Nanoparticle Effects on Human Platelets in Vitro: A Comparison between PAMAM and Triazine Dendrimers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Zeta Potential
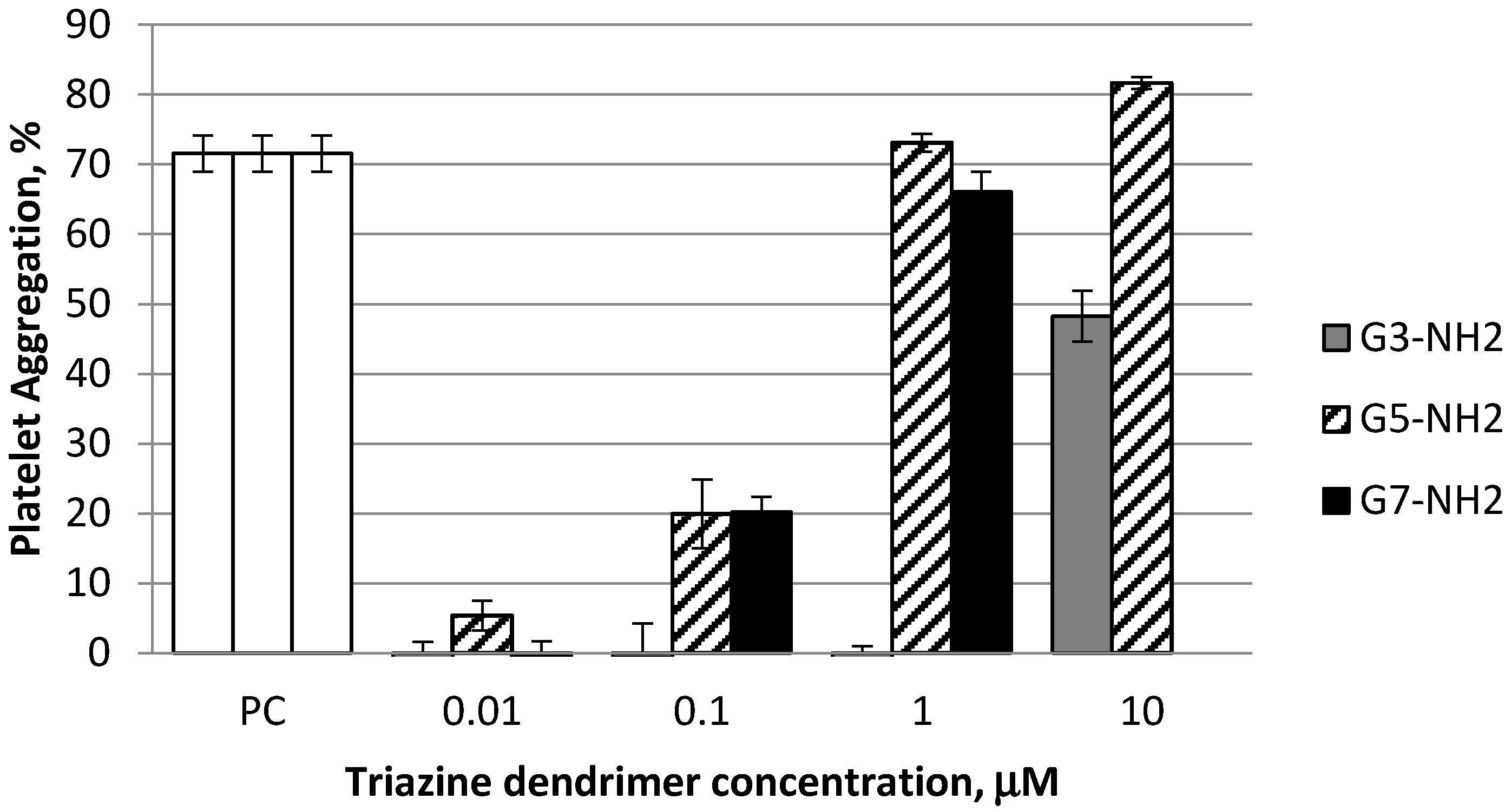
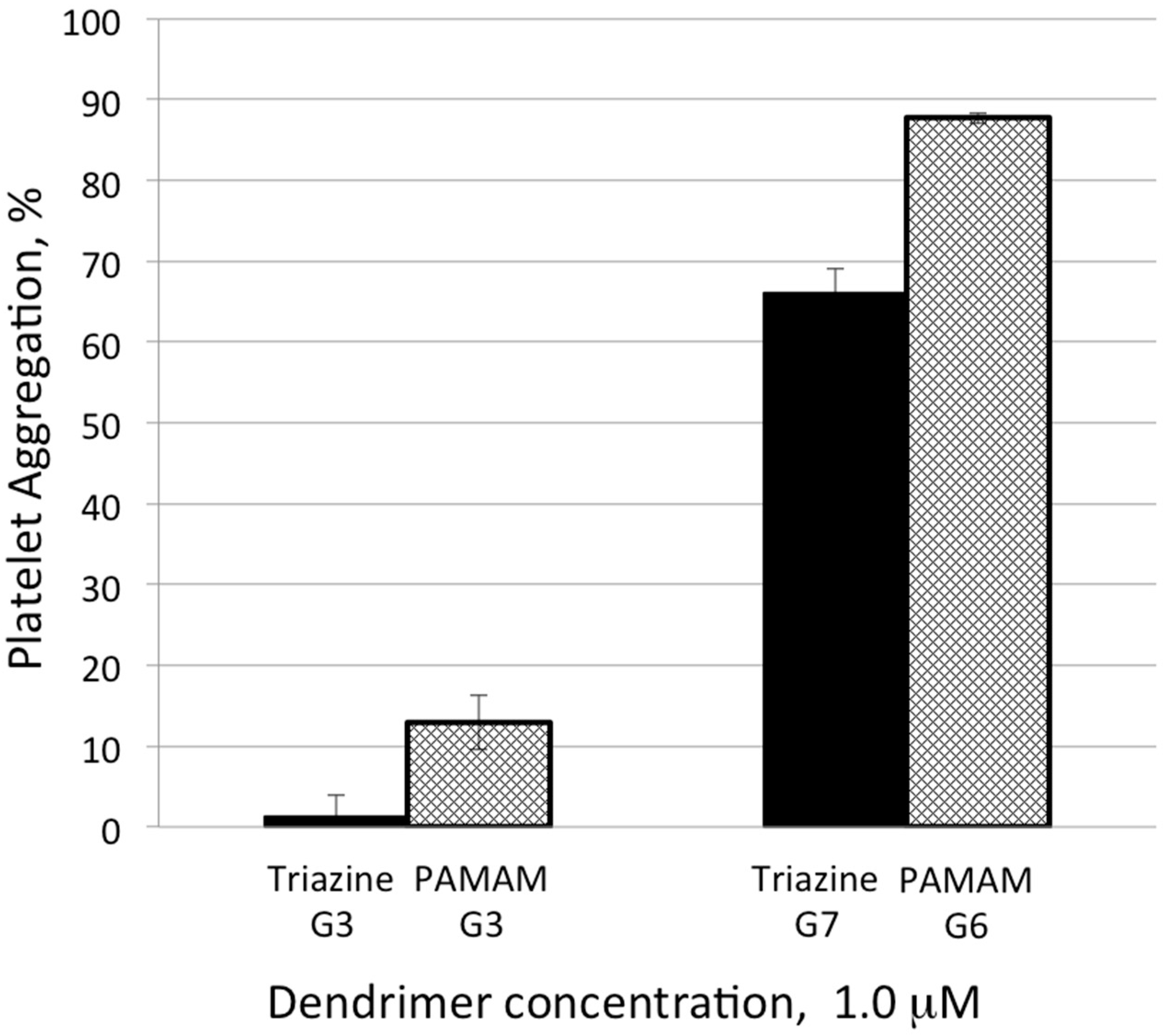
2.2. Platelet Aggregation
3. Materials and Methods
3.1. Reagents
3.2. Research Donor Blood
3.3. Platelet Aggregation
3.4. Zeta Potential Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sowinska, M.; Urbanczyk-Lipkowska, Z. Advances in the chemistry of dendrimers. New J. Chem. 2014, 38, 2168–2203. [Google Scholar] [CrossRef]
- Deng, X.-X.; Du, F.-S.; Li, Z.-C. Combination of orthogonal ABB and ABC multicomponent reactions toward efficient divergent synthesis of dendrimers with structural diversity. ACS Macro Lett. 2014, 3, 667–670. [Google Scholar] [CrossRef]
- Patra, S.; Kozura, B.; Huang, A.Y.-T.; Enciso, A.E.; Sun, X.; Hsieh, J.-T.; Kao, C.-L.; Chen, H.-T.; Simanek, E.E. Dendrimers terminated with dichlorotriazine groups provide a rout to compositional diversity. Org. Lett. 2013, 15, 3808–3811. [Google Scholar] [CrossRef] [PubMed]
- Simanek, E.E.; Abdou, H.; Lalwani, S.; Lim, J.; Mintzer, M.; Venditto, V.J.; Vittur, B. The 8 year thicket of triazine dendrimers: Strategies, targets and applications. Proc. R. Soc. A 2010, 466, 1445–1468. [Google Scholar] [CrossRef]
- Liu, Y.; Ng, Y.; Toh, M.R.; Chiu, G.N.C. Lipid-dendrimer hybrid nanosystem as a novel delivery system for paclitaxel to treat ovarian cancer. J. Control. Release 2015, 220, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Jie, X.; Chen, C.; Shen, W.; Cao, Y.; Lian, G.; Qi, R. Recent advances in dendrimer research for cardiovascular diseases. Biomacromolecules 2015, 16, 2588–2598. [Google Scholar] [CrossRef] [PubMed]
