Trapa japonica Pericarp Extract Reduces LPS-Induced Inflammation in Macrophages and Acute Lung Injury in Mice
Abstract
:1. Introduction
2. Results
2.1. Active Compounds
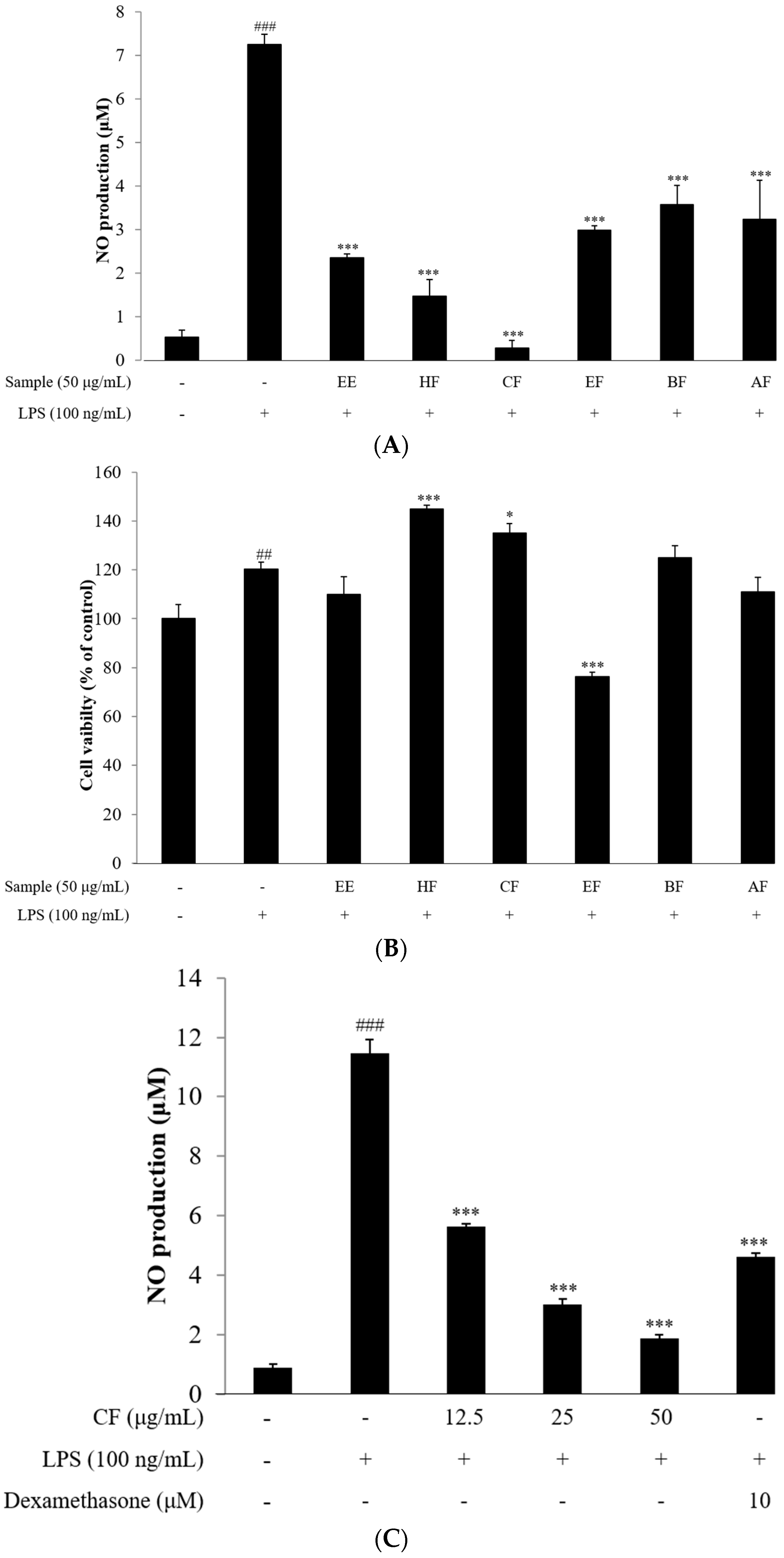
2.2. NO Assay
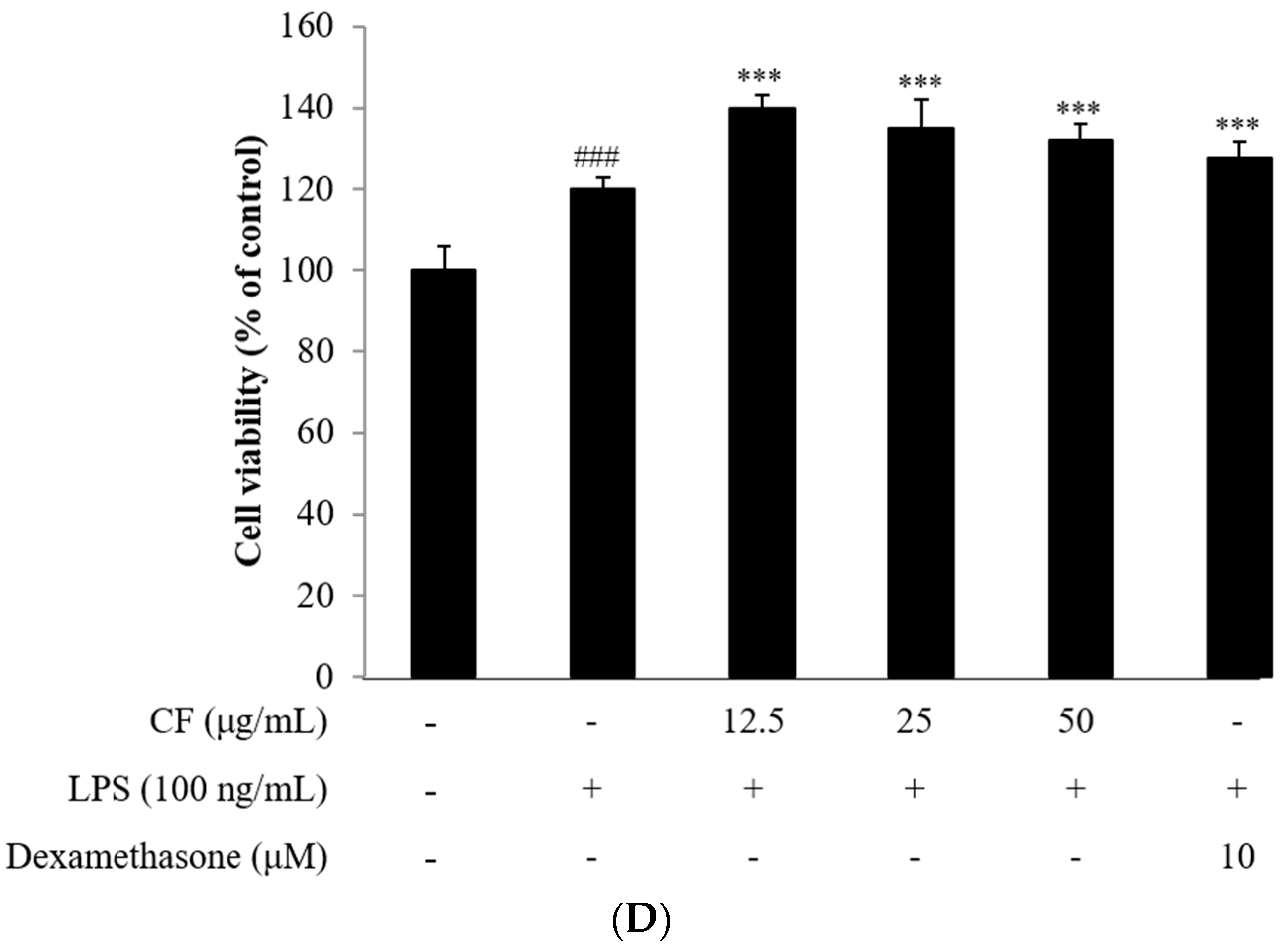
2.3. Cell Viability
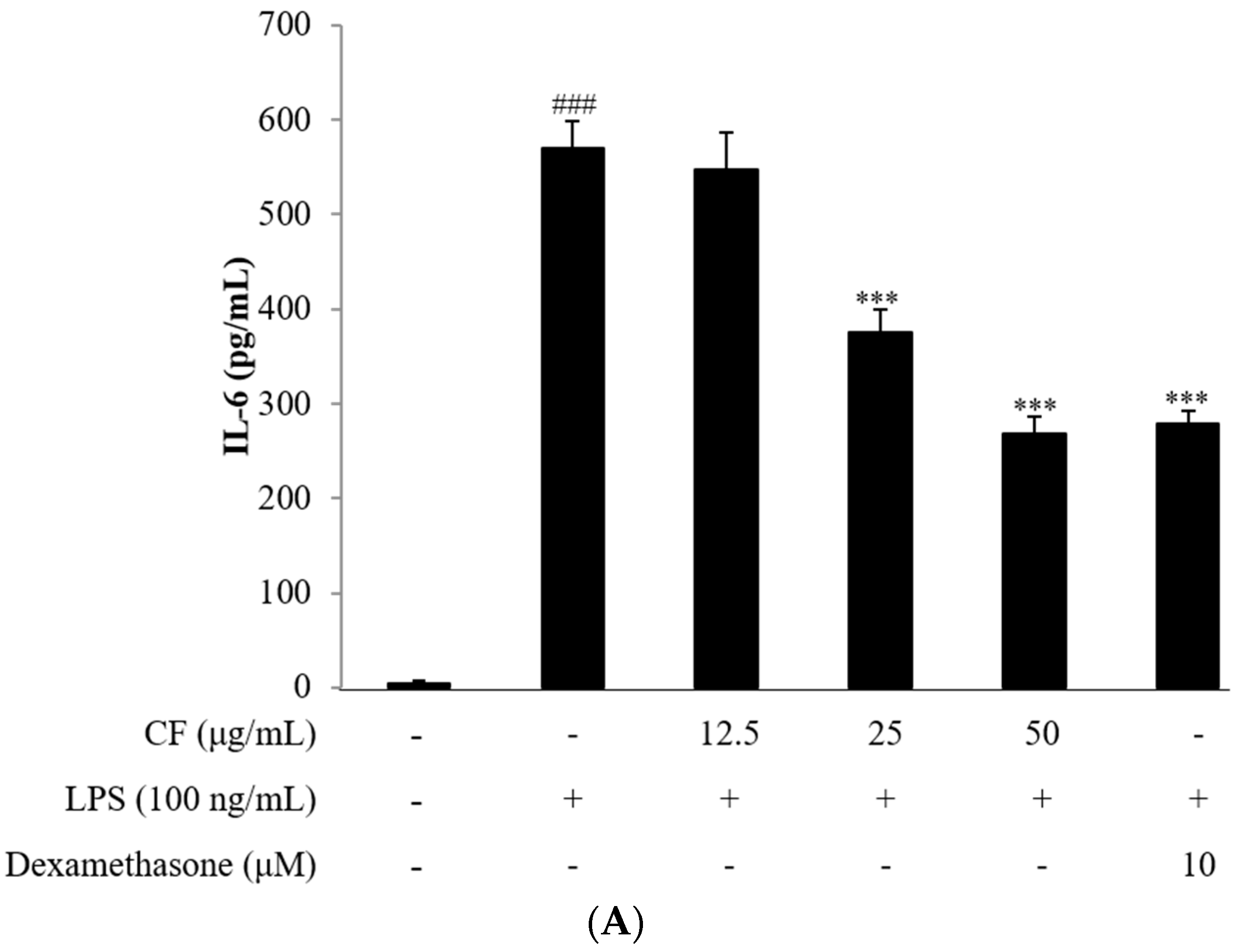
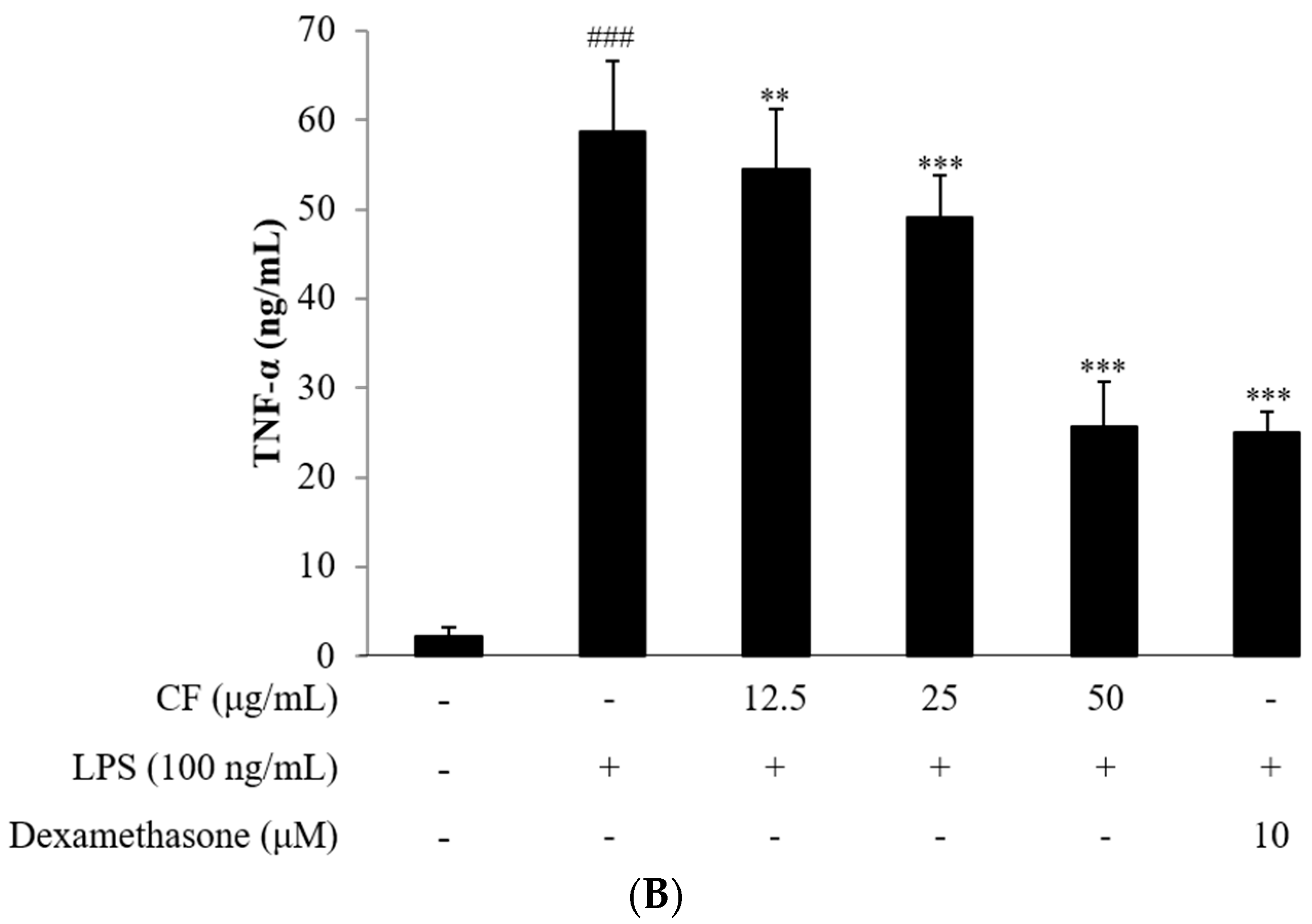
2.4. Cytokine Assay
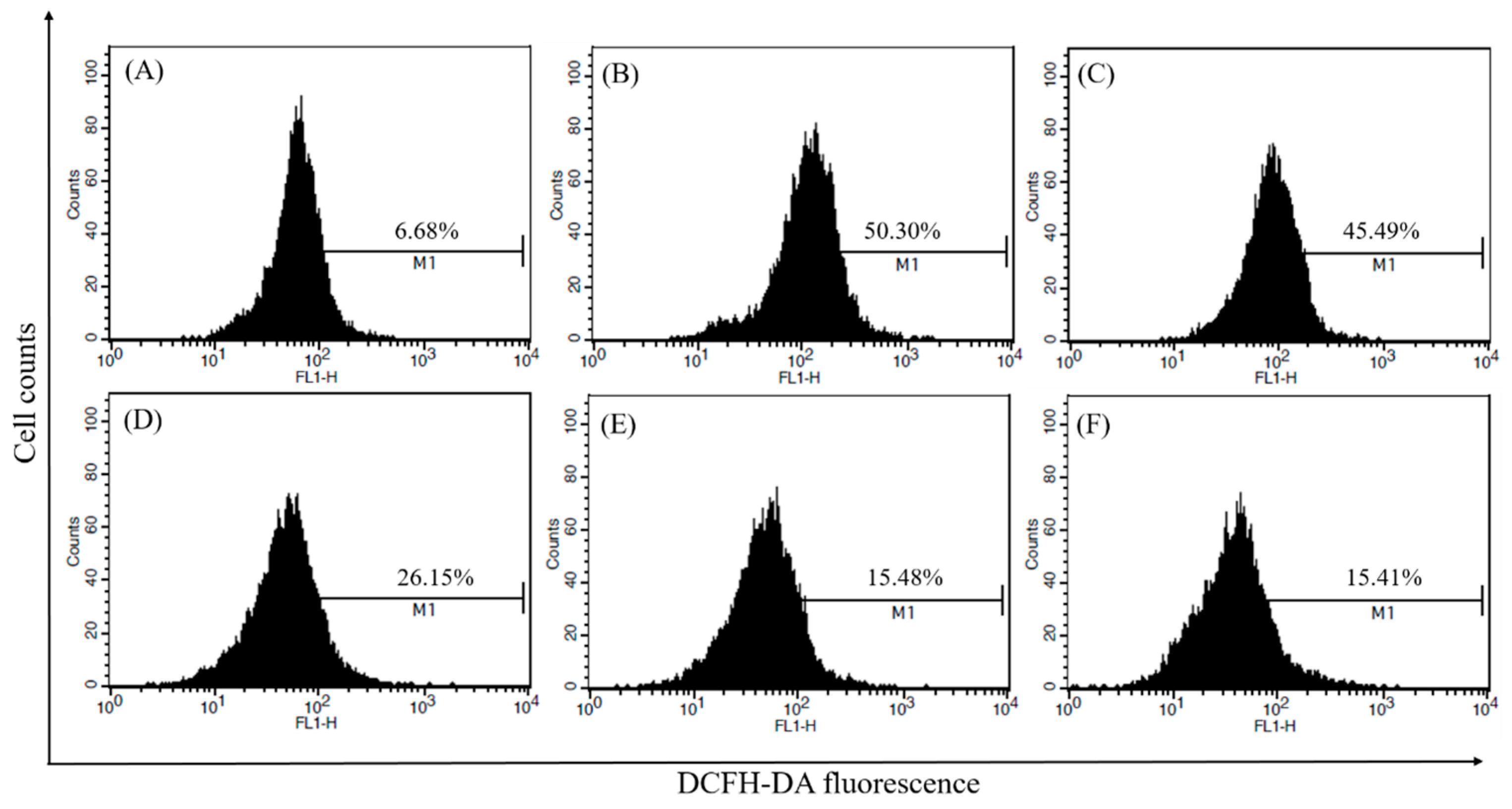
2.5. Measurement of Intracellular ROS
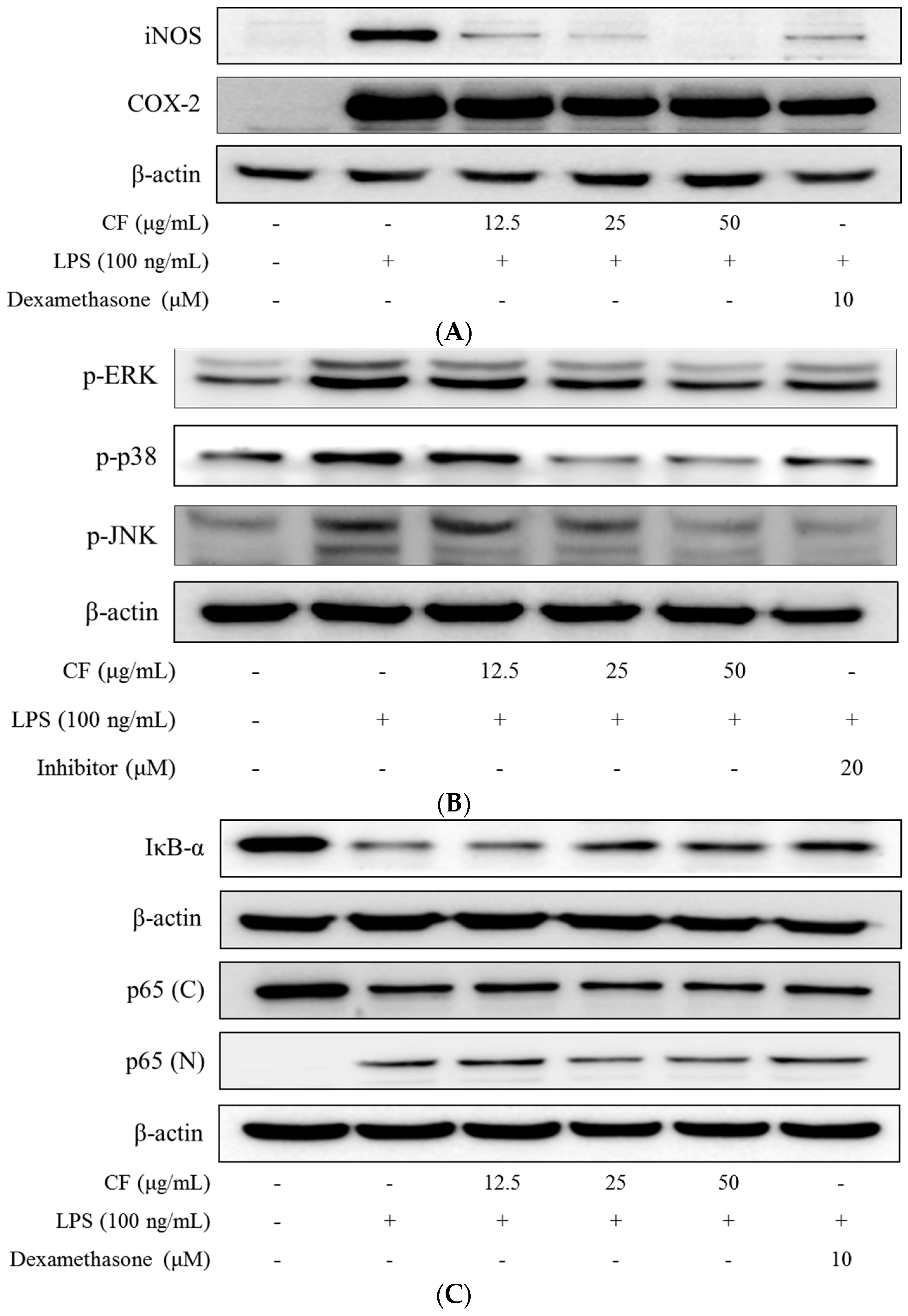
2.6. iNOS and COX-2 Expression
2.7. Inhibition of MAPKs Phosphorylation
2.8. IκB-α Degradation
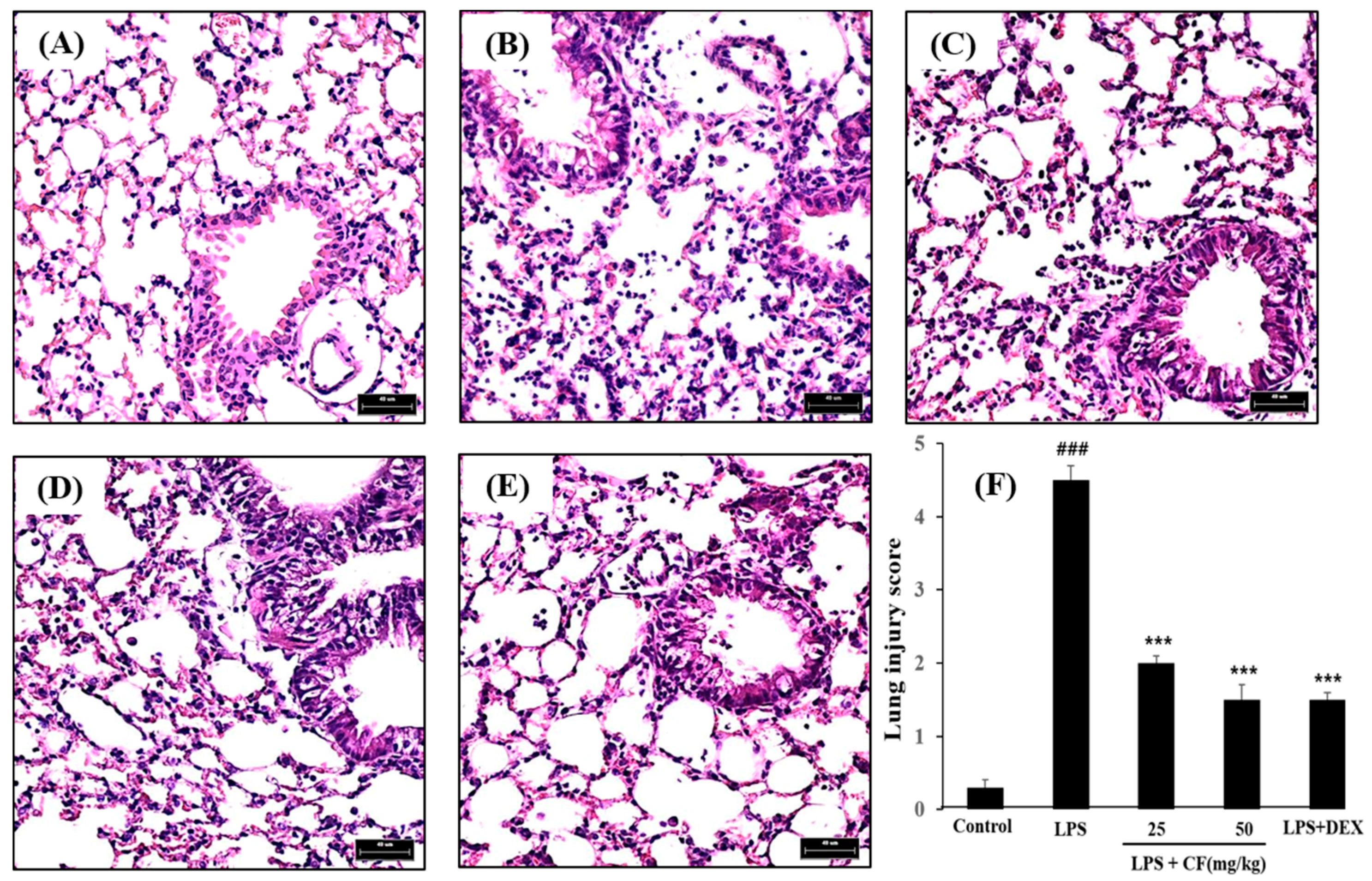
2.9. Histological Study
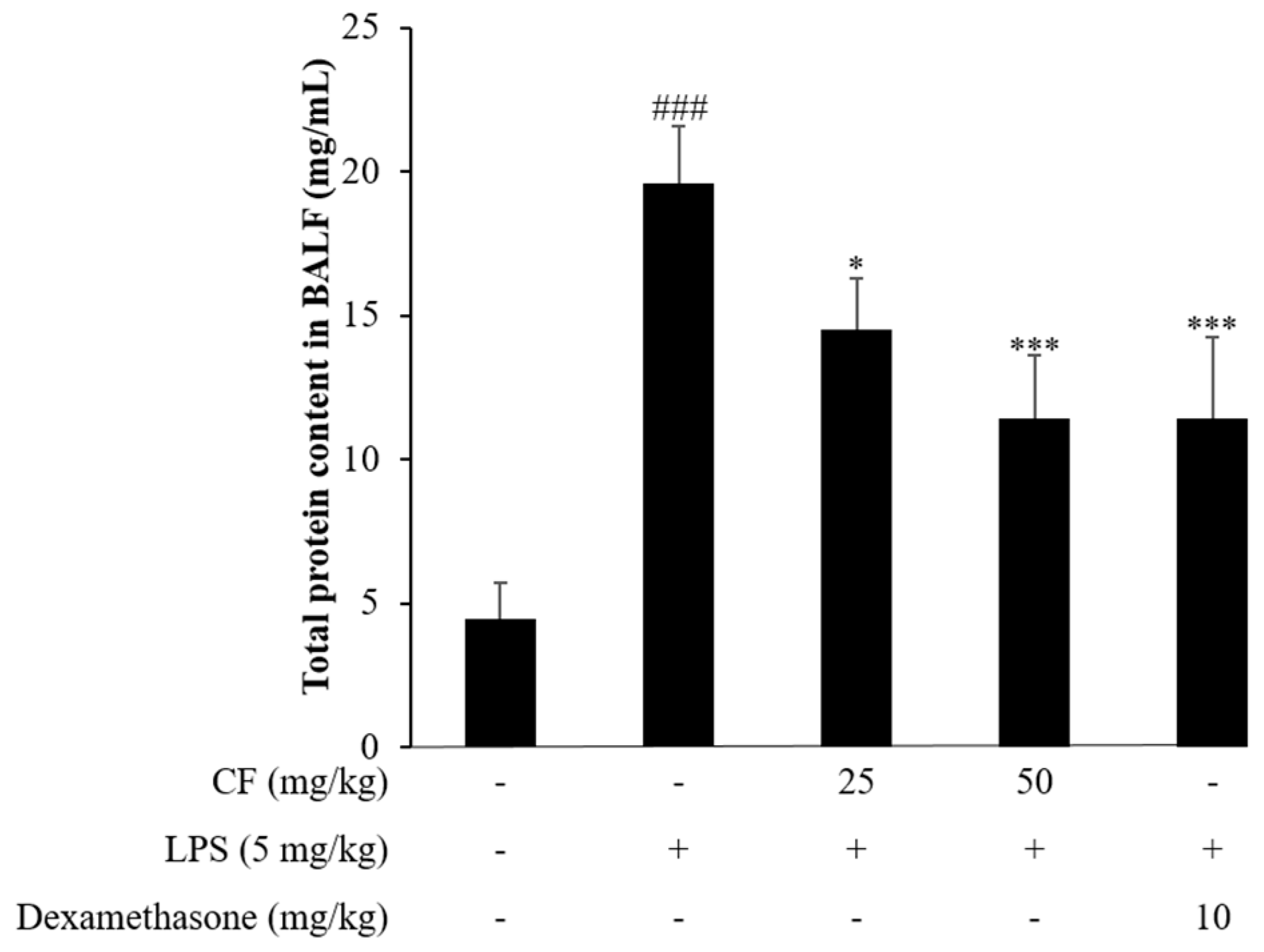
2.10. Total Protein Content Determination in BALF
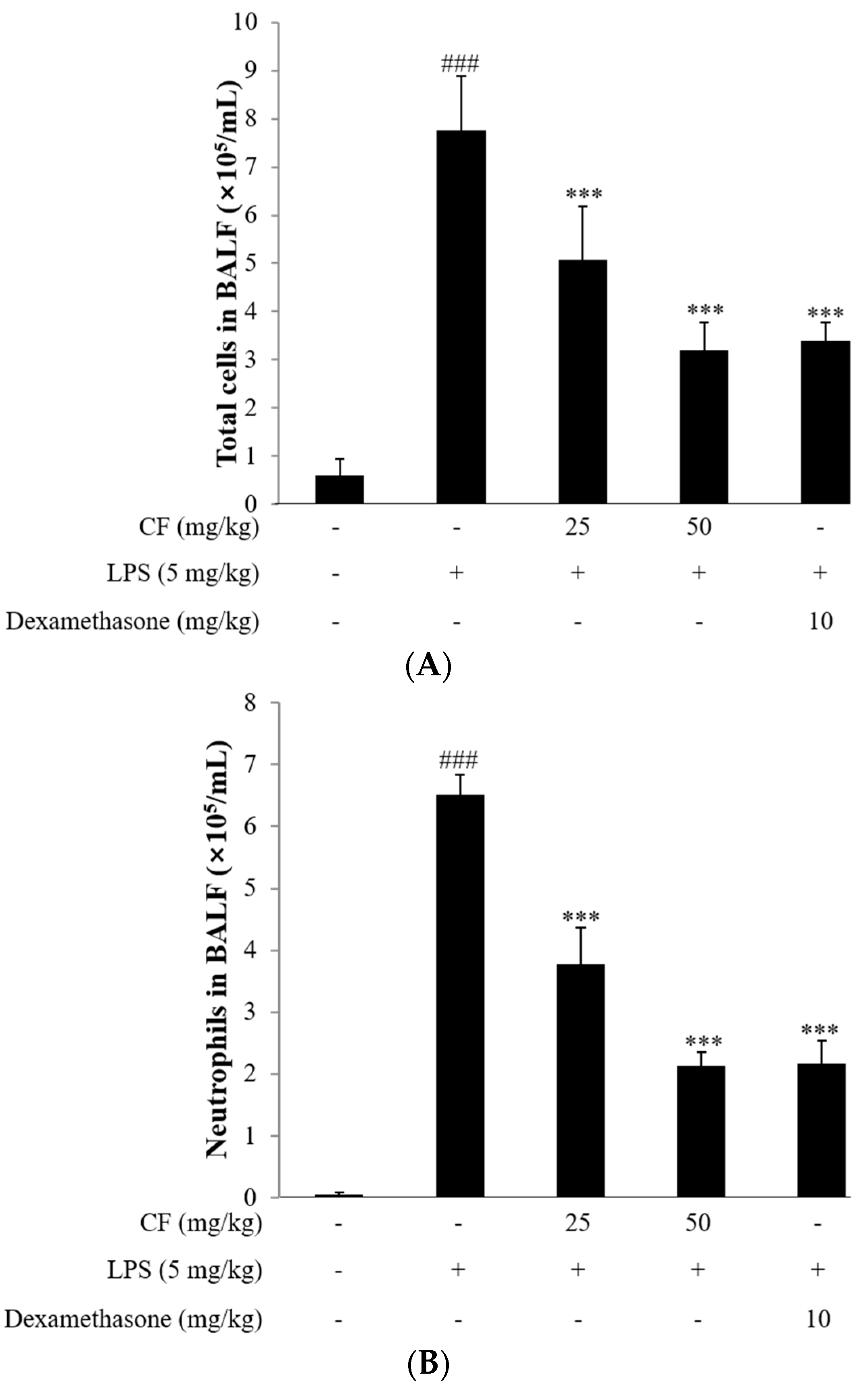
2.11. Cell Counts in BALF
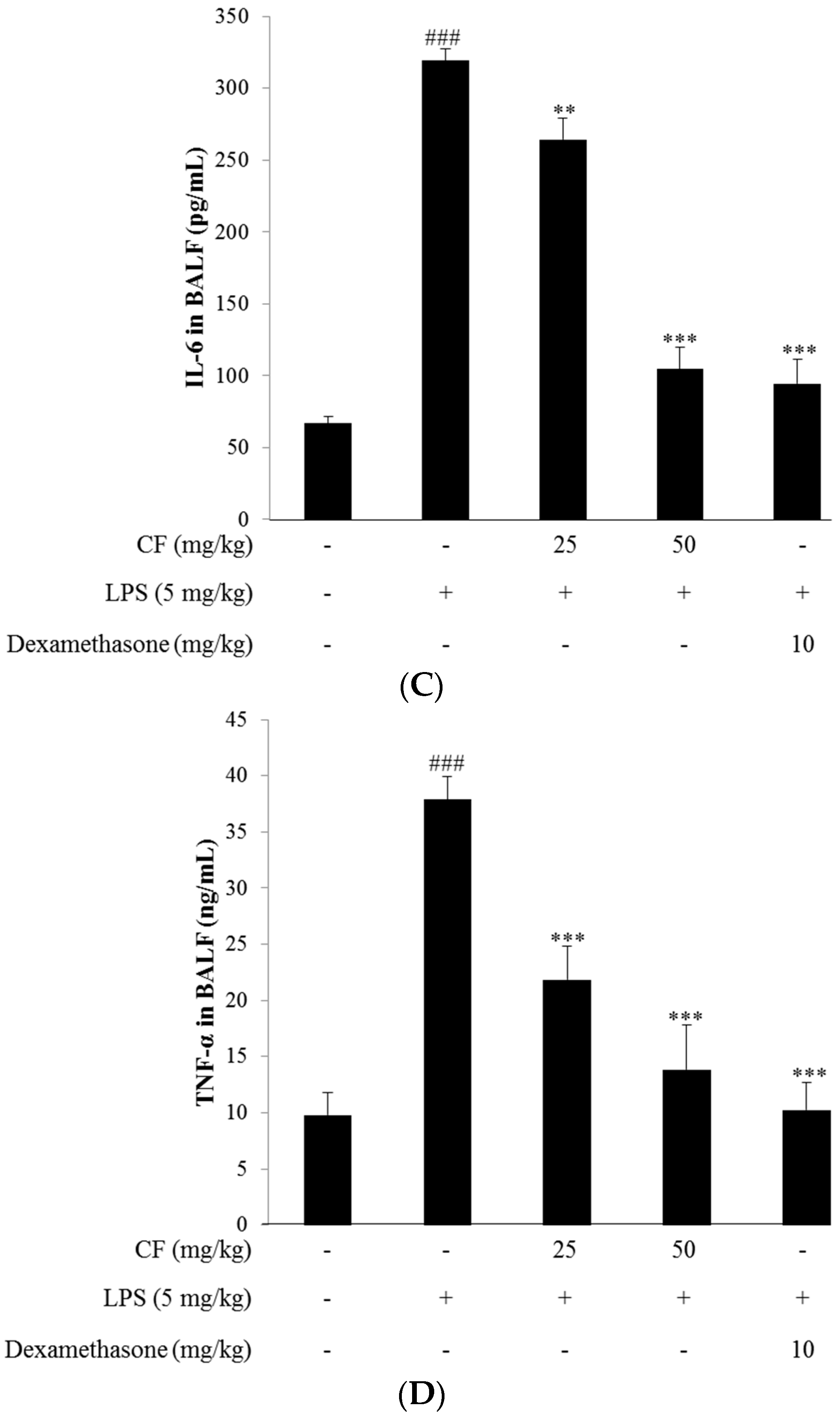
2.12. Cytokine Measurement in BALF
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extraction and Isolation
4.3. In Vitro Study
4.3.1. Cell Culture
4.3.2. Cell Viability
4.3.3. Measurement of NO Production
4.3.4. Measurement of Pro-Inflammatory Cytokine Production
