo, F.; Perich, F.; Quiroz, A. Electroantennographic and behavioral responses of adults of raspberry weevil Aegorhinus superciliosus (Coleoptera: Curculionidae) to odors released from conspecific females. Environ. Entomol. 2010, 39, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, M.H. The potential of secondary metabolites in plant material as deterents against insect pests: A review. Afr. J. Pure Appl. Chem. 2010, 4, 243–246. [Google Scholar]
- Kim, S.-I.; Yoon, J.-S.; Jung, J.W.; Hong, K.-B.; Ahn, Y.-J.; Kwon, H.W. Toxicity and repellency of origanum essential oil and its components against Tribolium castaneum (Coleoptera: Tenebrionidae) adults. J. Asia Pac. Entomol. 2010, 13, 369–373. [Google Scholar] [CrossRef]
- Yáñez, M.; France, A. Effects of fungicides on the development of the entomopathogenic fungus Metarhizium anisopliae var. Anisopliae. Chil. J. Agric. Res. 2010, 70, 390–398. [Google Scholar]
- Parra, L.; Mutis, A.; Ceballos, R.; Lizama, M.; Pardo, F.; Perich, F.; Quiroz, A. Volatiles released from Vaccinium corymbosum were attractive to Aegorhinus superciliosus (Coleoptera: Curculionidae) in an olfactometric bioassay. Environ. Entomol. 2009, 38, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Urzúa, A.; Santander, R.; Echeverría, J.; Villalobos, C.; Palacios, S.M.; Rossi, Y. Insecticidal properties of Peumus Boldus Mol. essential oil on the House Fly, Musca domestica L. Bol. Latinoam. Caribe Plant Med. Aromat. 2010, 9, 465–469. [Google Scholar]
- Olivero-Verbel, J.; Tirado-Ballestas, I.; Caballero-Gallardo, K.; Stashenko, E.E. Essential oils applied to the food act as repellents toward Tribolium castaneum. J. Stored Prod. Res. 2013, 55, 145–147. [Google Scholar] [CrossRef]
- Kalita, B.; Bora, S.; Sharma, A.K. Plant essential oils as mosquito repellent—A review. Int. J. Res. Dev. Pharm. Life Sci. 2013, 3, 741–747. [Google Scholar]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Stefanazzi, N.; Stadler, T.; Ferrero, A. Composition and toxic, repellent and feeding deterrent activity of essential oils against the stored-grain pests Tribolium castaneum (Coleoptera: Tenebrionidae) and Sitophilus oryzae (Coleoptera: Curculionidae). Pest Manag. Sci. 2011, 67, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Solís, C.; Becerra, J.; Flores, C.; Robledo, J.; Silva, M. Antibacterial and antifungal terpenes from Pilgerodendron uviferum (D. Don) Florin. J. Chil. Chem. Soc. 2004, 49, 157–161. [Google Scholar] [CrossRef]
- Donoso, C.A.; Becerra, J.; Bittner, M.; Elissetche, J.P.; Freer, J.; Mendoza, R.; Sterner, O.; Silva, M. Allelochemicals and natural durability in Chilean Cupressaceae heartwoods. Allelopath. J. 2008, 21, 119–132. [Google Scholar]
- Oyarzún, M.L.; Garbarino, J.A. Sesquiterpenoids from Pilgerodendron uvífera. Phytochemistry 1988, 27, 1121–1123. [Google Scholar] [CrossRef]
- Malizia, R.A.; Cardell, D.A.; Molli, J.S.; González, S.; Guerra, P.E.; Grau, R.J. Volatile constituents of leaf oils from the Cupressacea family: Part II. Austrocedrus chilensis, Fitzroya cupressoides and Pilgerodendron uviferum species growing in Argentina. J. Essent. Oil Res. 2000, 12, 233–237. [Google Scholar] [CrossRef]
- Mutis, A.; Parra, L.; Palma, R.; Pardo, F.; Perich, F.; Quiroz, A. Evidence of contact pheromone use in mating behavior of the raspberry weevil (Coleoptera: Curculionidae). Environ. Entomol. 2009, 38, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Lancelle, H.G.; Giordano, O.S.; Sosa, M.E.; Tonn, C.E. Chemical composition of four essential oils from Eupatorium spp. Biological activities toward Tribolium castaneum (Coleoptera: Tenebrionidae). Rev. Soc. Entomol. Argent 2009, 68, 329–338. [Google Scholar]
- Khani, A.; Heydarian, M. Fumigant and repellent properties of sesquiterpene-rich essential oil from Teucrium polium subsp. Capitatum (L.). Asian Pac. J. Trop. Med. 2014, 7, 956–961. [Google Scholar] [CrossRef]
- Wang, C.-F.; Yang, K.; You, C.-X.; Zhang, W.-J.; Guo, S.-S.; Geng, Z.-F.; Du, S.-S.; Wang, Y.-Y. Chemical composition and insecticidal activity of essential oils from Zanthoxylum dissitum leaves and roots against three species of storage pests. Molecules 2015, 20, 7990–7999. [Google Scholar] [CrossRef] [PubMed]
