Identification of Subnanometric Ag Species, Their Interaction with Supports and Role in Catalytic CO Oxidation
Abstract
:1. Introduction
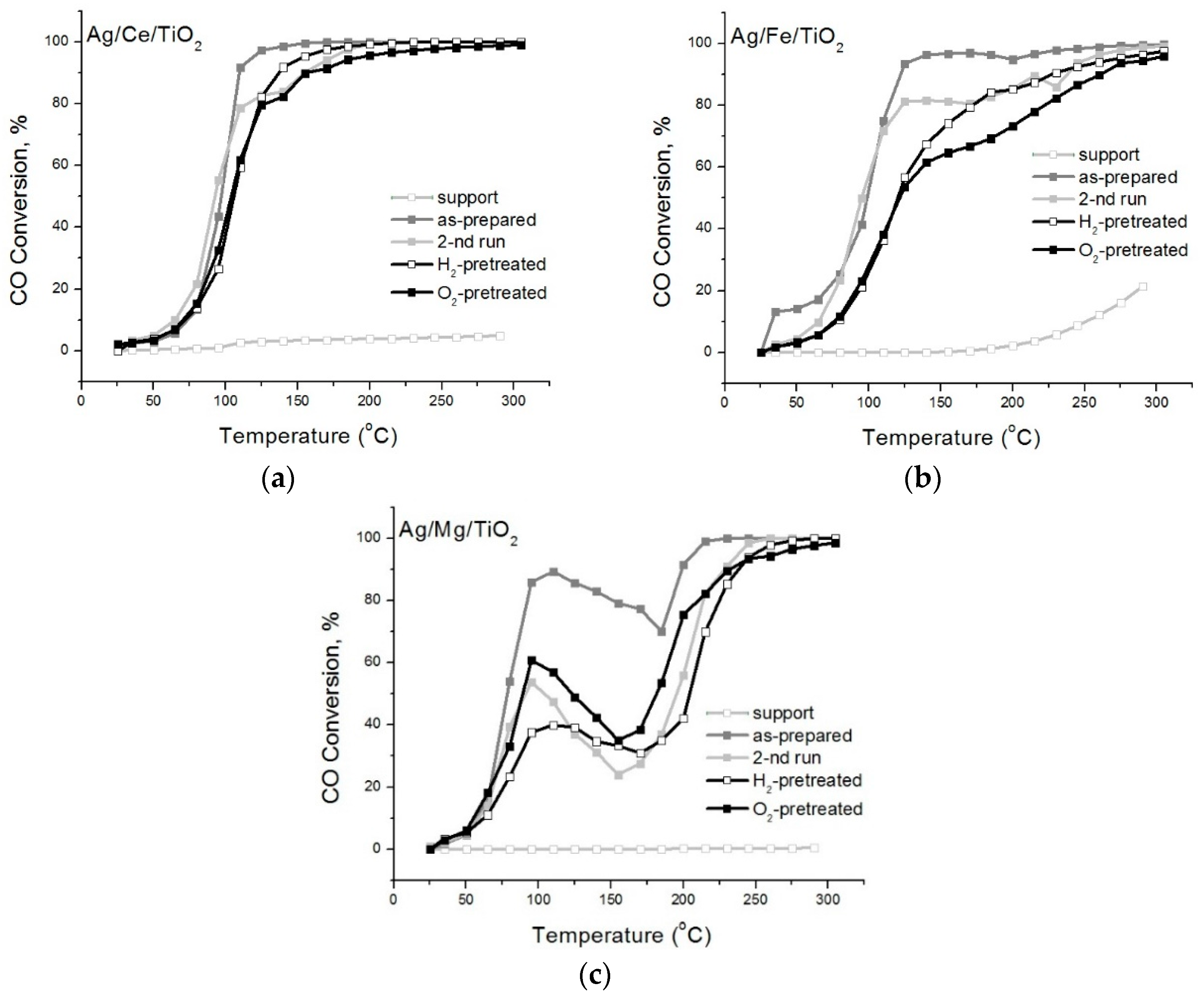
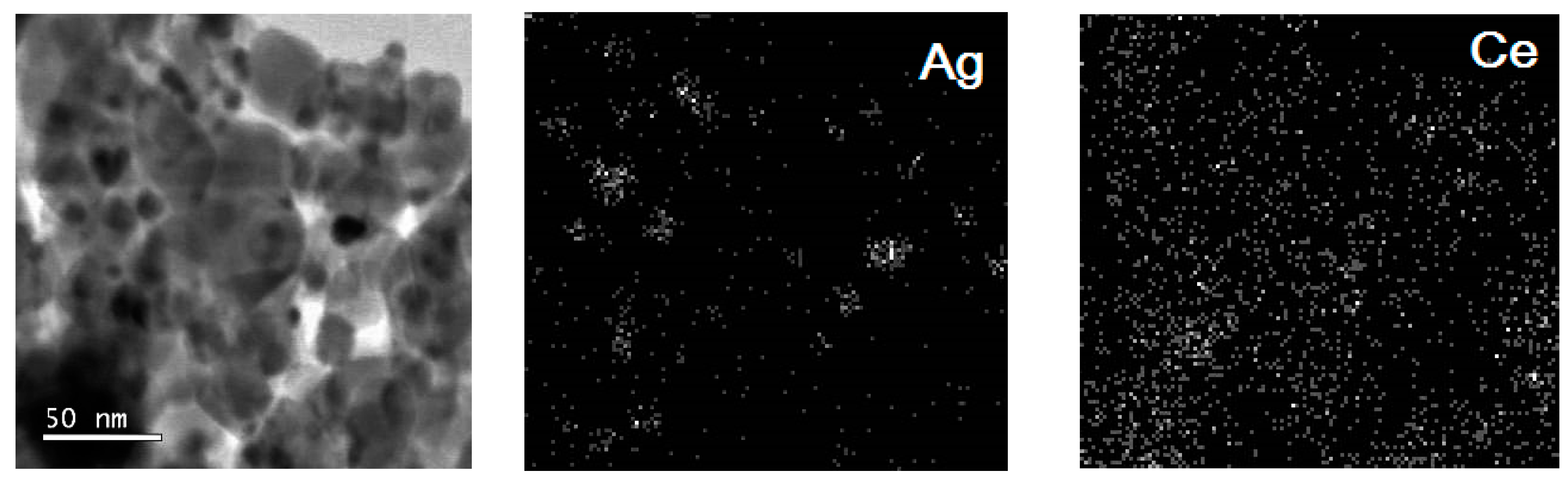
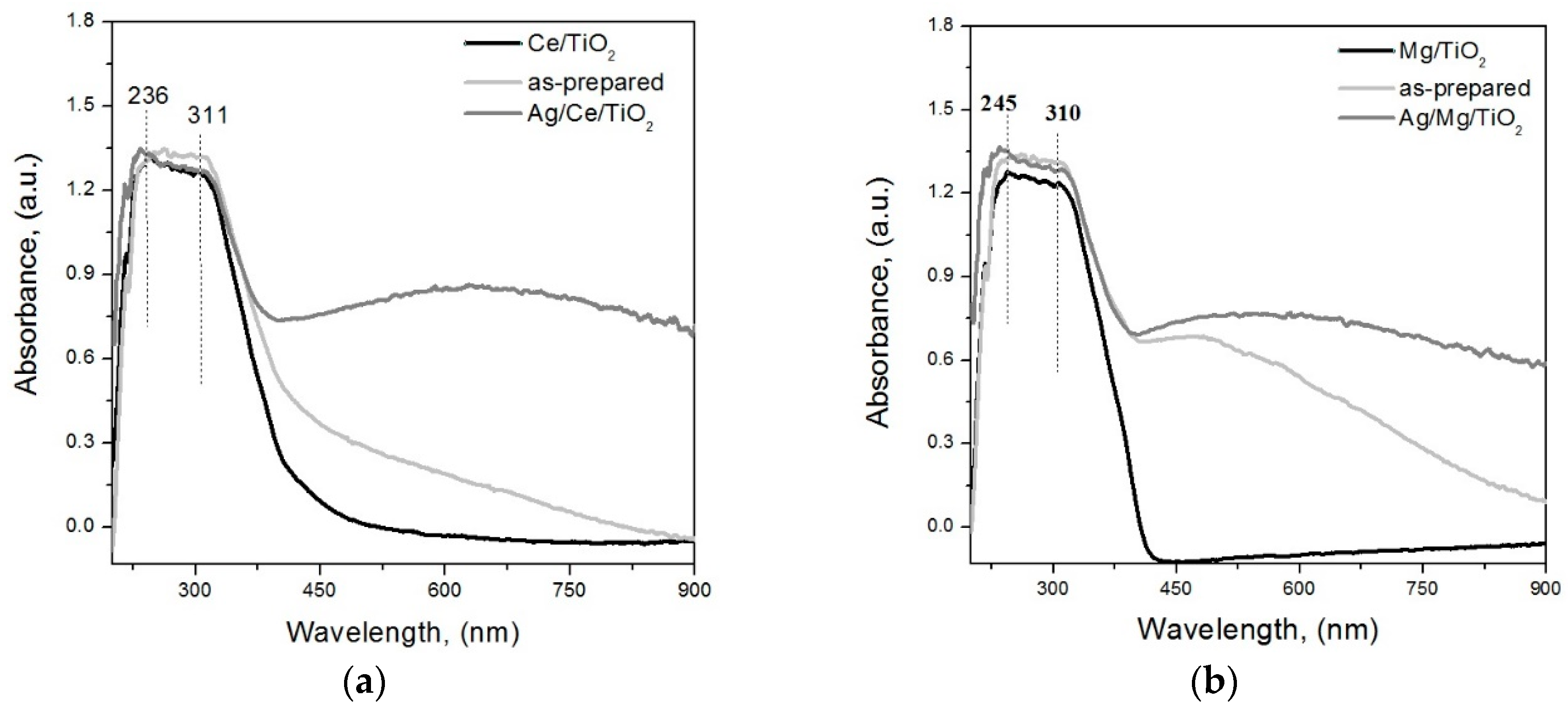
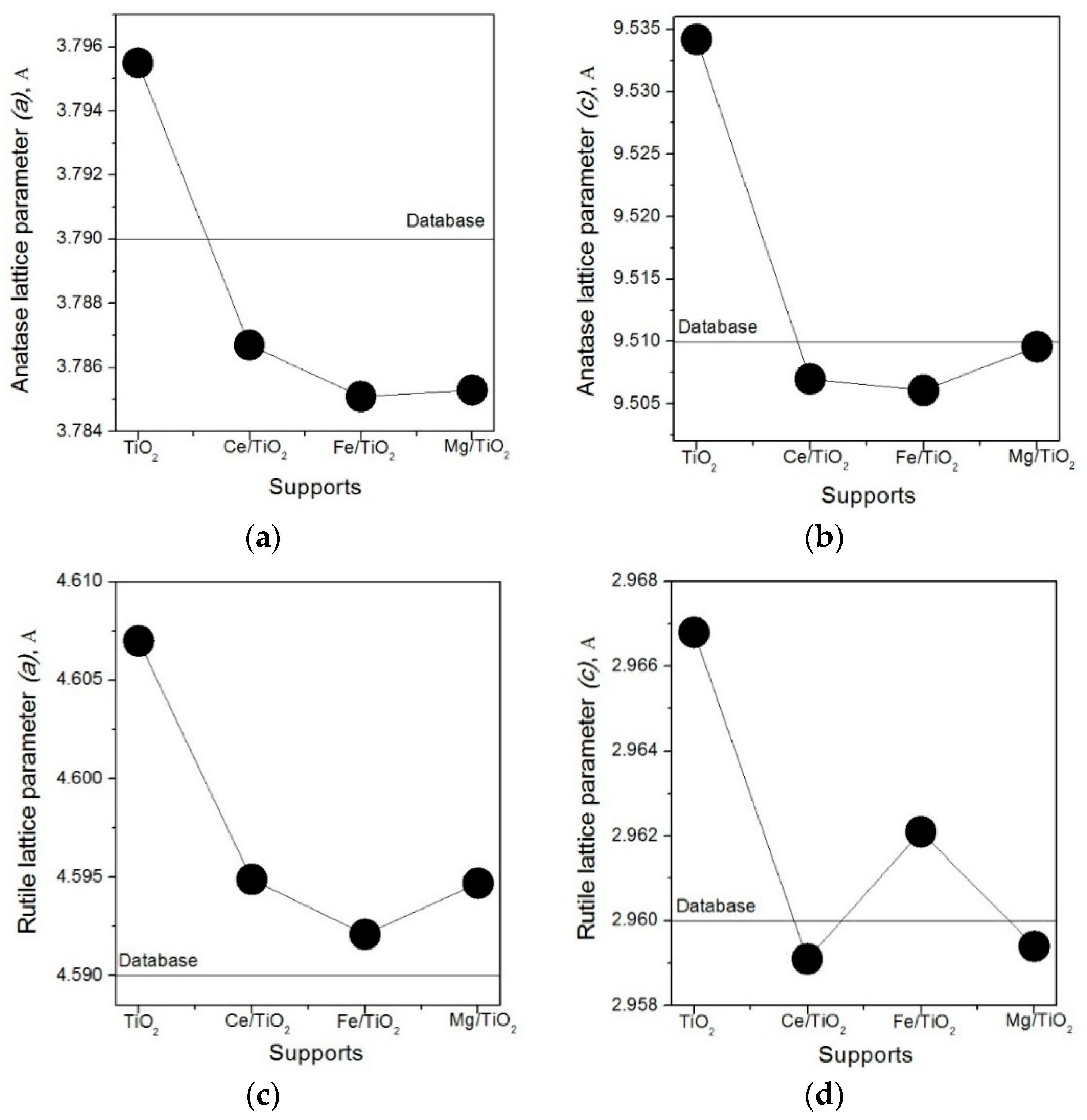
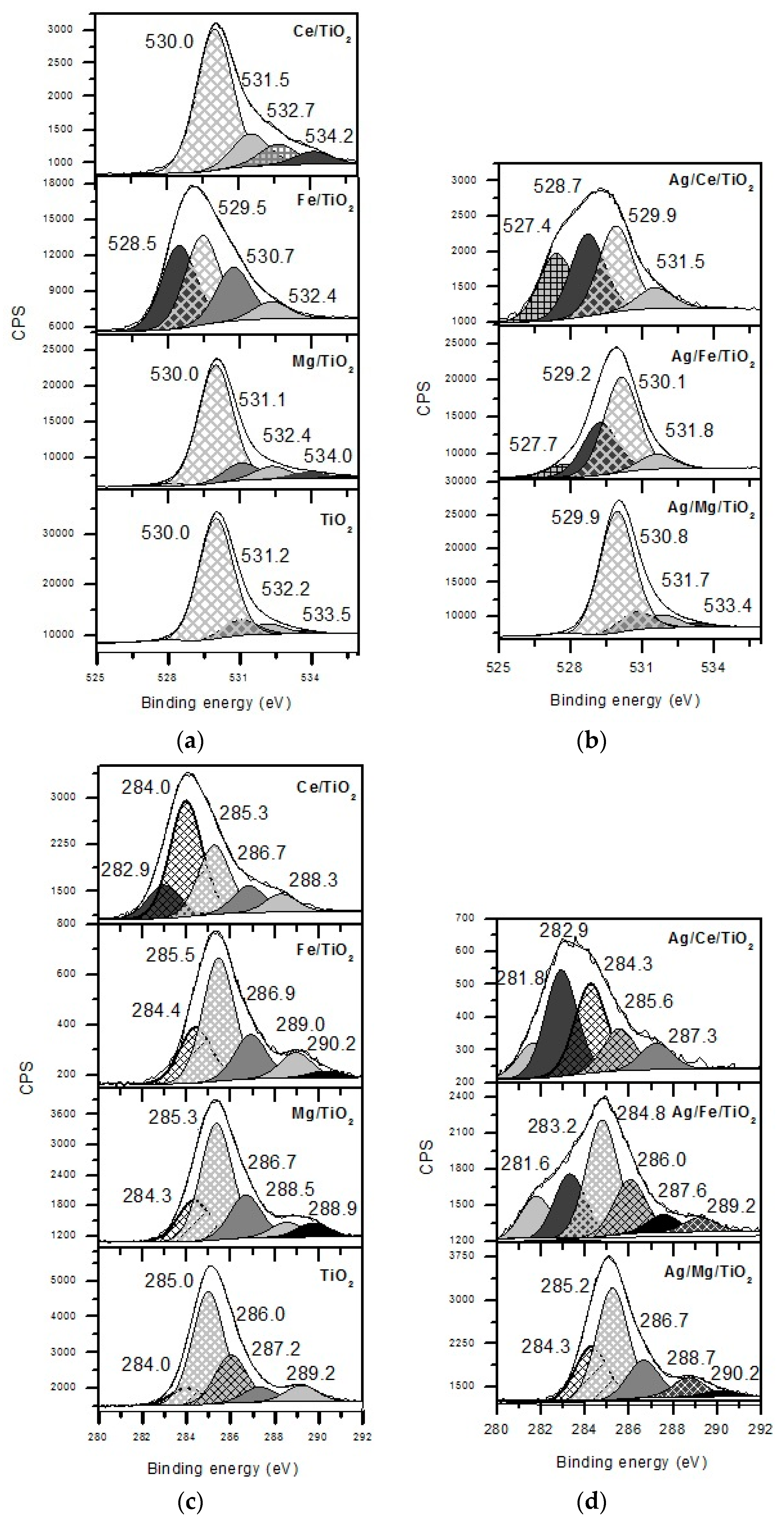
2. Results and Discussion
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Catalytic Tests
3.3. Samples’ Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ac-HAADF/STEM | aberration-corrected high angle annular dark field scanning transmission electron microscopy |
| BE | binding energy |
| CPS | Counts per second. |
| DFT | density functional theory |
| DRS | diffuse reflectance spectroscopy in ultraviolet and visible diapason |
| EDS | energy dispersive spectroscopy |
| EXAFS | extended X-ray absorption fine structure |
| FTIR CO | Fourier-transform infrared spectroscopy of adsorbed CO. |
| HRTEM | high resolution transmission electron microscopy |
| SMSI | strong metal-support interaction |
| SR-XRD | synchrotron radiation X-ray diffraction |
| TCD | thermal conductivity detector |
| TPR | temperature-programmed reduction |
| UV-VIS | ultraviolet-visible spectroscopy |
| XPS | X-ray photoelectron spectroscopy, |
| XANES | X-ray absorption near edge structure |
| WGS | water-gas shift reaction |
References
- Zhu, H.; Ma, Z.; Clark, J.C.; Pan, Z.; Overbury, S.H.; Dai, S. Low-temperature CO oxidation on Au/fumed SiO2-based catalysts prepared from Au(en)2Cl3 precursor. Appl. Catal. A. 2007, 326, 89–99. [Google Scholar] [CrossRef]
- Bondarchuk, I.S.; Mamontov, G.V. Role of PdAg Interface in Pd–Ag/SiO2 Bimetallic Catalysts in Low Temperature Oxidation of Carbon Monoxide. Kinet. Catal. 2015, 56, 382–388. [Google Scholar] [CrossRef]
- Mamontov, G.V.; Knyazev, A.S.; Paukshtis, E.A.; Vodyankina, O.V. Adsorption and conversion of ethylene glycol on the surface of Ag containing catalyst modified with phosphate. Kinet. Catal. 2013, 54, 735–743. [Google Scholar] [CrossRef]
- Martynova, D.O.; Kibis, L.S.; Stonkus, O.A.; Vodyankina, O.V.; Izaak, T.I.; Slavinskaya, E.M.; Boronin, A.I. Synthesis and Catalytic Activity of Porous Blocked Ag/SiO2 Composites in Low Temperature Carbon Monoxide Oxidation. Kinet. Catal. 2013, 54, 519–523. [Google Scholar] [CrossRef]
