Isoquercitrin Inhibits Hydrogen Peroxide-Induced Apoptosis of EA.hy926 Cells via the PI3K/Akt/GSK3β Signaling Pathway
Abstract
:1. Introduction
2. Results
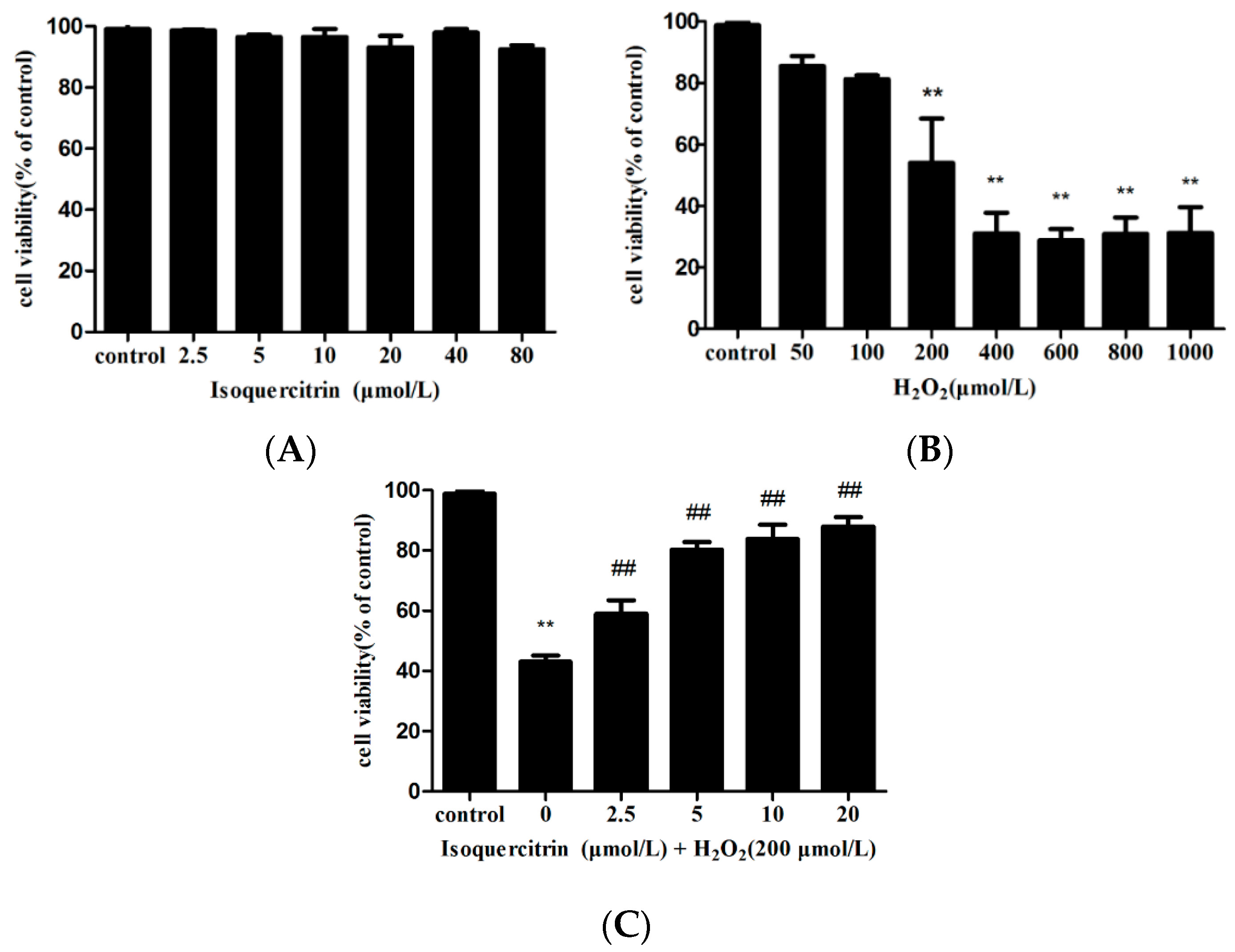
2.1. Isoquercitrin Inhibits H2O2-Induced Loss of Cell Viability in EA.hy926 Cells
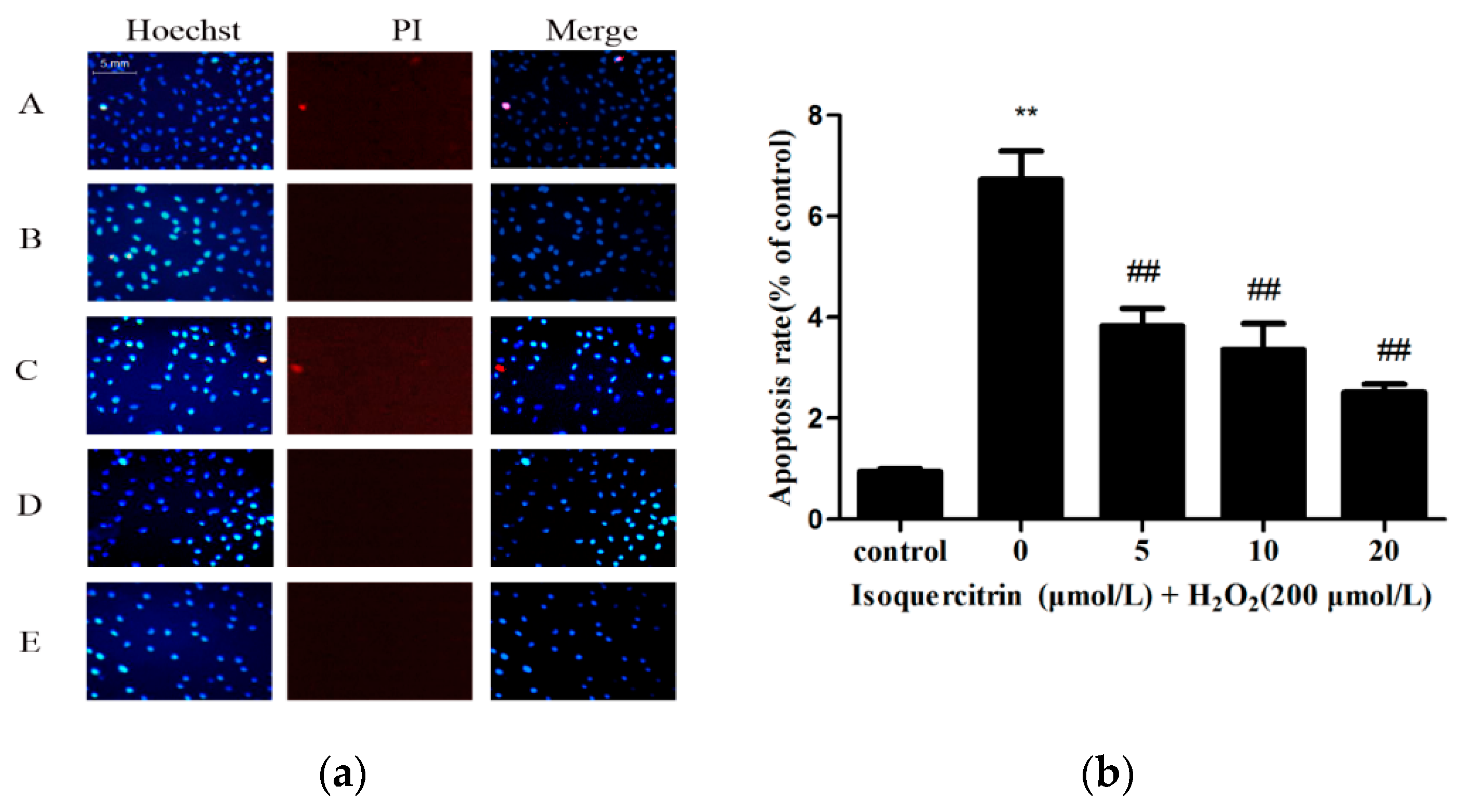
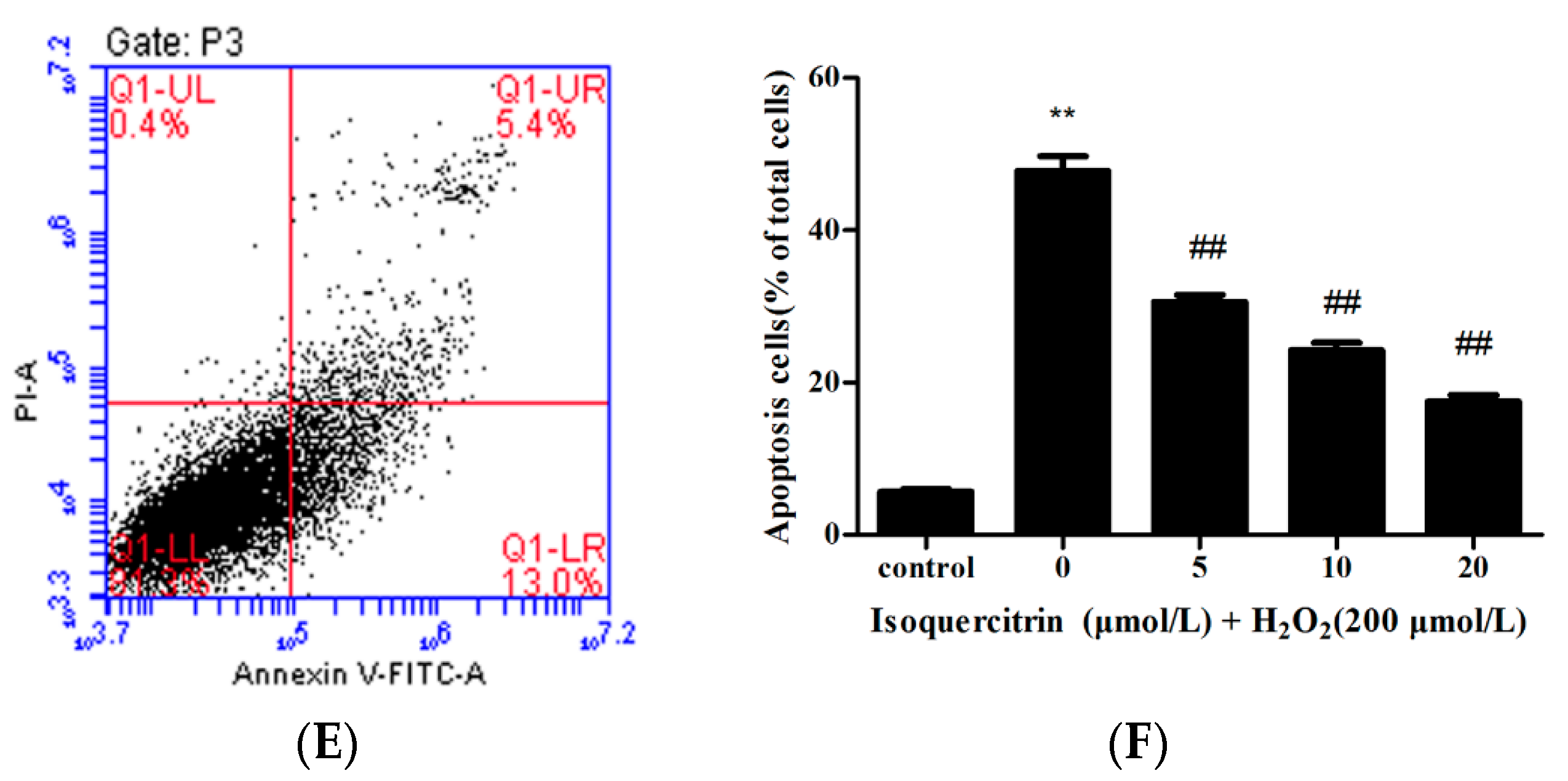
2.2. Isoquercitrin Inhibits H2O2-Induced Apoptosis of EA.hy926 Cells
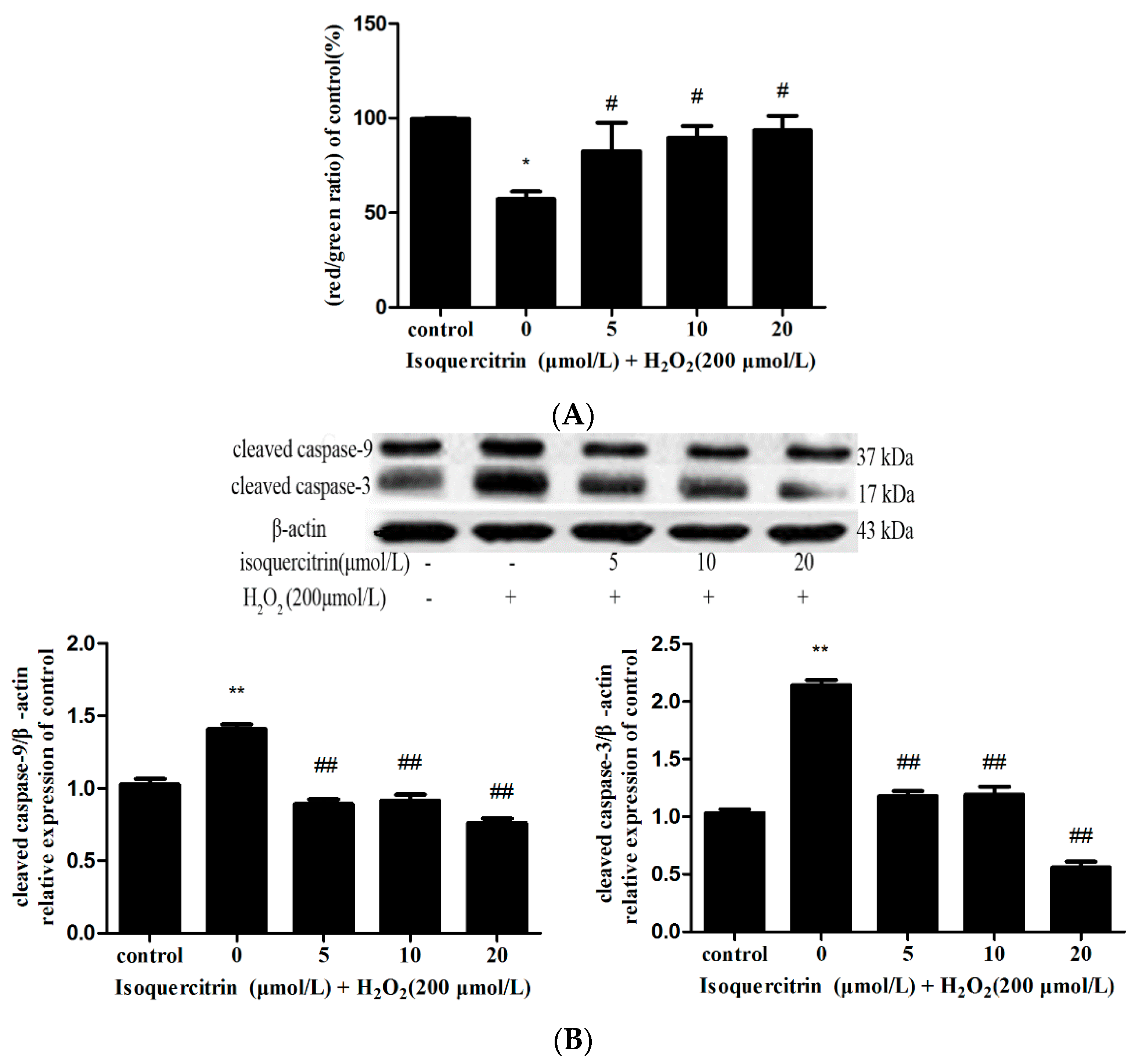
2.3. Isoquercitrin Inhibits H2O2-Induced Decreases in Mitochondrial Membrane Potential in EA.hy926 Cells
2.4. Isoquercitrin Inhibited H2O2-Induced Increases in Cleaved Caspase-3 and Cleaved Caspase-9 Expression in EA.hy926 Cells
2.5. Isoquercitrin Enhanced Mcl-1 Expression in H2O2-Induced EA.hy926 Cells
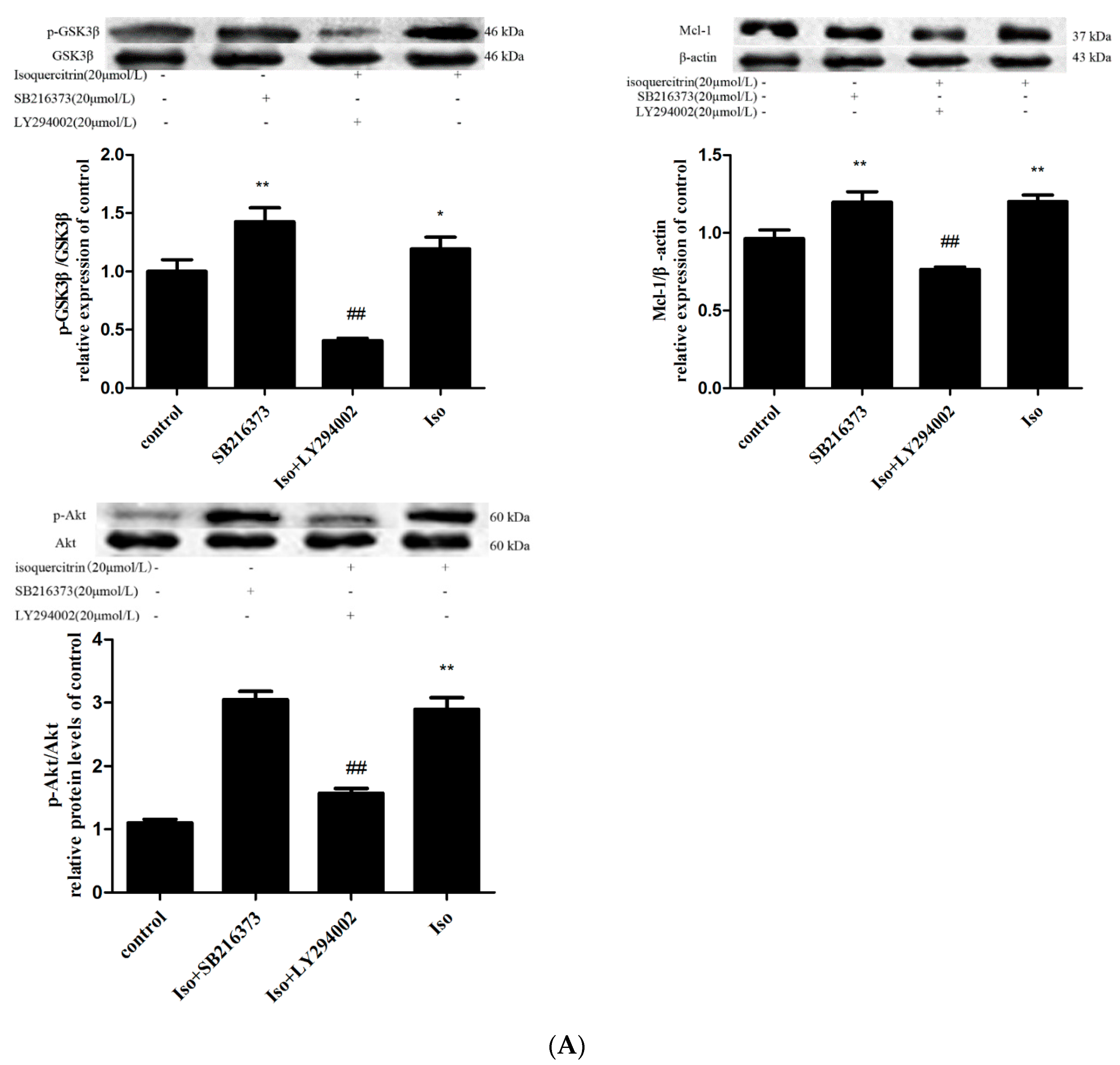
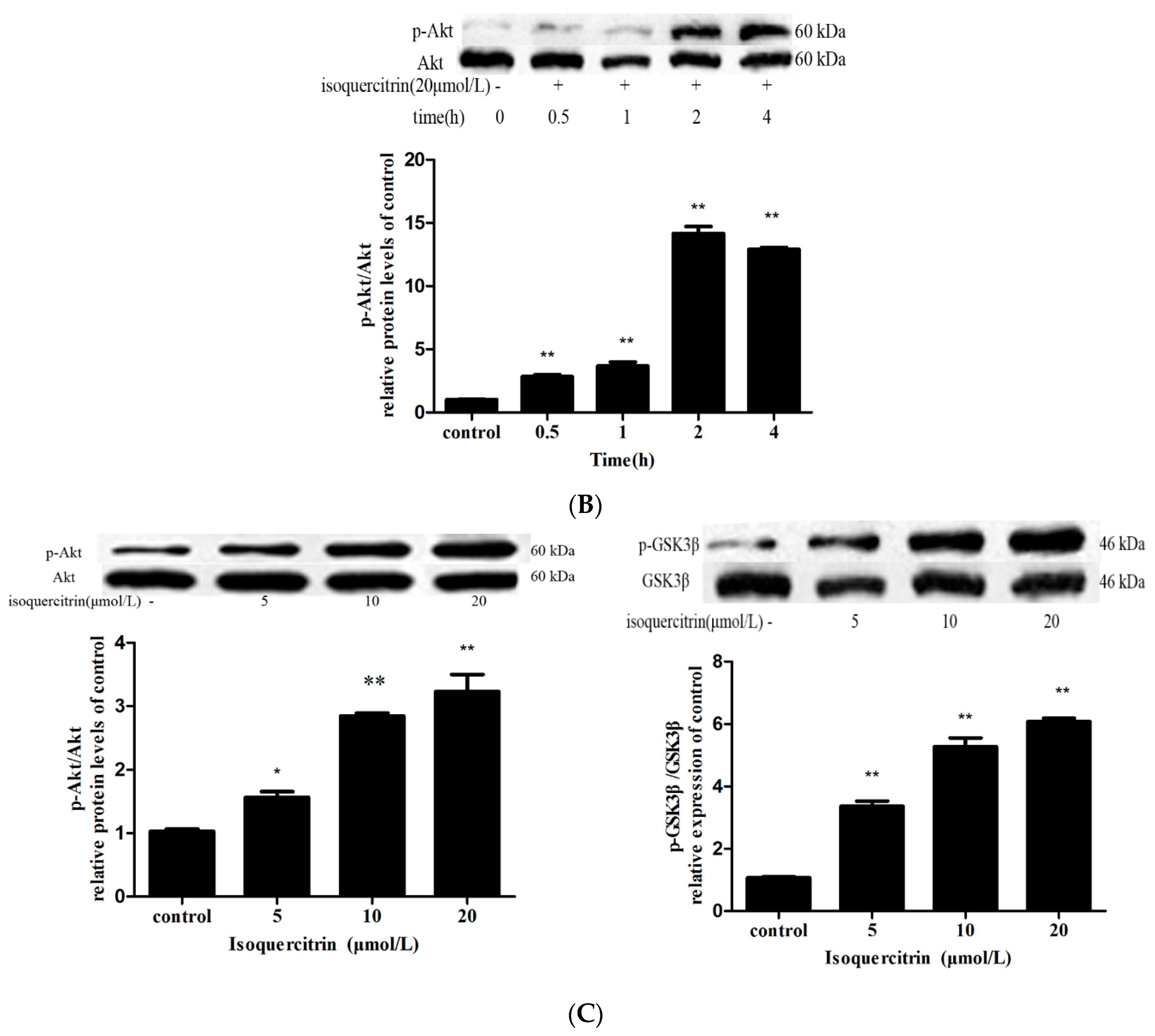
2.6. Isoquercitrin Treatment Increased Mcl-1 Expression in a PI3K/Akt/GSK3β-Dependent Manner
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Cell Culture
4.3. Cell Viability Analysis
4.4. Hoechst33342/PI Fluorescent Staining
4.5. Flow Cytometric Analysis of Apoptotic Cells
4.6. Mitochondrial Membrane Potential Assay
4.7. Western Blotting
4.8. Fluorescence Quantitative PCR
4.9. StatisticalAnalysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| DMEM | Dulbecco’s modified Eagle’s medium |
| FBS | Fetal bovine serum |
| DMSO | Dimethyl sulfoxide |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-dephenyltetrazolium bromide |
| HUVEC | Human umbilical vein endothelial cell |
| FBS | Fetal bovine serum |
| PI | Propidium iodide |
| TBST | Tris-buffered saline containing 0.05% Tween-20 |
| HRP | Horseradish peroxidase |
| FQ-PCR | Fluorescence quantitative polymerase chain reaction |
| Iso | Isoquercitrin |
References