4.3.5. Measurement of Intracellular ROS
4.3.6. Western Blotting
4.4. In Vivo Study
4.4.1. Animals
4.4.2. Murine Model and Grouping of LPS-Induced ALI
| Group I | Negative control |
| Group II | LPS control (5 mg/kg) |
| Group III | LPS + CF (25 mg/kg) |
| Group IV | LPS + CF (50 mg/kg) |
| Group V | Positive control (LPS + Dexamethasone 10 mg/kg) |
4.4.3. Bronchoalveolar Lavage Fluid (BALF) Collection
4.4.4. Histopathological Analysis
4.4.5. Measurement of Cytokines
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, S.H.; Jeong, H.; Kim, Y.K.; Cho, S.H.; Min, K.U.; Kim, Y.Y. IgE-mediated occupational asthma induced by herbal medicine, Banha (Pinellia ternata). Clin. Exp. Allergy 2001, 31, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.C.; Crotti, T.N.; Goldring, S.R.; Gravallese, E.M. Rheumatic diseases: The effects of inflammation on bone. Immunol. Rev. 2005, 208, 228–251. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.M.; Kim, Y.H.; Park, W.S.; Kang, I.; Ha, J.; Choi, J.W.; Park, H.J.; Lee, K.T. Inhibition of methanol extract from the fruits of Kochia scoparia on