- Morgan, K.; Inceesungvorn, B.; Goguet, A.; Hardacre, C.; Meunier, F.C.; Shekhtman, S.O. TAP studies on 2% Ag/c–Al2O3 catalyst for selective reduction of oxygen in a H2-rich ethylene feed. Catal. Sci. Technol. 2012, 2, 2128–2133. [Google Scholar] [CrossRef]
- Guerba, H.; Djellouli, B.; Petit, C.; Pitchon, V. CO oxidation catalyzed by Ag/SBA-15 catalysts: Influence of the hydrothermal treatment. C. R. Chim. 2014, 17, 775–784. [Google Scholar] [CrossRef]
- Inceesungvorn, B.; López-Castro, J.; Calvino, J.J.; Bernal, S.; Meunier, F.C.; Hardacre, C.; Griffin, K.; Delgado, J.J. Nano-structural investigation of Ag/Al2O3 catalyst for selective removal of O2 with excess H2 in the presence of C2H4. Appl. Catal. A Gene. 2011, 391, 187–193. [Google Scholar] [CrossRef]
- Lippits, M.J.; Gluhoi, A.C.; Nieuwenhuys, B.E. A comparative study of the effect of addition of CeOx and Li2O on c-Al2O3 supported copper, silver and gold catalysts in the preferential oxidation of CO. Top. Catal. 2007, 44, 159–165. [Google Scholar] [CrossRef]
- Imaoka, T.; Kitazawa, H.; Chun, W.-J.; Yamamoto, K. Finding the Most Catalytically Active Platinum Clusters With Low Atomicity. Angew. Chem. Int. Ed. 2015, 54, 9810–9815. [Google Scholar] [CrossRef] [PubMed]
- Berr, M.J.; Schweinberger, F.F.; Döblinger, M.; Sanwald, K.E.; Wolff, C.; Breimeier, J.; Crampton, A.S.; Ridge, C.J.; Tschurl, M.; Heiz, U. Size-selected Subnanometer Cluster Catalysts on Semiconductor Nanocrystal Films for Atomic Scale Insight into Photocatalysis. Nano Lett. 2012, 12, 5903–5906. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Vidal, N.; Rivas, J.; Lopez-Quintela, M.A. Size dependent catalytic activity of reusable subnanometer copper(0) clusters. ACS Catal. 2012, 2, 1693–1697. [Google Scholar] [CrossRef]
- Pestryakov, A.N.; Lunin, V.V.; Kharlanov, A.N.; Bogdanchikova, N.E.; Tuzovskaya, I.V. Electronic state of gold in supported clusters. Eur. Phys. J. D 2003, 24, 307–309. [Google Scholar] [CrossRef]
- Bogdanchikova, N.; Pestryakov, A.; Farias, M.H.; Diaz, J.A.; Avalos, M.; Navarrete, J. Formation of TEM- and XRD-undetectable gold clusters accompanying big gold particles on TiO2-SiO2 supports. Solid State Sci. 2008, 10, 908–914. [Google Scholar] [CrossRef]