- Kumar, P.; Singh, C.; Agarwal, N.; Pandey, S.; Ranjan, A.; Singh, G. Prevalence of risk factors for non-communicable disease in a rural area of Patna, Bihar—A WHO step wise approach. Indian J. Prev. Soc. Med. 2013, 44, 47–53. [Google Scholar]
- Străchinariu, R.T. The role of endothelial dysfunction in the pathogenesis of vascular complications of diabetes mellitus—A high priority area of investigation. Romanian J. Diabetes Nutr. Metab. Dis. 2015, 22, 61–66. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Davignon, J.; Ganz, P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004, 109 (Suppl. S1), III27–III32. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.J.; Uehata, A.; Gerhard, M.D.; Meredith, I.T.; Knab, S.; Delagrange, D.; Lieberman, E.H.; Ganz, P.; Creager, M.A.; Yeung, A.C. Close relation of endothelial function in the human coronary and peripheral circulations. J. Am. Coll. Cardiol. 1995, 26, 1235–1241. [Google Scholar] [CrossRef]
- Barton, M.; Vos, I.; Shaw, S.; Boer, P.; D’uscio, L.V.; Gröne, H.J.; Rabelink, T.J.; Lattmann, T.; Moreau, P.; Lüscher, T.F. Dysfunctional renal nitric oxide synthase as a determinant of salt-sensitive hypertension mechanisms of renal artery endothelial dysfunction and role of endothelin for vascular hypertrophy and glomerulosclerosis. J. Am. Soc. Nephrol. 2000, 11, 835–845. [Google Scholar] [PubMed]
- Badran, M.; Ayas, N.; Laher, I. Cardiovascular complications of sleep apnea: Role of oxidative stress. Oxid. Med. Cell. Longev. 2014, 2014, 985258. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, C.; Wu, X.; Zheng, W.; Sandberg, K.; Ji, H.; Welch, W.J.; Wilcox, C.S. Endothelial dysfunction and enhanced contractility in microvessels from ovariectomized rats: Roles of oxidative stress and perivascular adipose tissue. Hypertension 2014, 63, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Aranguren, L.C.; Prada, C.E.; Riano-Medina, C.E.; Lopez, M. Endothelial dysfunction and preeclampsia: Role of oxidative stress. Front. Phys. 2014, 5, 372. [Google Scholar] [CrossRef] [PubMed]