- Witte, A.B.; Timmer, C.M.; Gam, J.J.; Choi, S.K.; Banaszak, M.M.; Orr, B.G.; Baker, J.R., Jr.; Sinniah, K. Biophysical characterization of a riboflavin-conjugated dendrimer platform for targeted drug delivery. Biomacromolecules 2012, 13, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Luo, K.; Lai, Y.; Pu, Y.; He, B.; Wang, G.; Wu, Y.; Gu, Z. A novel poly(l-glutamic acid) dendrimer based drug delivery system with both pH-sensitive and targeting functions. Mol. Pharm. 2010, 7, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.N.; Yuan, Q.; Yang, H. Synthesis and characterization of photocurable polyamidoamine dendrimer hydrogels as a versatile platform for tissue engineering and drug delivery. Biomacromolecules 2010, 11, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Simanek, E.E. Triazine dendrimers as drug delivery systems: From synthesis to therapy. Adv. Drug Deliv. Rev. 2012, 64, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Lo, S.-T.; Hill, S.; Pavan, G.M.; Sun, X.; Simanek, E.E. Antitumor activity and molecular dynamics simulations of paclitaxel-laden triazine dendrimers. Mol. Pharm. 2012, 9, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Simanek, E.E.; Enciso, A.E.; Pavan, G.M. Computational design principles for the Discovery of bioactive dendrimers: [s]-triazines and other examples. Exp. Opin. Drug Disc. 2013, 9, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Lo, S.-T.; Lim, J.; da Costa, V.C.; Ramezani, S.; Öz, O.K.; Pavan, G.M.; Annunziata, O.; Sun, X.; Simanek, E.E. Design, synthesis and biological assessment of a triazine dendrimer with approximately 16 paclitaxel groups and 8 PEG groups. Mol. Pharm. 2013, 10, 4452–4461. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.F.; Campbell, R.A.; Franks, Z.; Gibson, C.C.; Thiagarajan, G.; Vieira-de-Abreu, A.; Sukavaneshvar, S.; Mohammad, S.F.; Li, D.Y.; Ghandehari, H.; et al. Cationic PAMAM dendrimers disrupt key platelet functions. Mol. Pharm. 2012, 9, 1599–1611. [Google Scholar] [CrossRef] [PubMed]
- Simak, J. Nanotoxicity in Blood: Effects of Engineered Nanomaterials on Platelets. In Nanotoxicity: From in Vivo and in Vitro Models to Health Risks, 1st ed.; Sahu, S.C., Casciano, D.A., Eds.; John Wiley and Sons Ltd.: Chichester, UK, 2009; pp. 191–225. [Google Scholar]
- Dong, H.-P.; Wu, H.-M.; Chen, S.-J.; Chen, C.-Y. The effect of butanolides from Cinnamomum tenuifolium on platelet aggregation. Molecules 2013, 18, 11836–11841. [Google Scholar] [CrossRef] [PubMed]
- Cejas, M.A.; Chen, C.; Kinney, W.A.; Maryanoff, B.E. Nanoparticles that display short collagen-related peptides. Potent stimulation of human platelet aggregation by triple helical motifs. Bioconjugate Chem. 2007, 18, 1025–1027. [Google Scholar] [CrossRef] [PubMed]
- Okamura, Y.; Handa, M.; Suzuki, H.; Ikeda, Y.; Takeoka, S. New strategy of platelet substitutes for enhancing platelet aggregation at high shear rates: Cooperative effects of a mixed system of fibrinogen gamma-chain dodecapeptide- or glycoprotein Ibalpha-conjugated latex beads under flow conditions. J. Artif. Organs 2006, 9, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xue, J.; Guo, Z.; Zhang, L.; Marchant, R.E. Biomimetic glycoliposomes as nanocarriers for targeting P-selectin on activated platelets. Bioconjugate Chem. 2007, 18, 1366–1369. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.G.; de Queiroz, A.A.; Abraham, G.A.; San Roman, J. Antithrombogenic properties of bioconjugate streptokinase-polyglycerol dendrimers. J. Mater. Sci. Mater. Med. 2006, 17, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Klutz, A.M.; Hechler, B.; Gao, Z.G.; Gachet, C.; Jacobson, K.A. Application of the functionalized congener approach to dendrimer-based signaling agents acting through A(2A) adenosine receptors. Purinergic Signal. 2009, 5, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.F.; Campbell, R.A.; Brooks, A.E.; Assemi, S.; Tadjiki, S.; Thiagarajan, G.; Mulcock, C.; Weyrich, A.S.; Brooks, B.D.; Ghandehari, H.; et al. Cationic PAMAM dendrimers aggressively initiate blood clot formation. ACS Nano 2012, 6, 9900–9910. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Patri, A.K.; Simak, J.; Hall, J.B.; Semberova, J.; de Paoli Lacerda, S.H.; McNeil, S.E. Nanoparticle size and surface charge determine effects of PAMAM dendrimers on human platelets in vitro. Mol. Pharm. 2012, 9, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Esfand, R.; Tomalia, D.A. Poly(amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications—Reflections on the field. Adv. Drug Deliv. Rev. 2005, 57, 2106–2129. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Reyna, L.A.; Svenson, S. Dendrimers as multi-purpose nanodevices for oncology drug delivery and diagnostic imaging. Biochem. Soc. Trans. 2007, 35, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Menjoge, A.R.; Kannan, R.M.; Tomalia, D.A. Dendrimer-based drug and imaging conjugates: Design considerations for nanomedical applications. Drug Discov. Today 2010, 15, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J. Intern. Med. 2014, 276, 579–617. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Patri, A.K.; Potter, T.M.; Rodriguez, J.C.; Hall, J.B.; McNeil, S.E. Dendrimer-induced leukocyte procoagulant activity depends on particle size and surface charge. Nanomedicine 2012, 7, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.T.; Stern, S.; Clogston, J.D.; Zheng, J.; Adiseshaiah, P.P.; Dobrovolskaia, M.; Lim, J.; Patri, A.K.; Sun, X.; Simanek, E.E. Biological assessment of triazine dendrimer:toxicological profiles, solution behavior, biodistribution, drug release andefficacy in a PEGylated, paclitaxel construct. Mol. Pharm. 2010, 7, 993–1006. [Google Scholar] [CrossRef] [PubMed]
- Enciso, A.E.; Abid, Z.M.; Simanek, E.E. Rapid, semi-automated convergent synthesis of low generation triazine dendrimers using microwave assisted reactions. Polym. Chem. 2014, 5, 4635–4640. [Google Scholar] [CrossRef]
- Lim, J.; Kostiainen, M.; Maly, J.; da Costa, V.C.; Annunziata, O.; Pavan, G.M.; Simanek, E.E. Synthesis of Large Dendrimers with the Dimensions of Small Viruses. J. Am. Chem. Soc. 2013, 135, 4660–4663. [Google Scholar] [CrossRef] [PubMed]
- Assay Cascade Protocols, Frederick National Lab, Nanotechnology Characterization Laboratory. Available online: http://ncl.cancer.gov/NCL_Method_ITA-2.pdf (accessed on 28 March 2016).
- Assay Cascade Protocols, Frederick National Lab, Nanotechnology Characterization Laboratory. Available online: http://ncl.cancer.gov/NCL_Method_STE-1.2.pdf (accessed on 28 March 2016).
- Tomalia, D.A. Dendritic effects: Dependecy of dendritic nano-periodic property patterns on critical nanoscale design parameters (CNDPs). New J. Chem. 2012, 36, 264–281. [Google Scholar] [CrossRef]