- Flytzani-Stephanopoulos, M.; Gates, B.C. Atomically Dispersed Supported Metal Catalysts. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 545–574. [Google Scholar]
- Sun, S.; Zhang, G.; Gauquelin, N.; Chen, N.; Zhou, J.; Yang, S.; Chen, W.; Meng, X.; Geng, D.; Banis, M.N.; et al. Single-atom Catalysis Using Pt/Graphene Achieved through Atomic Layer Deposition. Sci. Rep. 2013, 3, 1–9. [Google Scholar] [CrossRef]
- Allard, L.F.; Flytzani-Stephanopoulos, M.; Overbury, S.H. Behavior of Au Species in Au/Fe2O3 Catalysts Characterized by Novel In Situ Heating Techniques and Aberration-Corrected STEM Imaging. Microsc. Microanal. 2010, 16, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Lessard, J.D.; Valsamakis, I.; Flytzani-Stephanopoulos, M. Novel Au/La2O3 and Au/La2O2SO4 catalysts for the water–gas shift reaction prepared via an anion adsorption method. Chem. Commun. 2012, 48, 4857–4859. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; He, G.; Akey, A.J.; Si, R.; Flytzani-Stephanopoulos, M.; Herman, I.P. Raman Analysis of Mode Softening in Nanoparticle CeO2-δ and Au-CeO2-δ during CO Oxidation. J. Am. Chem. Soc. 2011, 133, 12952–12955. [Google Scholar] [CrossRef] [PubMed]
- Flytzani-Stephanopoulos, M. Gold Atoms Stabilized on Various Supports Catalyze the Water Gas Shift Reaction. Acc. Chem. Res. 2014, 47, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, S.; Wang, Y.; Herron, J.A.; Xu, Y.; Allard, L.F.; Lee, S.; Huang, J.; Mavrikakis, M.; Flytzani-Stephanopoulos, M. Catalytically active Au-O(OH)x species stabilized by alkali ions on zeolites and mesoporous oxides. Science 2014, 364, 1498–1501. [Google Scholar] [CrossRef] [PubMed]
- Carabineiro, S.A.C.; Chen, X.; Martynyuk, O.; Bogdanchikova, N.; Avalos-Borja, M.; Pestryakov, A.; Tavares, P.B.; Orfao, J.J.M.; Pereira, M.F.R.; Figueiredo, J.L. Gold supported on metal oxides for volatile organic compounds total oxidation. Catal. Today 2015, 244, 103–114. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Bogdanchikova, N.; Pestryakov, A.; Tavares, P.B.; Fernandes, L.; Figueiredo, J.L. Gold nanoparticles supported on magnesium oxide for CO oxidation. Nanoscale Res. Lett. 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Carabineiro, S.A.C.; Bogdanchikova, N.; Avalos-Borja, M.; Pestryakov, A.; Tavares, P.B.; Figueiredo, J.L. Gold supported on metal oxides for carbon monoxide oxidation. Nano Res. 2011, 4, 180–193. [Google Scholar] [CrossRef]