- Jurikova, T.; Sochor, J.; Rop, O.; Mlcek, J.; Balla, S.; Szekeres, L.; Adam, V.; Kizek, R. Polyphenolic profile and biological activity of Chinese hawthorn (Crataegus pinnatifida BUNGE) fruits. Molecules 2012, 17, 14490–14509. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, K.S.; Rupasinghe, H.P. Antioxidant and cytoprotective properties of partridgeberry polyphenols. Food Chem. 2015, 168, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.G.; Raulino, R.J.; Cerqueira, D.M.; Mannarino, S.C.; Pereira, M.D.; Panek, A.D.; Silva, J.F.; Menezes, F.S.; Eleutherio, E.C. In vitro and in vivo determination of antioxidant activity and mode of action of isoquercitrin and Hyptis fasciculata. Phytomed. Int. J. Phytother. Phytopharm. 2009, 16, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Plumb, G.W.; Uda, Y.; Price, K.R.; Rhodes, M.J. Dietary quercetin glycosides: Antioxidant activity and induction of the anticarcinogenic phase II marker enzyme quinone reductase in Hepalclc7 cells. Carcinogenesis 1996, 17, 2385–2387. [Google Scholar] [CrossRef] [PubMed]
- Amado, N.G.; Cerqueira, D.M.; Menezes, F.S.; da Silva, J.F.; Neto, V.M.; Abreu, J.G. Isoquercitrin isolated from Hyptis fasciculata reduces glioblastoma cell proliferation and changes beta-catenin cellular localization. Anti Cancer Drugs 2009, 20, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.E.; Kuster, R.M.; Yamamoto, K.A.; Salles, T.S.; Campos, R.; de Meneses, M.D.; Soares, M.R.; Ferreira, D. Quercetin and quercetin 3-O-glycosides from Bauhinia longifolia (Bong.) Steud. Show anti-Mayaro virus activity. Parasite Vectors 2014, 7, 130. [Google Scholar] [CrossRef] [PubMed]