- Tomalia, D.A. In quest of a systematic framework for unifying and defining nanoscience. J. Nanopart. Res. 2009, 11, 1251–1310. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Khanna, S.N. A systematic framework and nanoperiodic concept for unifying nanoscience: Hard/Soft nanoelements, superatoms, meta-atoms, new emerging properties, periodic property patterns, and predictive mendeleev-like nanoperiodic tables. Chem. Rev. 2016, 116, 2705–2774. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds G1–G9 triazine dendrimers are available from the authors.
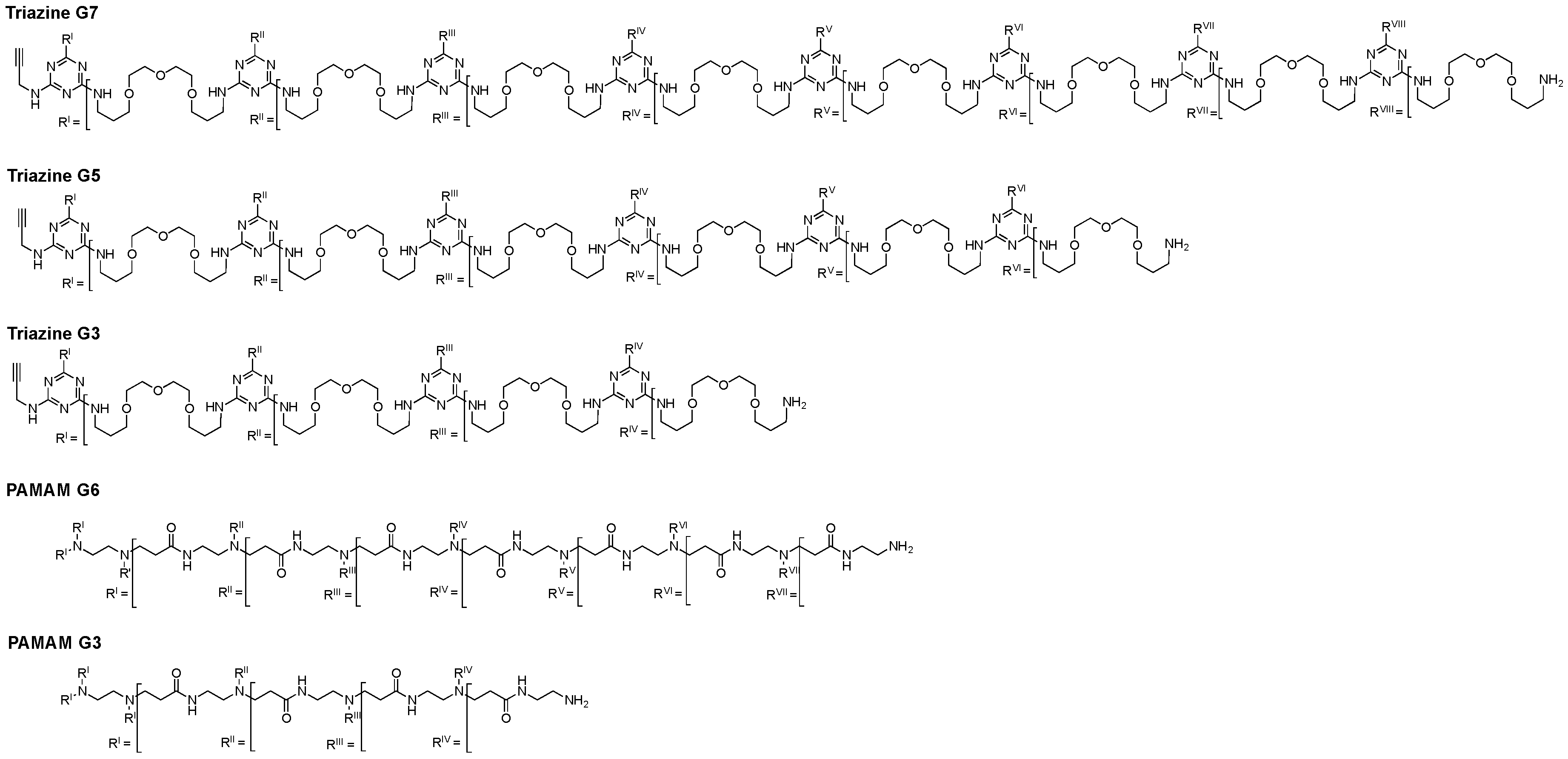
| Dendrimer | Z-Potential (mV) | Monomer Size (nm) | Peripheral Amines | Internal Tertiary Amines | MW (Da) |
|---|---|---|---|---|---|
| G3-Triazine | 23.1 | 3.7 | 16 | - | 7785 |
| G3-PAMAM | 43.3 | 3.1 | 32 | 30 | 6910 |
| G5-Triazine | 16.5 | 8.0 | 64 | - | 33 K |
| G6-PAMAM | 46.2 | 7.5 | 256 | 254 | 58 K |
| G7-Triazine | 19.8 | 13.7 | 256 | - | 130 K |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enciso, A.E.; Neun, B.; Rodriguez, J.; Ranjan, A.P.; Dobrovolskaia, M.A.; Simanek, E.E. Nanoparticle Effects on Human Platelets in Vitro: A Comparison between PAMAM and Triazine Dendrimers. Molecules 2016, 21, 428. https://doi.org/10.3390/molecules21040428
Enciso AE, Neun B, Rodriguez J, Ranjan AP, Dobrovolskaia MA, Simanek EE. Nanoparticle Effects on Human Platelets in Vitro: A Comparison between PAMAM and Triazine Dendrimers. Molecules. 2016; 21(4):428. https://doi.org/10.3390/molecules21040428
Chicago/Turabian StyleEnciso, Alan E., Barry Neun, Jamie Rodriguez, Amalendu P. Ranjan, Marina A. Dobrovolskaia, and Eric E. Simanek. 2016. "Nanoparticle Effects on Human Platelets in Vitro: A Comparison between PAMAM and Triazine Dendrimers" Molecules 21, no. 4: 428. https://doi.org/10.3390/molecules21040428