- Shekhar, M.; Wang, J.; Lee, W.-S.; Williams, W.D.; Min Kim, S.; Stach, E.A.; Miller, J.T.; Delgass, W.N.; Ribeiro, F.H. Size and Support Effects for the Water-Gas Shift Catalysis over Gold Nanoparticles Supported on Model Al2O3 and TiO2. J. Am. Chem. Soc. 2012, 134, 4700–4708. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.A.; Senanayake, S.D.; Stacchiola, D.; Liu, P. Hrbek, The Activation of gold and the water-gas shift reaction: Insights from Studies with model catalysts. J. Acc. Chem. Res. 2014, 47, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Gracia, J.; Nieuwenhuys, B.E.; Niemantsverdriet, J.W. (Hans) Explicit roles of Au and TiO2 in a bifunctional Au/TiO2 catalyst for the water-gas shift reaction: A DFT Study. ChemCatChem 2013, 5, 2479–2488. [Google Scholar] [CrossRef]
- Boccuzzi, F.; Chiorino, A.; Manzoli, M.; Andreeva, D.; Tabakova, T. FTIR Study of the low-temperature water-gas shift reaction on Au/Fe2O3 and Au/TiO2 Catalysts. J. Catal. 1999, 188, 176–185. [Google Scholar] [CrossRef]
- Qu, Z.; Huang, W.; Cheng, M.; Bao, X. Restructuring and Redispersion of Silver on SiO2 under Oxidizing/Reducing Atmospheres and Its Activity toward CO Oxidation. J. Phys. Chem. B 2005, 109, 15842–15848. [Google Scholar] [CrossRef] [PubMed]
- Pestryakov, A.N.; Lunin, V.V. Physicochemical study of active sites of metal catalysts for alcohol partial oxidation. J. Mol. Catal. A Chem. 2000, 158, 325–329. [Google Scholar] [CrossRef]
- Tabakova, T.; Boccuzzi, F.; Manzoli, M.; Chiorino, A.; Andreeva, D. Characterization of nanosized gold, silver and copper catalysts supported on ceria. Stud. Surf. Sci. Catal. 2005, 155, 493–500. [Google Scholar]
- Boccuzzi, F.; Chiorino, A.; Manzoli, M.; Andreeva, D.; Tabakova, T.; Ilieva, L.; Iadakiev, V. Gold, silver and copper catalysts supported on TiO2 for pure hydrogen production. Catal. Today 2002, 75, 169–175. [Google Scholar] [CrossRef]