- Rogerio, A.P.; Kanashiro, A.; Fontanari, C.; da Silva, E.V.; Lucisano-Valim, Y.M.; Soares, E.G.; Faccioli, L.H. Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm. Res. 2007, 56, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.W.; Juang, L.J.; Wang, B.S.; Wang, M.Y.; Tai, H.M.; Hung, W.J.; Chen, Y.J.; Huang, M.H. Antioxidant and antityrosinase activity of mulberry (Morus alba L.) twigs and root bark. Food Chem. Toxicol. 2011, 49, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.H.; Kim, B.J.; Lee, E.H.; Osborne, N.N. Isoquercitrin is the most effective antioxidant in the plant Thuja orientalis and able to counteract oxidative-induced damage to a transformed cell line (RGC-5 cells). Neurochem. Int. 2010, 57, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Gasparotto Junior, A.; Gasparotto, F.M.; Lourenco, E.L.; Crestani, S.; Stefanello, M.E.; Salvador, M.J.; da Silva-Santos, J.E.; Marques, M.C.; Kassuya, C.A. Antihypertensive effects of isoquercitrin and extracts from Tropaeolum majus L.: Evidence for the inhibition of angiotensin converting enzyme. J. Ethnopharmacol. 2011, 134, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Paulo, A.; Martins, S.; Branco, P.; Dias, T.; Borges, C.; Rodrigues, A.I.; Costa Mdo, C.; Teixeira, A.; Mota-Filipe, H. The opposing effects of the flavonoids isoquercitrin and sissotrin, isolated from Pterospartum tridentatum, on oral glucose tolerance in rats. Phytother. Res. 2008, 22, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Gasparotto Junior, A.; Prando, T.B.; LemeTdos, S.; Gasparotto, F.M.; Lourenco, E.L.; Rattmann, Y.D.; Da Silva-Santos, J.E.; Kassuya, C.A.; Marques, M.C. Mechanisms underlying the diuretic effects of Tropaeolum majus L. extracts and its main component isoquercitrin. J. Ethnopharmacol. 2012, 141, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Gasparotto Junior, A.; Gasparotto, F.M.; Boffo, M.A.; Lourenco, E.L.; Stefanello, M.E.; Salvador, M.J.; da Silva-Santos, J.E.; Marques, M.C.; Kassuya, C.A. Diuretic and potassium-sparing effect of isoquercitrin—An active flavonoid of Tropaeolum majus L. J. Ethnopharmacol. 