Formation and Transformation Behavior of Sodium Dehydroacetate Hydrates
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Effect of Cooling Rate on Hydrate Forms
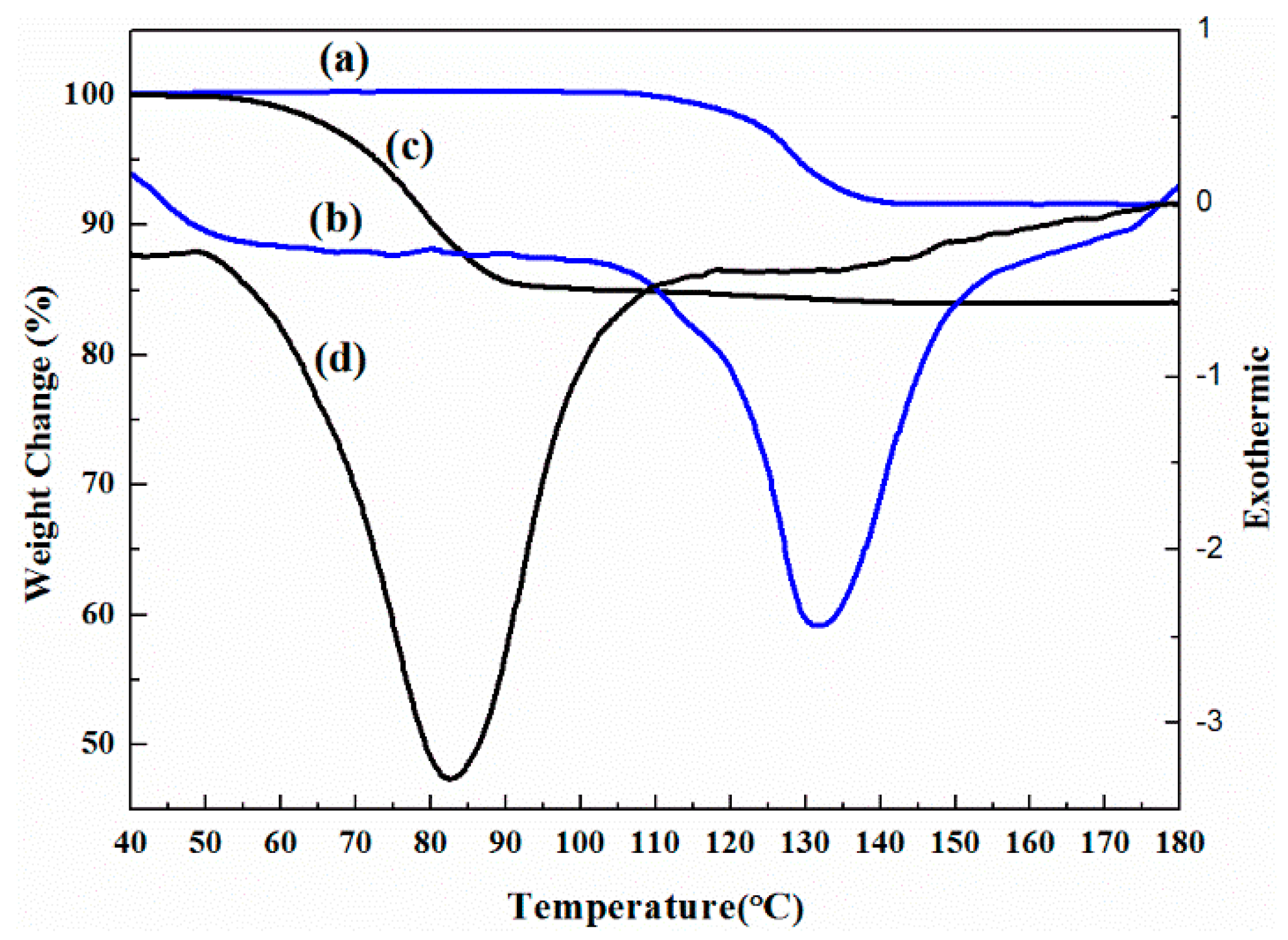
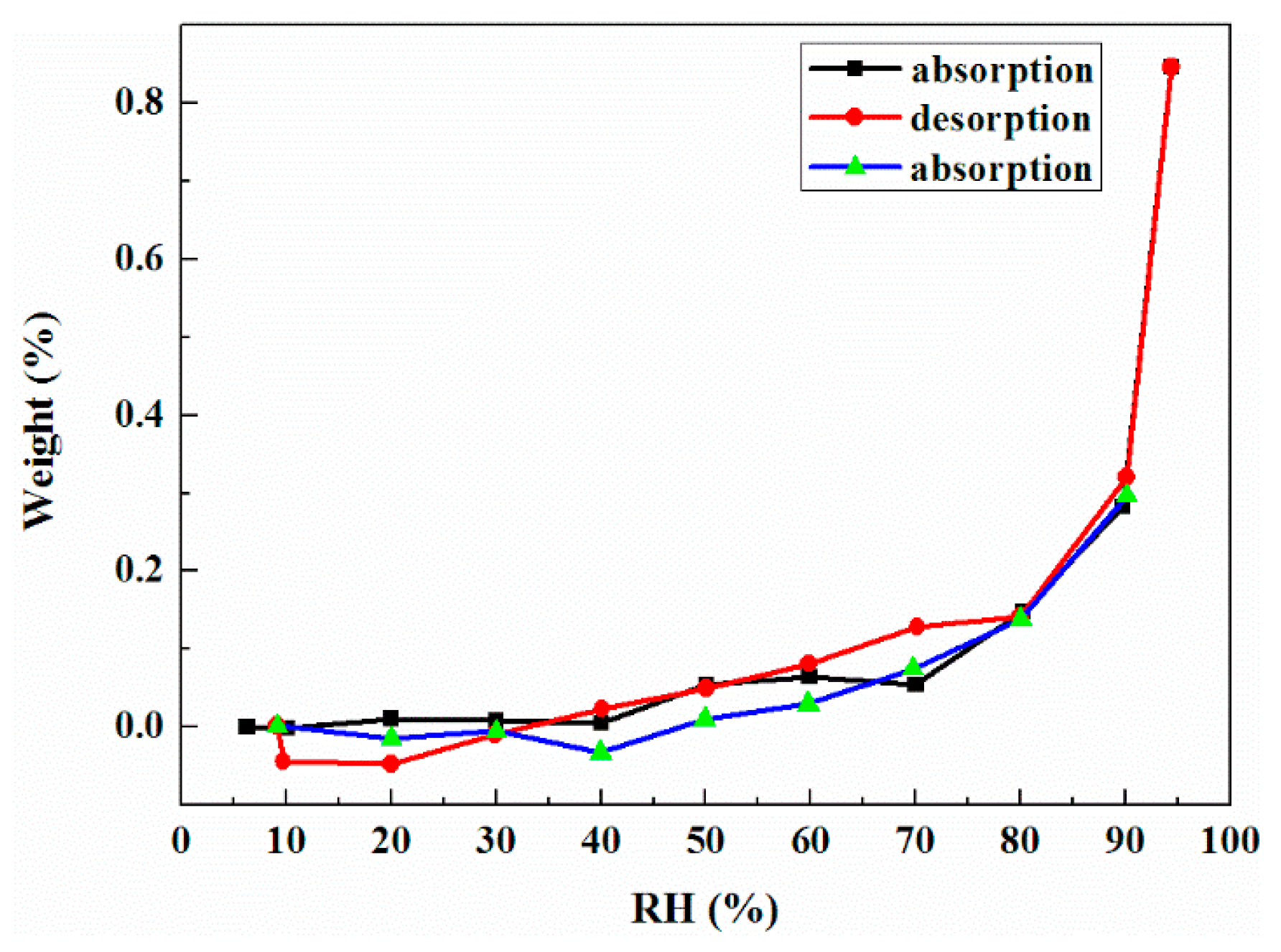
2.2. Phase Transformation Behaviour of DHA-S Hydrates
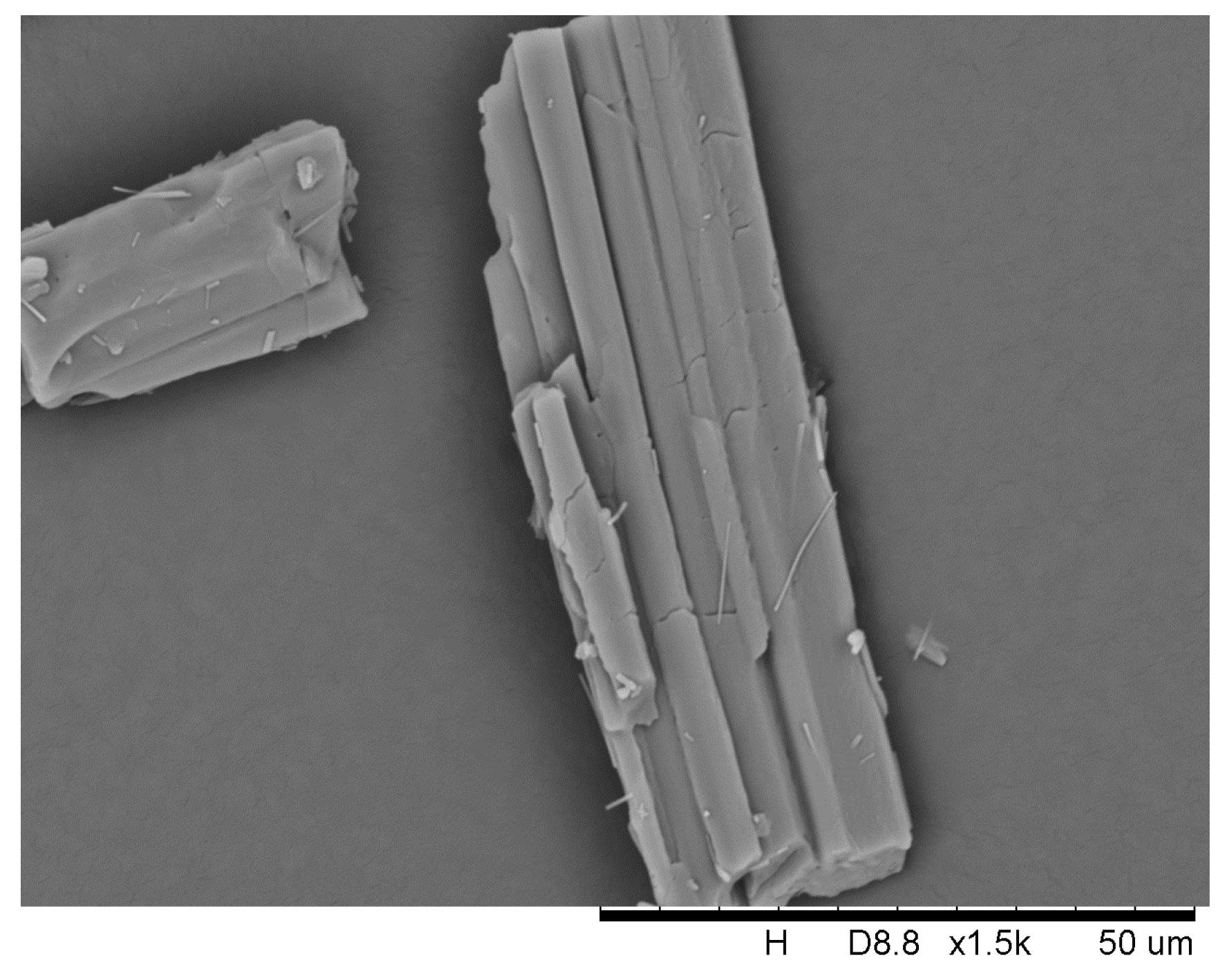
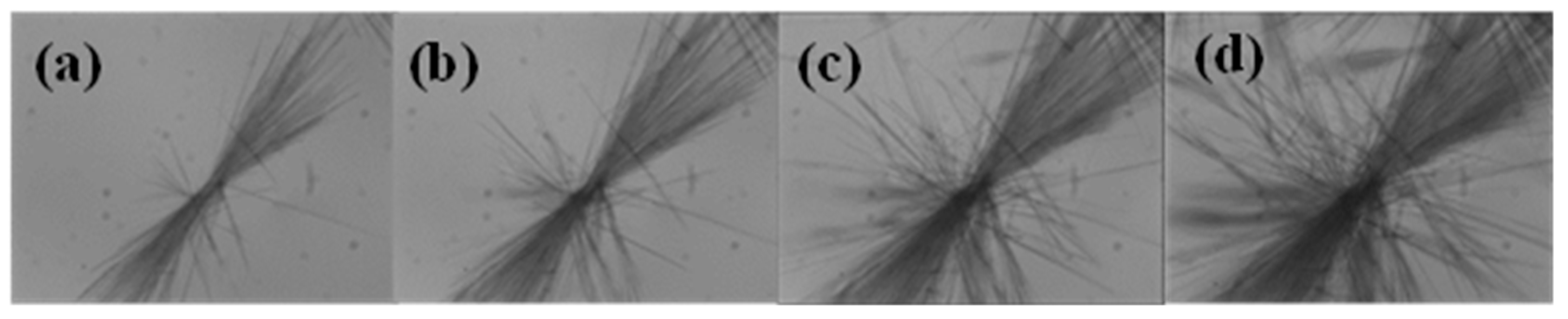
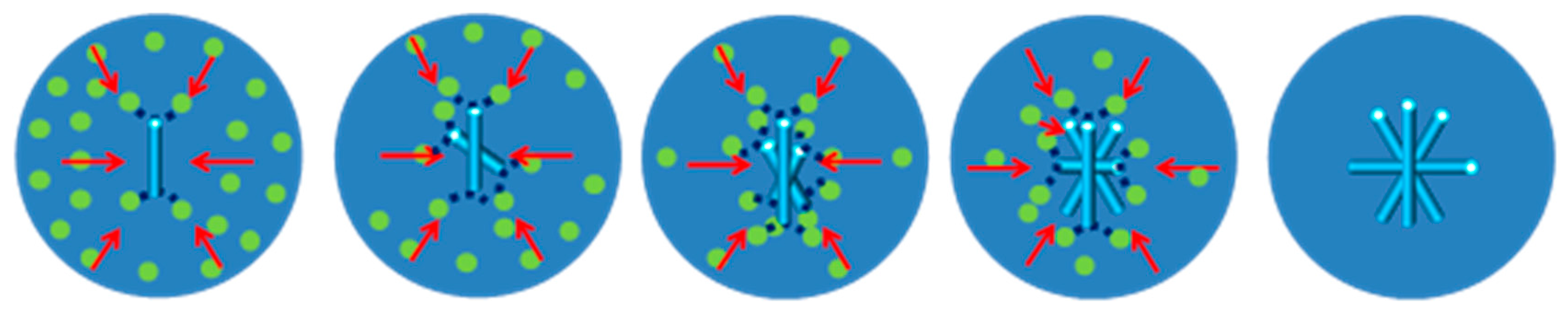
2.3. Formation Mechanism of DHA-S Hydrates
3. Materials and Methods
3.1. Materials
3.2. Solid–State Characterization
3.3. Controlled Cooling Crystallization Experiments
3.4. Solid–Solid Polymorphic Transformation of DHA-S Hydrates
3.5. Crystallization Behaviour of DHA-S Dihydrate