- Keshavaraja, A.; She, X.; Flytzani-Stephanopoulos, M. Selective catalytic reduction of NO with methane over Ag-alumina catalysts. Appl. Catal. B Environ. 2000, 27, L1–L9. [Google Scholar] [CrossRef]
- Kolobova, E.; Pestryakov, A.; Shemeryankina, A.; Kotolevich, Y.; Martynyuk, O.; Tiznado Vazquez, H.J.; Bogdanchikova, N. Formation of silver active states in Ag/ZSM-5 catalysts for CO oxidation. Fuel 2014, 138, 65–71. [Google Scholar] [CrossRef]
- Bogdanchikova, N.; Tuzovskaya, T.; Pestryakov, A.; Susarrey-Arce, A. Comparative study of formation and stabilization of gold and silver clusters and nanoparticles in mordenites. J. Nanosci. Nanotechnol. 2011, 11, 5476–5482. [Google Scholar] [CrossRef] [PubMed]
- Green, I.X.; Tang, W.; Neurock, M.; Yates Jr, J.T. Spectroscopic Observation of Dual Catalytic Sites During Oxidation of CO on a Au/TiO2 Catalyst. Science 2011, 333, 736–739. [Google Scholar] [CrossRef] [PubMed]
- Parka, J.B.; Graciani, J.; Evans, J.; Stacchiola, D.; Ma, S.; Liu, P.; Nambu, A.; Fernández Sanz, J.; Hrbeka, J.; Rodriguez, J.A. High catalytic activity of Au/CeOx/TiO2(110) controlled by the nature of the mixed-metal oxide at the nanometer level. PNAS 2009, 106, 4975–4980. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Yin, H.; Dai, S. Influence of Preparation Methods on the performance of metal phosphate-supported gold catalysts in CO Oxidation. Catal. Lett. 2010, 138, 40–45. [Google Scholar] [CrossRef]
- Ma, Z.; Yin, H.; Overbury, S.H.; Dai, S. Metal Phosphates as a New Class of Supports for Gold Nanocatalysts. Catal. Lett. 2008, 126, 20–30. [Google Scholar] [CrossRef]
- Pestryakov, A.N.; Bogdanchikova, N.; Simakov, A.; Tuzovskaya, I.; Jentoft, F.; Farias, M.; Díaz, A. Catalytically active gold clusters and nanoparticles for CO oxidation. Surf. Sci. 2007, 601, 3792–3795. [Google Scholar] [CrossRef]
- Pestryakov, A.; Tuzovskaya, I.; Smolentseva, E.; Bogdanchikova, N.; Jentoft, F.C.; Knop-Gericke, A. Formation of gold nanoparticles in zeolites. Int. J. Modern Phys. B 2005, 19, 2321–2326. [Google Scholar] [CrossRef]
- Smolentseva, E.; Bogdanchikova, N.; Simakov, A.; Pestryakov, A.; Avalos, M.; Farias, M.H.; Tompos, A.; Gurin, V. Catalytic activity of gold nanoparticles incorporated into modified zeolites. J. Nanosci. Nanotechnol. 2007, 7, 1882–1886. [Google Scholar] [CrossRef] [PubMed]