2011, 134, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Kanemaru, M.; Okuyama, S.; Shimizu, R.; Tanaka, H.; Mizukami, H. Anti-allergic effects of enzymatically modified isoquercitrin (α-oligoglucosyl quercetin 3-O-glucoside), quercetin 3-O-glucoside, α-oligoglucosyl rutin, and quercetin, when administered orally to mice. J. Nat. Med. 2013, 67, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Slee, E.A.; Harte, M.T.; Kluck, R.M.; Wolf, B.B.; Casiano, C.A.; Newmeyer, D.D.; Wang, H.G.; Reed, J.C.; Nicholson, D.W.; Alnemri, E.S.; et al. Ordering the cytochrome c–initiated caspase cascade: Hierarchical activation of caspases-2,-3,-6,-7,-8, and-10 in a caspase-9—Dependent manner. J. Cell Biol. 1999, 144, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Browne, G.; Melino, G.; Cohen, G.M. Ordering of caspases in cells undergoing apoptosis by the intrinsic pathway. Cell Death Differ. 2009, 16, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.W.; Lam, C.; Edwards, S.W. Mcl-1; the molecular regulation of protein function. FEBS Lett. 2010, 584, 2981–2989. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; He, X.; Hsu, J.M.; Xia, W.; Chen, C.T.; Li, L.Y.; Lee, D.F.; Liu, J.C.; Zhong, Q.; Wang, X.D.; Hung, M.C. Degradation of Mcl-1 by β-TrCP mediates glycogen synthase kinase 3-induced tumor suppression and chemosensitization. Mol. Cell. Biol. 2007, 27, 4006–4017. [Google Scholar] [CrossRef] [PubMed]
- Hybertson, B. M.; Gao, B.; Bose, S.K.; McCord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Asp. Med. 2011, 32, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Vuleta, A.; Jovanović, S.M.; Tucić, B. Adaptive flexibility of enzymatic antioxidants SOD, APX and CAT to high light stress: The clonal perennial monocot Iris pumila as a study case. Plant Physiol. Biochem. 