lipopolysaccharide-induced nitric oxide, prostaglandin E2, and tumor necrosis factor-alpha production from murine macrophage RAW264.7 cells. Biol. Pharm. Bull. 2004, 27, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.H.; Lim, J.H.; Kim, J.H. Lipopolysaccharide induces matrix metalloproteinase-9 expression via a mitochondrial reactive oxygen species p38 kinase-activator protein-1 pathway in RAW264.7 cells. J. Immunol. 2004, 173, 6973–6980. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Hwang, J.W.; Han, Y.K.; Kwon, H.J.; Hong, H.; Kim, E.H.; Moon, S.H.; Jeon, B.T.; Park, P.J. Antioxidant activity and protective effects of Trapa japonica pericarp extracts against tert-butylhydroperoxide-induced oxidative damage in Chang cells. Food Chem. Toxicol. 2014, 64, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Yasutake, K.; Hino, M.; Ohwatari, H.; Ohmagari, N.; Takedomi, K.; Tanaka, T.; Nonaka, G.I. Inhibitory effects of polyphenols from water chestnut (Trapa japonica) husk on glycolytic enzymes and postprandial blood glucose elevation in mice. Food Chem. 2014, 165, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.R.; Ciou, J.Y.; Chiang, P.Y. Effect of micronization on functional properties of the water caltrop (Trapa taiwanensis Nakai) pericarp. Food Chem. 2009, 113, 970–974. [Google Scholar] [CrossRef]
- Shindo, K.; Kuroki, E.; Toyoda, M. Antioxidative compounds contained in the seed with hard shell of Trapa japonica Flerov. and its herbal tea. J. Home Econ. Jpn. 2013, 64, 353–359. [Google Scholar]
- Kawasaki, M.; Kuwano, K.; Hagimoto, N.; Matsuba, T.; Kunitake, R.; Tanaka, T.; Maeyama, T.; Hara, N. Protection from lethal apoptosis in lipopolysaccharide induced acute lung injury in mice by a caspase inhibitor. Am. J. Pathol. 2000, 157, 597–603. [Google Scholar] [CrossRef]
- Ware, L.B.; Matthay, M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1334–1349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, K.; Xiong, H.; Li, H.; Chu, X.; Deng, X. Protective effect of florfenicol on acute lung injury induced by lipopolysaccharide in mice. Int. Immunopharmacol. 2009, 9, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Looney, M.R.; Su, X.; van Ziffle, J.A.; Lowell, C.A.; Matthay, M.A. Neutrophils and their Fcγ receptors are essential in a mouse model of transfusion-related acute lung injury. J. Clin. Investig. 