- Bogdanchikova, N.; Meunier, F.C.; Avalos-Borja, M.; Breen, J.P.; Pestryakov, A. On the nature of the silver phases of Ag/Al2O3 catalysts for reactions involving nitric oxide. Appl. Catal. B 2002, 36, 287–297. [Google Scholar] [CrossRef]
- Pestryakov, A.N.; Lunin, V.V.; Bogdanchikova, N.E.; Petranovskii, V.P.; Knop-Gericke, A. Supported foam-silver catalysts for alcohol partial oxidation. Catal. Commun. 2003, 4, 327–331. [Google Scholar] [CrossRef]
- Pestryakov, A.N.; Bogdanchikova, N.E.; Knop-Gericke, A. Alcohol selective oxidation over modified foam-silver catalysts. Catal. Today. 2004, 91–92C, 49–52. [Google Scholar] [CrossRef]
- Ershov, B.; Abkhalimov, E.; Sukhov, N. Formation of Long-Lived Clusters and Silver Nucleation in the g-Irradiation of Aqueous Silver Perchlorate Solutions Containing Polyphosphate. High Energ. Chem. 2005, 39, 83–87. [Google Scholar] [CrossRef]
- Rtimi, S.; Baghrichea, O.; Sanjines, R.; Pulgarina, C.; Bensimonc, M.; Kiwi, J. TiON and TiON-Ag sputtered surfaces leading to bacterial inactivation under indoor actinic light. J. Photochem. Photobiol. A 2013, 256, 52–63. [Google Scholar]
- Mejia, I.M.; Marín, M.; Sanjines, R.; Pulgarín, C.; Mielczarski, E.; Mielczarski, J.; Kiwi, L. Magnetron-sputtered Ag-modified cotton textiles active in the inactivation of airborne bacteria. ACS Appl. Mater. Interfaces 2010, 2, 230–235. [Google Scholar]
- Wagner, C.D.; Riggs, M.; Davis, E.; Müllenberg, G. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corp. Physical Electronics Division: Eden Prairie, MN, USA, 1979; pp. 1–190. [Google Scholar]
- Pestryakov, A.N.; Davydov, A.A. Active electronic states of silver catalysts for methanol selective oxidation. Appl. Catal. A 1994, 120, 7–15. [Google Scholar] [CrossRef]
- Pestryakov, A. Modification of silver catalysts for oxidation of methanol to formaldehyde. Catal. Today 1996, 239–244. [Google Scholar] [CrossRef]