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fujii, K.; Aoki, M.; Uekusa, H. Solid-State Hydration/Dehydration of Erythromycin A Investigated by ab Initio Powder X-ray Diffraction Analysis: Stoichiometric and Nonstoichiometric Dehydrated Hydrate. Cryst. Growth Des. 2013, 13, 2060–2066. [Google Scholar] [CrossRef]
- Croker, D.; Hodnett, B.K. Mechanistic Features of Polymorphic Transformations: The Role of Surfaces. Cryst. Growth Des. 2010, 10, 2806–2816. [Google Scholar] [CrossRef]
- Infantes, L.; Fábián, L.; Motherwell, W.D.S. Organic crystal hydrates: What are the important factors for formation. CrystEngComm 2007, 9, 65–71. [Google Scholar] [CrossRef]
- Du, W.; Yin, Q.; Hao, H.; Bao, Y.; Zhang, X.; Huang, J.; Li, X.; Xie, C.; Gong, J. Solution-Mediated Polymorphic Transformation of Prasugrel Hydrochloride from Form II to Form I. Ind. Eng. Chem. Res. 2014, 53, 5652–5659. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, Q.; Du, W.; Gong, J.; Bao, Y.; Zhang, M.; Hou, B.; Hao, H. Phase Transformation between Anhydrate and Monohydrate of Sodium Dehydroacetate. Ind. Eng. Chem. Res. 2015, 54, 3438–3444. [Google Scholar] [CrossRef]
- Ulrich, J.; Strege, C. Some aspects of the importance of metastable zone width and nucleation in industrial crystallizers. J. Cryst. Growth 2002, 237–239, 2130–2135. [Google Scholar] [CrossRef]
- He, G.; Wong, A.B.H.; Chow, P.S.; Tan, R.B.H. Effects of the rate of supersaturation generation on polymorphic crystallization of m-hydroxybenzoic acid and o-aminobenzoic acid. J. Cryst. Growth 2011, 314, 220–226. [Google Scholar] [CrossRef]
- Barrett, M.; Hao, H.; Maher, A.; Hodnett, K.; Glennon, B.; Croker, D. In Situ Monitoring of Supersaturation and Polymorphic Form of Piracetam during Batch Cooling Crystallization. Org. Process Res. Dev. 2011, 15, 681–687. [Google Scholar] [CrossRef]