2006, 116, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Song, K.; Xu, K.; Zhang, X.; Zhang, X.; Song, Y.; Wang, D.; Liu, S.; Deng, X. Ceftiofur attenuates lipopolysaccharide-induced acute lung injury. Int. Immunopharmacol. 2010, 10, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Hoidal, J.R.; Mukherjee, T.K. Role of TNF alpha in pulmonary pathophysiology. Respir. Res. 2006, 7, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Geiser, T.; Atabai, K.; Jarreau, P.H.; Ware, L.B.; Pugin, J.; Matthay, M.A. Pulmonary edema fluid from patients with acute lung injury augments in vitro alveolar epithelial repair by an IL-1β-dependent mechanism. Am. J. Respir. Crit. Care Med. 2001, 163, 1384–1388. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Abdelmageed, M.E.; El-Awady, M.S.; Suddek, G.M. Apocynin ameliorates endotoxin-induced acute lung injury in rats. Int. Immunopharmacol. 2016, 30, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Peng, W.H.; Tsai, K.D.; Hsu, S.L. Luteolin suppresses inflammation-associated gene expression by blocking NF-κB and AP-1 activation pathway in mouse alveolar macrophages. Life Sci. 2007, 81, 1602–1614. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Suh, S.J.; Yang, J.H.; Lu, Y.; Kim, S.J.; Kwon, S.; Jo, T.H.; Kim, J.W.; Park, Y.I.; Ahn, G.W.; et al. Anti-inflammatory activity of bark of Dioscorea batatas DECNE through the inhibition of iNOS and COX-2 expressions in RAW264.7 cells via NF-κB and ERK1/2 inactivation. Food Chem. Toxicol. 2010, 48, 3073–3079. [Google Scholar] [CrossRef] [PubMed]
- Laskin, D.L.; Pendino, K.J. Macrophages and inflammatory mediators in tissue injury. Annu. Rev. Pharmacol. Toxicol. 1995, 35, 655–677. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.W.; Kashiwabara, Y.; Nathan, C. Role of transcription factor NF-κB/Rel in induction of nitric oxide synthase. J. Biol. Chem. 1994, 269, 4705–4708. [Google Scholar] [PubMed]
- Craig, R.; Larkin, A.; Mingo, A.M.; Thuerauf, D.J.; Andrews, C.; McDonough, P.M.; Glembotski, C.C. P38 MAPK and NF-kappa B collaborate to induce interleukin-6 gene expression and release. Evidence for a cytoprotectiveautocrine signaling pathway in a cardiac myocyte model system. J. Biol. Chem. 2000, 275, 23814–23824. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Verma, I.M. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, E.L.; Karavitis, J.; Deburghgraeve, C.; Ramirez, L.; Kovacs, E.J. Prolonged chemokine expression and excessive neutrophil infiltration in the lungs of burn-injured mice exposed to ethanol and pulmonary infection. Shock 2011, 35, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Parsons, P.E.; Matthay, M.A.; Ware, L.B.; Eisner, M.D. Elevated plasma levels of soluble TNF receptors are associated with morbidity and mortality in patients with acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 288, L426–L431. [Google Scholar] [CrossRef] [PubMed]
- Niu, N.; Li, B.; Hu, Y.; Li, X.; Li, J.; Zhang, H. Protective effects of scoparone against lipopolysaccharide-induced acute lung injury. Int. Immunopharmacol. 2014, 23, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Xue, M.Y.; Geng, Z.R.; Chen, P.Y. The suppressive effects of Bursopentine (BP5) on oxidative stress and NF-κB activation in lipopolysaccharide-activated murine peritoneal macrophages. Cell. Physiol. Biochem. 2012, 29, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-S.; Hwang, J.-W.; Jang, J.-H.; Son, S.; Seo, I.-B.; Jeong, J.-H.; Kim, E.-H.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Trapa japonica Pericarp Extract Reduces LPS-Induced Inflammation in Macrophages and Acute Lung Injury in Mice. Molecules 2016, 21, 392. https://doi.org/10.3390/molecules21030392
Kim Y-S, Hwang J-W, Jang J-H, Son S, Seo I-B, Jeong J-H, Kim E-H, Moon S-H, Jeon B-T, Park P-J. Trapa japonica Pericarp Extract Reduces LPS-Induced Inflammation in Macrophages and Acute Lung Injury in Mice. Molecules. 2016; 21(3):392. https://doi.org/10.3390/molecules21030392
Chicago/Turabian StyleKim, Yon-Suk, Jin-Woo Hwang, Jae-Hyuk Jang, Sangkeun Son, Il-Bok Seo, Jae-Hyun Jeong, Ee-Hwa Kim, Sang-Ho Moon, Byong-Tae Jeon, and Pyo-Jam Park. 2016. "Trapa japonica Pericarp Extract Reduces LPS-Induced Inflammation in Macrophages and Acute Lung Injury in Mice" Molecules 21, no. 3: 392. https://doi.org/10.3390/molecules21030392