2016, 100, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Inagi, R. Oxidative stress in cardiovascular disease: A new avenue toward future therapeutic approaches. Recent Pat. Cardiovasc. Drug Discov. 2006, 1, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Slezak, J.; Tribulova, N.; Pristacova, J.; Uhrik, B.; Thomas, T.; Khaper, N.; Kaul, N.; Singal, P. Hydrogen peroxide changes in ischemic and reperfused heart. Cytochemistry and biochemical and X-ray microanalysis. Am. J. Pathol. 1995, 147, 772–781. [Google Scholar] [PubMed]
- Chen, Q.M.; Tu, V.C.; Wu, Y.; Bahl, J.J. Hydrogen peroxide dose dependent induction of cell death or hypertrophy in cardiomyocytes. Arch. Biochem. Biophys. 2000, 373, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Giampieri, F.; González-Paramás, A.M.; Damiani, E.; Astolfi, P.; Martinez-Sanchez, G.; Bompadre, S.; Quiles, J.L.; Santos-Buelga, C.; Battino, M. Phenolics from monofloral honeys protect human erythrocyte membranes against oxidative damage. Food Chem. Toxicol. 2012, 50, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Valentová, K.; Vrba, J.; Bancířová, M.; Ulrichová, J.; Křen, V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Batista, Â.G.; Ferrari, A.S.; da Cunha, D.C.; da Silva, J.K.; Cazarin, C.B.B.; Correa, L.C.; Prado, M.A.; de Carvalho-Silva, L.B.; Esteves, E.A.; Júnior, M.R.M. Polyphenols, antioxidants, and antimutagenic effects of Copaifera langsdorffii fruit. Food Chem. 2016, 197, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Forbes-Hernández, T.Y.; Giampieri, F.; Gasparrini, M.; Mazzoni, L.; Quiles, J.L.; Alvarez-Suarez, J.M.; Battino, M. The effects of bioactive compounds from plant foods on mitochondrial function: A focus on apoptotic mechanisms. Food Chem. Toxicol. 2014, 68, 154–182. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Du, L.; Xu, C.; Cao, J.; Wang, Q.; Liu, Q.; Fan, F. Radiation‑induced cytochrome c release and the neuroprotective effects of the pan-caspase inhibitor z‑VAD‑fmk in the hypoglossal nucleus. Exp. Ther. Med. 2014, 7, 383–388. [Google Scholar] [PubMed]