- Martins, D.; Stelzer, T.; Ulrich, J.; Coquerel, G. Formation of Crystalline Hollow Whiskers as Relics of Organic Dissipative Structures. Cryst. Growth Des. 2011, 11, 3020–3026. [Google Scholar] [CrossRef]
- Schuster, A.; Stelzer, T.; Petersen, S.; Ulrich, J. Closed Crystalline Tubes as a Container System. Chem. Eng. Technol. 2010, 33, 787–790. [Google Scholar] [CrossRef]
- Mallet, F.; Petit, S.; Lafont, S.; Billot, P.; Lemarchand, D.; Coquerel, G. Crystal Growth Mechanism in a Solution of Hollow Whiskers of Molecular Compounds. Cryst. Growth Des. 2004, 4, 965–969. [Google Scholar] [CrossRef]
- Dette, S.S.; Stelzer, T.; Römbach, E.; Jones, M.J.; Ulrich, J. Controlling the Internal Diameter of Nanotubes by Changing the Concentration of the Antisolvent. Cryst. Growth Des. 2007, 7, 1615–1617. [Google Scholar] [CrossRef]
- Eddleston, M.D.; Jones, W. Formation of Tubular Crystals of Pharmaceutical Compounds. Cryst. Growth Des. 2010, 10, 365–370. [Google Scholar] [CrossRef]
- Paulino, A.S.; Rauber, G.S.; Campos, C.E.M.; Maurício, M.H.P.; Avillez, R.R.; Cuffini, S.L.; Cardoso, S.G. Hollow crystal anti-solvent preparation process as a promising technique to improve dissolution of poorly soluble drugs. J. Cryst. Growth 2013, 366, 76–81. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; Socrates, G., Ed.; Wiley: New York, NY, USA, 2001; pp. 95–142. [Google Scholar]
- Kim, Y.S.; Paskow, H.C.; Rousseau, R.W. Propagation of Solid-State Transformations by Dehydration and Stabilization of Pseudopolymorphic Crystals of Sodium Naproxen. Cryst. Growth Des. 2005, 5, 1623–1632. [Google Scholar] [CrossRef]