- Yang, F.; Graciani, J.; Evans, J.; Liu, P.; Hrbek, J.; Sanz, J.F.; Rodriguez, J.A. CO Oxidation on inverse CeOx/Cu(111) catalysts: High catalytic activity and ceria-promoted dissociation of O2. J. Am. Chem. Soc. 2011, 133, 3444–3451. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.N.; Bharali, P.; Thrimurthulu, G.; Reddy, B.M. Supported copper–ceria catalysts for low temperature CO oxidation. Catal. Commun. 2011, 11, 863–866. [Google Scholar] [CrossRef]
- Kuzmicheva, G.M. Nanosized phases with titanium(iv) oxides. Preparation. Characterisation. Properties. Fine Chem. Technol. 2015, 10, 5–36. [Google Scholar]
- Lopez-Salido, I.; Lim, D.C.; Kim, Y.D. Ag nanoparticles on highly ordered pyrolytic graphite (HOPG) surfaces studied using STM and XPS. Surf. Sci. 2005, 588, 6–18. [Google Scholar] [CrossRef]
- Lim, D.C.; Lopez-Salido, I.; Kim, T.D. Size selectivity for CO-oxidation of Ag nanoparticles on highly ordered pyrolytic graphite (HOPG). Surf. Sci. 2005, 598, 96–103. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Golovko, V.B.; Adnan, R.H.; Abu Bakar, F.; Ruzicka, J.; Anderson, D.P.; Andersson, G.G.; Wlodarski, W. Hydrogen sensing using gold nanoclusters supported on tungsten trioxide thin film. Int. J. Hydrog. Energy 2013, 38, 2865–2877. [Google Scholar] [CrossRef]
- Zanella, R.; Giorgio, S.; Henry, C.R.; Louis, C. Alternative Methods for the Preparation of Gold Nanoparticles Supported on TiO2. Phys. Chem. B 2002, 106, 7634. [Google Scholar] [CrossRef]
- Zanella, R.; Delannoy, L.; Louis, C. Mechanism of deposition of gold precursors onto TiO2 during the preparation by cation adsorption and deposition-precipitation with NaOH and urea. Appl. Catal. A 2005, 291, 62–72. [Google Scholar] [CrossRef]
- Zanella, R.; Louis, C. Influence of the conditions of thermal treatments and of storage on the size of the gold particles in Au/TiO2 samples. Catal. Today 2005, 107–108, 768–777. [Google Scholar] [CrossRef]
- Chernyshov, A.A.; Veligzhanin, A.A.; Zubavichus, Y.V. Structural materials science end-station at the Kurchatov synchrotron radiation source: Recent instrumentation upgrades and experimental results. Nucl. Instrum. Methods Phys. Res. A 2009, 603, 95–98. [Google Scholar] [CrossRef]
- Petricek, V.; Dusek, M.; Palatinus, L. Jana2006: The CRYSTALLOGRAPHIC computing System. Institute of Physics: Praha, Czech Republic, 2006. Available online: http://jana.fzu.cz/ (accessed on 25 January 2016).