- Tingey, D.T.; Manning, M.; Grothaus, L.C.; Burns, W.F. Influence of light and temperature on monoterpene emission rates from slash pine. Plant Physiol. 1980, 65, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Fichan, I.; Larroche, C.; Gros, J.B. Water solubility, vapor pressure, and activity coefficients of terpenes and terpenoids. J. Chem. Eng. Data 1999, 44, 56–62. [Google Scholar] [CrossRef]
- Ortega, J.; Helmig, D. Approaches for quantifying reactive and low-volatility biogenic organic compound emissions by vegetation enclosure techniques—Part A. Chemosphere 2008, 72, 343–364. [Google Scholar] [CrossRef] [PubMed]
- Paluch, G.; Grodnitzky, J.; Bartholomay, L.; Coats, J. Quantitative structure-activity relationship of botanical sesquiterpenes: Spatial and contact repellency to the yellow fever mosquito, Aedes aegypti. J. Agric. Food Chem. 2009, 57, 7618–7625. [Google Scholar] [CrossRef] [PubMed]
- Koul, O.; Walia, S.; Dhaliwal, G. Essential oils as green pesticides: Potential and constraints. Biopestic. Int. 2008, 4, 63–84. [Google Scholar]
- Rattan, R.S. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 2010, 29, 913–920. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Tampe, J.; Parra, L.; Huaiquil, K.; Mutis, A.; Quiroz, A. Repellent effect and metabolite volatile profile of the essential oil of Achillea millefolium against Aegorhinus nodipennis (Hope) (Coleoptera: Curculionidae). Neotrop. Entomol. 2015, 44, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Tapia, S.; Pardo, F.; Perich, F.; Quiroz, A. Clover root borer Hylastinus obscurus (Marsham) (Coleoptera: Scolytidae) has no preference for volatiles from root extracts of disease infected red clover. Acta Agric. Scand. B 2005, 55, 158–160. [Google Scholar]
- Noldus, L.P.J.J.; Spink, A.J.; Tegelenbosch, R.A.J. Computerised video tracking, movement analysis and behaviour recognition in insects. Comput. Electron. Agric. 2002, 35, 201–227. [Google Scholar] [CrossRef]
- StatsDirect Ltd. StatsDirect Statistical Software; StatsDirect Ltd.: Cheshire, UK, 2013. [Google Scholar]
- Sample Availability: Samples of the EOs are not available from the authors.
| RT | RI | Compound | % | Identification |
|---|---|---|---|---|
| 19.64 | 1033 | p-Cymene | 0.12 | RI, MS |
| 19.78 | 1037 | Limonene | 0.24 | RI, MS |
| 19.92 | 1042 | Eucalyptol | 0.06 | RI, MS |
| 29.54 | 1365 | α-Cubebene | 0.05 | RI, MS |
| 30.34 | 1394 | Copaene | 0.71 | RI, MS |
| 31.60 | 1444 | Caryophyllene | 1.27 | RI, MS |
| 31.93 | 1458 | α-Guaiene | 0.06 | RI, MS |
| 32.47 | 1479 | Humulene | 1.33 | RI, MS |
| 32.86 | 1494 | β-Cadinene | 1.37 | RI, MS |
| 33.49 | 1520 | α-Amorphene | 0.96 | RI, MS |
| 34.04 | 1544 | δ-Cadinene | 10.81 | RI, MS |
| 34.32 | 1556 | 1,4-Cadinadiene | 0.31 | RI, MS |
| 34.46 | 1561 | 4,5,9,10-Dehydroisolongifolene | 0.26 | RI, MS |
| 34.62 | 1568 | α-Calacorene | 0.95 | RI, MS |
| 35.56 | 1607 | γ-Elemene | 4.75 | RI, MS |
| 35.72 | 1615 | Caryophyllene oxide | 1.02 | RI, MS |
| 36.33 | 1642 | 1,2-Epoxyhumulene | 0.51 | RI, MS |
| 36.65 | 1656 | δ-Cadinol | 24.16 | RI, MS |
| 36.99 | 1671 | Cubenol | 22.64 | RI, MS |
| 37.16 | 1678 | 15-Copaenol | 15.46 | RI, MS |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza, J.; Urzúa, A.; Tampe, J.; Parra, L.; Quiroz, A. Repellent Activity of the Essential Oil from the Heartwood of Pilgerodendron uviferum (D. Don) Florin against Aegorhinus superciliosus (Coleoptera: Curculionidae). Molecules 2016, 21, 533. https://doi.org/10.3390/molecules21040533
Espinoza J, Urzúa A, Tampe J, Parra L, Quiroz A. Repellent Activity of the Essential Oil from the Heartwood of Pilgerodendron uviferum (D. Don) Florin against Aegorhinus superciliosus (Coleoptera: Curculionidae). Molecules. 2016; 21(4):533. https://doi.org/10.3390/molecules21040533
Chicago/Turabian StyleEspinoza, Javier, Alejandro Urzúa, Jocelyne Tampe, Leonardo Parra, and Andrés Quiroz. 2016. "Repellent Activity of the Essential Oil from the Heartwood of Pilgerodendron uviferum (D. Don) Florin against Aegorhinus superciliosus (Coleoptera: Curculionidae)" Molecules 21, no. 4: 533. https://doi.org/10.3390/molecules21040533
APA StyleEspinoza, J., Urzúa, A., Tampe, J., Parra, L., & Quiroz, A. (2016). Repellent Activity of the Essential Oil from the Heartwood of Pilgerodendron uviferum (D. Don) Florin against Aegorhinus superciliosus (Coleoptera: Curculionidae). Molecules, 21(4), 533. https://doi.org/10.3390/molecules21040533