- Jiang, G.B.; Zheng, X.; Yao, J.H.; Han, B.J.; Li, W.; Wang, J.; Huang, H.L.; Liu, Y.J. Ruthenium(II) polypyridyl complexes induce BEL-7402 cell apoptosis by ROS-mediated mitochondrial pathway. J. Inorg. Biochem. 2014, 141, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Brunelle, J.K.; Letai, A. Control of mitochondrial apoptosis by the Bcl-2 family. J. Cell Sci. 2009, 122, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Kuwana, T.; Bouchier-Hayes, L.; Chipuk, J.E.; Bonzon, C.; Sullivan, B.A.; Green, D.R.; Newmeyer, D.D. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol. Cell 2005, 17, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Srinivasula, S.M.; Ahmad, M.; Fernandes-Alnemri, T.; Alnemri, E.S. Autoactivation of Procaspase-9 by Apaf-1-Mediated Oligomerization. Mol. Cell 1998, 1, 949–957. [Google Scholar] [CrossRef]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef]
- Michels, J.; Johnson, P.W.; Packham, G. Mcl-1. Int. J. Biochem. Cell Biol. 2005, 37, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Willis, S.N.; Chen, L.; Dewson, G.; Wei, A.; Naik, E.; Fletcher, J.I.; Adams, J.M.; Huang, D.C. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005, 19, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Longo, P.G.; Laurenti, L.; Gobessi, S.; Sica, S.; Leone, G.; Efremov, D.G. The Akt/Mcl-1 pathway plays a prominent role in mediating antiapoptotic signals downstream of the B-cell receptor in chronic lymphocytic leukemia B cells. Blood 2008, 111, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Opferman, J. Unraveling MCL-1 degradation. Cell Death Differ. 2006, 13, 1260–1262. [Google Scholar] [CrossRef] [PubMed]