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Athena, R.B.; Hephaestus, A. Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad. 2005, 12, 537–541. [Google Scholar]
- Newville, M. IFEFFIT: Interactive XAFS analysis and IFEF fitting. J. Synchrotron Rad. 2001, 8, 322–324. [Google Scholar] [CrossRef]
- Ankudinov, A.L.; Ravel, B.; Rehr, J.J.; Conradson, S.D. Real-space multiple-scattering calculation and interpretation of X-ray-absorption near-edge structure. Phys. Rev. B 1998, 58, 7565–7576. [Google Scholar] [CrossRef]
- Sample Availability: All studied samples are available from the authors.





| Samples | SBET, m2/g | EDX | |
|---|---|---|---|
| Support | Catalyst | Ag Content, wt % | |
| Ag/Ce/TiO2 | 43.4 | 46.3 | 2.1 ± 0.4 |
| Ag/Fe/TiO2 | 45.5 | 44.0 | 1.9 ± 0.4 |
| Ag/Mg/TiO2 | 42.6 | 43.9 | 2.0 ± 0.3 |
| Catalysts | BE of Different gold Electronic States (eV) and Their Relative Atomic Concretions, % (Indicated in Parenthesis) | |||
|---|---|---|---|---|
| Ag+ | Ag2O | Ag° | Ag <2 nm | |
| 364.0–366.2 | 367.4–368.1 | 368.1–368.4 | >369.0 | |
| Ag/Mg/TiO2 | - | 367.5 (47%) | 368.3 (40%) | 369.9 (9%), 371.6 (4%) |
| Ag/Fe/TiO2 | 366.0 (14%) | 368.1 (65%) | 369.3 (21%) | |
| Ag/Ce/TiO2 | 365.7 (80%) | 367.5 (20%) | - | - |
| Sample | XANES | EXAFS | ||||
|---|---|---|---|---|---|---|
| Phase | Molar Fraction, % | R-Factor, % | Corresponding Single-Scattering Path | Coordination Number | Mean Squared Displacement | |
| Ag foil | Ag | 100 | - | Ag-Ag | 12 | 0.0097 |
| Ag2O | Ag2O | 100 | - | Ag-O | 4 | - |
| Ag/Ce/TiO2 | Ag | 26.4 | 0.030 | Ag-Ag | 2.7 | 0.0115 |
| Ag2O | 73.6 | Ag-O | 3.5 | 0.0183 | ||
| Ag/Mg/TiO2 | Ag | 9.8 | 0.024 | Ag-Ag | 2.2 | 0.0149 |
| Ag2O | 90.2 | Ag-O | 2.6 | 0.0127 | ||
| Ag/Fe/TiO2 | Ag | 38.3 | 0.025 | Ag-Ag | 5.0 | 0.0131 |
| Ag2O | 61.7 | Ag-O | 2.5 | 0.0170 | ||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotolevich, Y.; Kolobova, E.; Khramov, E.; Cabrera Ortega, J.E.; Farías, M.H.; Zubavichus, Y.; Zanella, R.; Mota-Morales, J.D.; Pestryakov, A.; Bogdanchikova, N.; et al. Identification of Subnanometric Ag Species, Their Interaction with Supports and Role in Catalytic CO Oxidation. Molecules 2016, 21, 532. https://doi.org/10.3390/molecules21040532
Kotolevich Y, Kolobova E, Khramov E, Cabrera Ortega JE, Farías MH, Zubavichus Y, Zanella R, Mota-Morales JD, Pestryakov A, Bogdanchikova N, et al. Identification of Subnanometric Ag Species, Their Interaction with Supports and Role in Catalytic CO Oxidation. Molecules. 2016; 21(4):532. https://doi.org/10.3390/molecules21040532
Chicago/Turabian StyleKotolevich, Yulia, Ekaterina Kolobova, Evgeniy Khramov, Jesús Efren Cabrera Ortega, Mario H. Farías, Yan Zubavichus, Rodolfo Zanella, Josué D. Mota-Morales, Alexey Pestryakov, Nina Bogdanchikova, and et al. 2016. "Identification of Subnanometric Ag Species, Their Interaction with Supports and Role in Catalytic CO Oxidation" Molecules 21, no. 4: 532. https://doi.org/10.3390/molecules21040532