- Brittain, H.G. Polymorphism in Pharmaceutical Solids; Brittain, H.G., Ed.; Informa Healthcare: New York, NY, USA, 2009; pp. 481–509. [Google Scholar]
- Griesser, U.J. Polymorphism in the Pharmaceutical Industry; Hilfiker, R., Ed.; Wiley-VCH: Weinheim, Germany, 2006; pp. 211–233. [Google Scholar]
- Galwey, A.K. Structure and Order in Thermal Dehydrations of Crystalline Solids. Thermochim. Acta 2000, 355, 181–238. [Google Scholar] [CrossRef]
- Llinàs, A.; Goodman, J.M. Polymorph control: Past, present and future. Drug Discov. Todays 2008, 13, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, M. Strategy for control of crystallization of polymorphs. Chem. Eng. Commun. 2009, 11, 949–964. [Google Scholar] [CrossRef]
- Jones, M.J.; Dette, S.S.; Ulrich, J. Rapid crystal growth without inherent supersaturation induced by nanoscale fluid flows? Cryst. Res. Technol. 2006, 41, 5–9. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the DHA-S monohydrate are available from the authors.
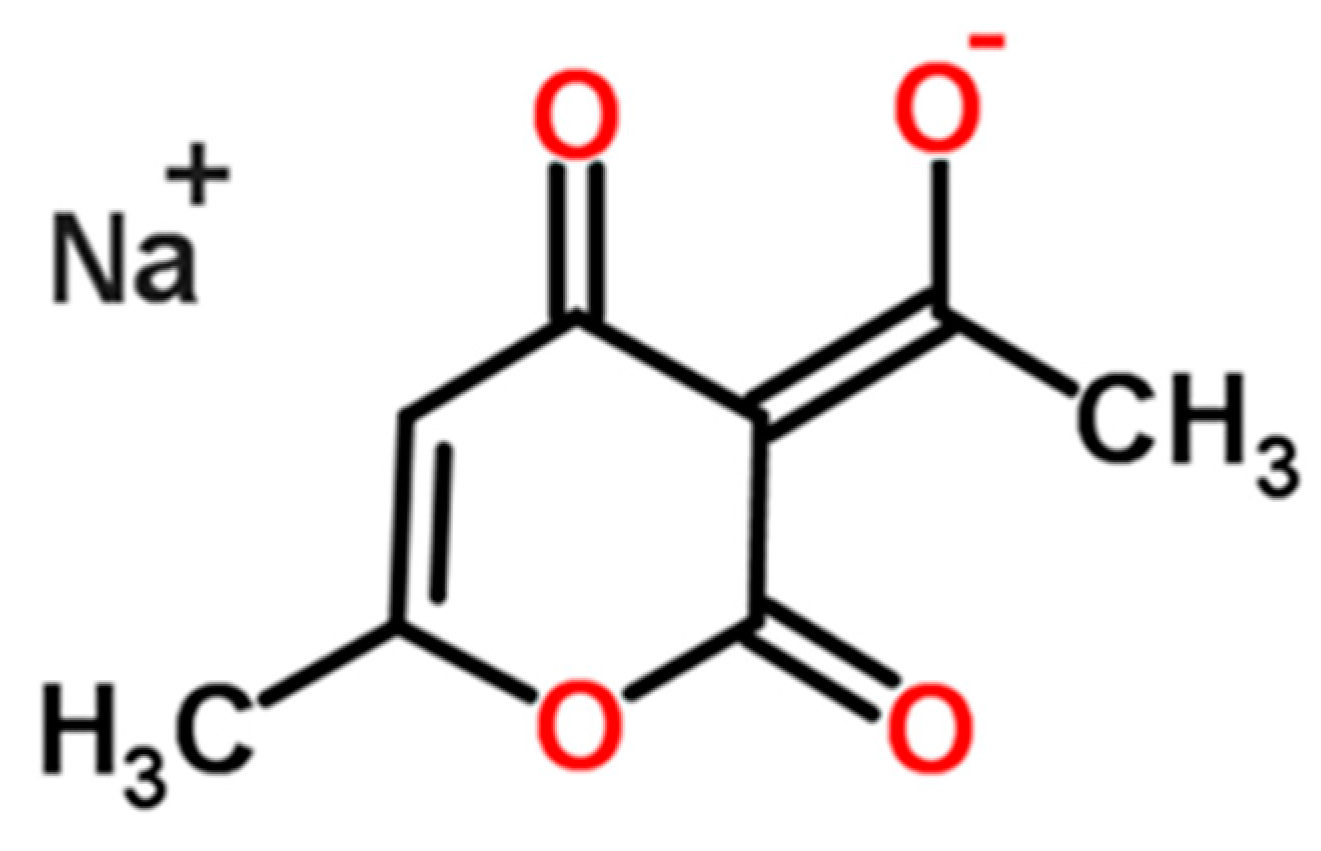

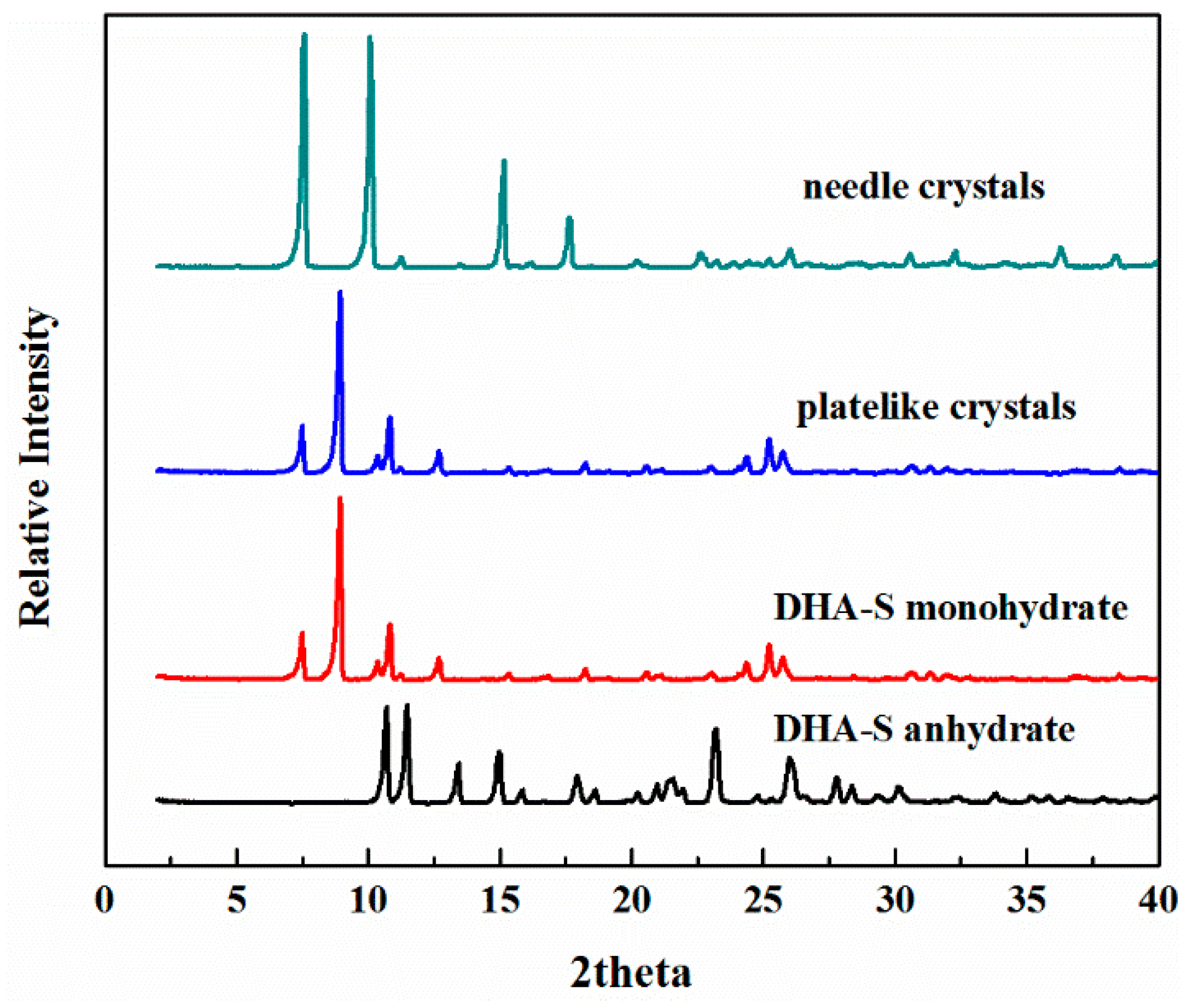
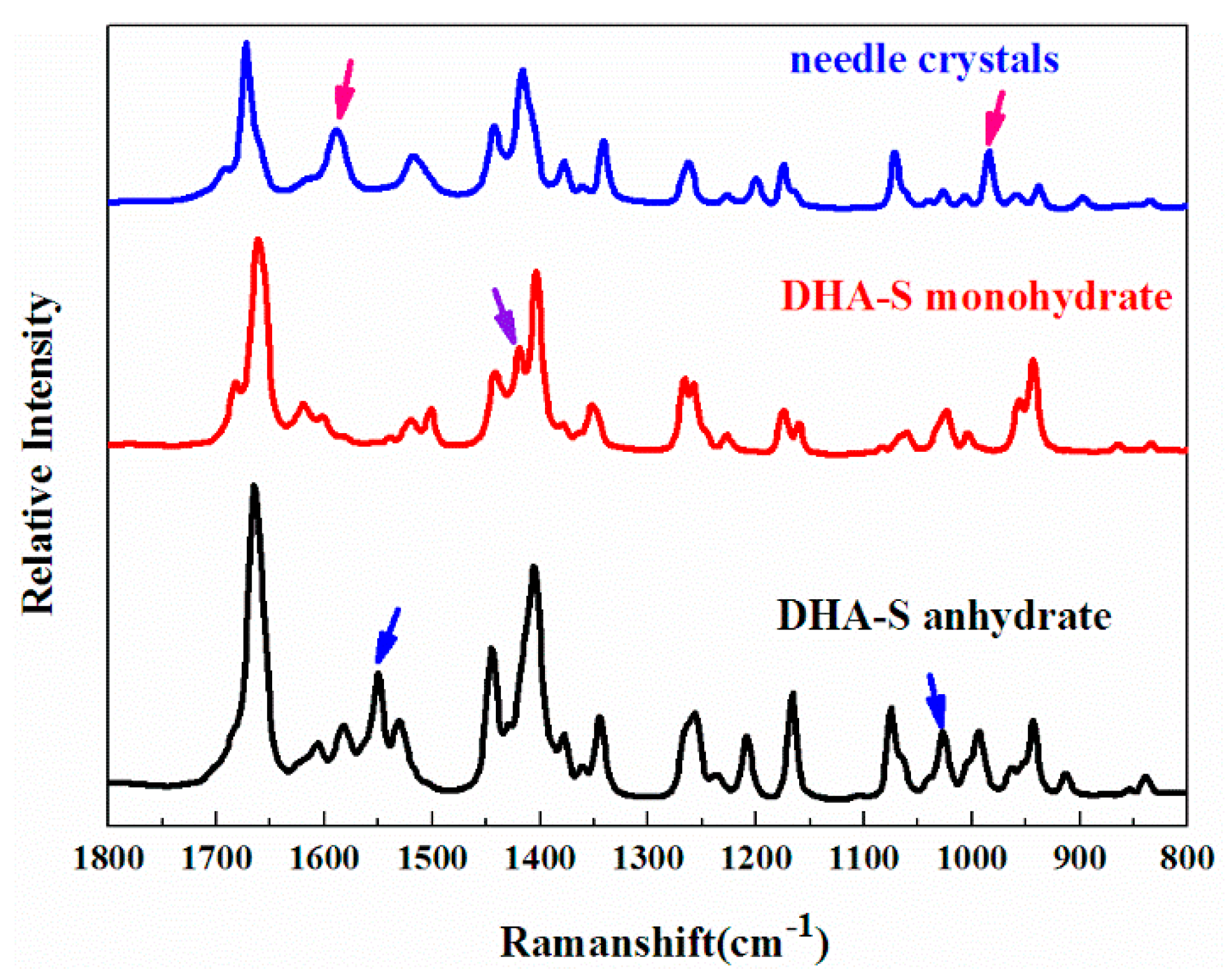
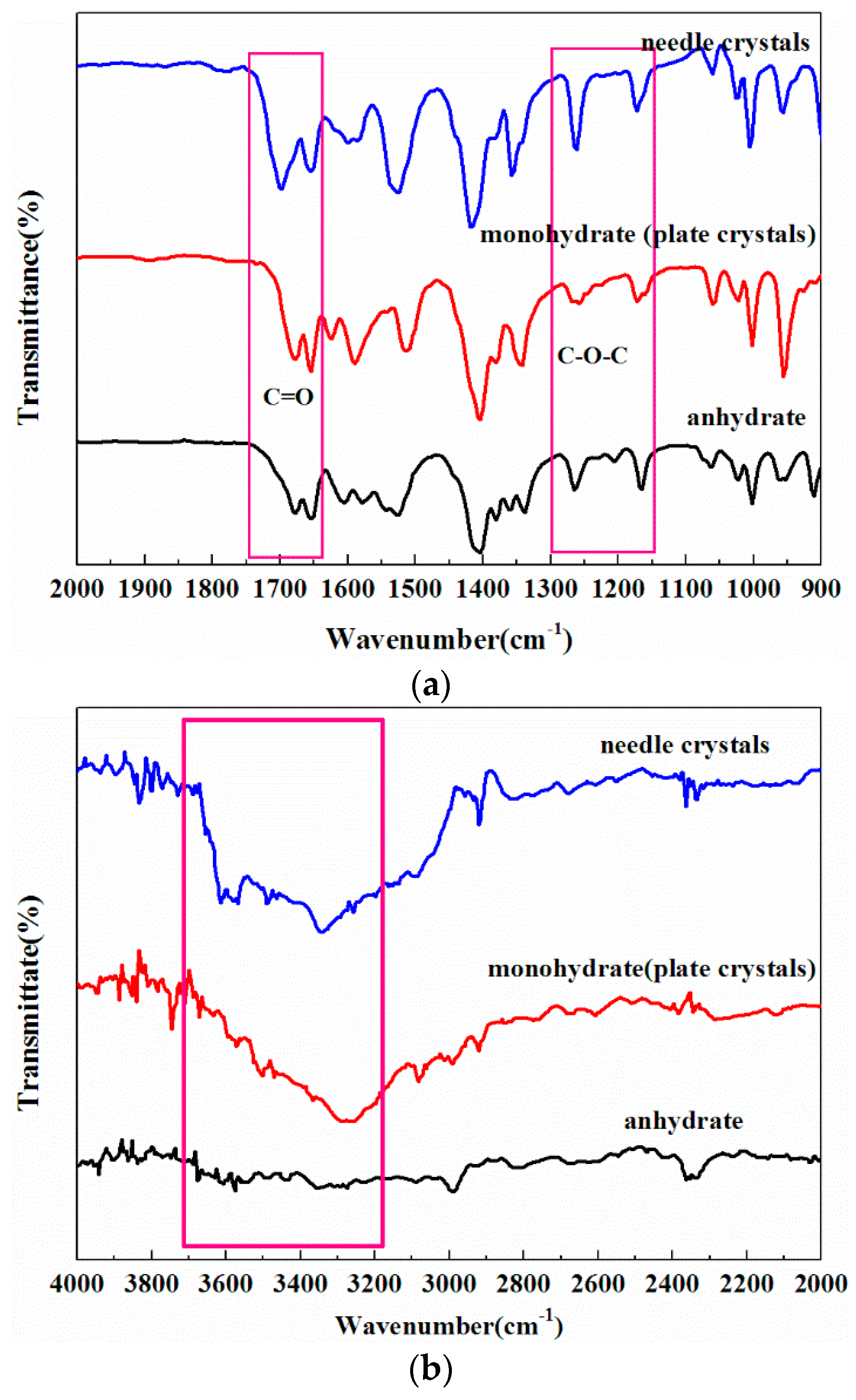


| Cooling Rate | 333.15 K Saturation Solution | 343.15 K Saturation Solution |
|---|---|---|
| 0.15 K/min | monohydrate | monohydrate |
| 0.20 K/min | monohydrate and dihydrate | monohydrate and dihydrate |
| 0.25 K/min | dihydrate | dihydrate |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Xie, C.; Huang, Y.; Hou, B.; Bao, Y.; Gong, J.; Yin, Q.; Rohani, S. Formation and Transformation Behavior of Sodium Dehydroacetate Hydrates. Molecules 2016, 21, 458. https://doi.org/10.3390/molecules21040458
Zhang X, Xie C, Huang Y, Hou B, Bao Y, Gong J, Yin Q, Rohani S. Formation and Transformation Behavior of Sodium Dehydroacetate Hydrates. Molecules. 2016; 21(4):458. https://doi.org/10.3390/molecules21040458
Chicago/Turabian StyleZhang, Xia, Chuang Xie, Yaohui Huang, Baohong Hou, Ying Bao, Junbo Gong, Qiuxiang Yin, and Sohrab Rohani. 2016. "Formation and Transformation Behavior of Sodium Dehydroacetate Hydrates" Molecules 21, no. 4: 458. https://doi.org/10.3390/molecules21040458
APA StyleZhang, X., Xie, C., Huang, Y., Hou, B., Bao, Y., Gong, J., Yin, Q., & Rohani, S. (2016). Formation and Transformation Behavior of Sodium Dehydroacetate Hydrates. Molecules, 21(4), 458. https://doi.org/10.3390/molecules21040458