- Maurer, U.; Charvet, C.; Wagman, A.S.; Dejardin, E.; Green, D.R. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol. Cell 2006, 21, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Frame, S. The renaissance of GSK3. Nat. Rev. Mol. Cell Biol. 2001, 2, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Maurer, U.; Preiss, F.; Brauns-Schubert, P.; Schlicher, L.; Charvet, C. GSK-3—At the crossroads of cell death and survival. J. Cell Sci. 2014, 127, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Cross, D.A.; Alessi, D.R.; Cohen, P.; Andjelkovich, M.; Hemmings, B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995, 378, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Geng, L.; Yazlovitskaya, E.M.; Hallahan, D.E. Protein kinase B/Akt-dependent phosphorylation of glycogen synthase kinase-3β in irradiated vascular endothelium. Cancer Res. 2006, 66, 2320–2327. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compound isoquercitrin are available from the authors.


© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.; Li, J.; Wang, K.; Hao, X.; Ge, R.; Li, Q. Isoquercitrin Inhibits Hydrogen Peroxide-Induced Apoptosis of EA.hy926 Cells via the PI3K/Akt/GSK3β Signaling Pathway. Molecules 2016, 21, 356. https://doi.org/10.3390/molecules21030356
Zhu M, Li J, Wang K, Hao X, Ge R, Li Q. Isoquercitrin Inhibits Hydrogen Peroxide-Induced Apoptosis of EA.hy926 Cells via the PI3K/Akt/GSK3β Signaling Pathway. Molecules. 2016; 21(3):356. https://doi.org/10.3390/molecules21030356
Chicago/Turabian StyleZhu, Meixia, Jiankuan Li, Ke Wang, Xuliang Hao, Rui Ge, and Qingshan Li. 2016. "Isoquercitrin Inhibits Hydrogen Peroxide-Induced Apoptosis of EA.hy926 Cells via the PI3K/Akt/GSK3β Signaling Pathway" Molecules 21, no. 3: 356. https://doi.org/10.3390/molecules21030356





