Elucidation of Transport Mechanism of Paeoniflorin and the Influence of Ligustilide, Senkyunolide I and Senkyunolide A on Paeoniflorin Transport through Mdck-Mdr1 Cells as Blood–Brain Barrier in Vitro Model
Abstract
:1. Introduction
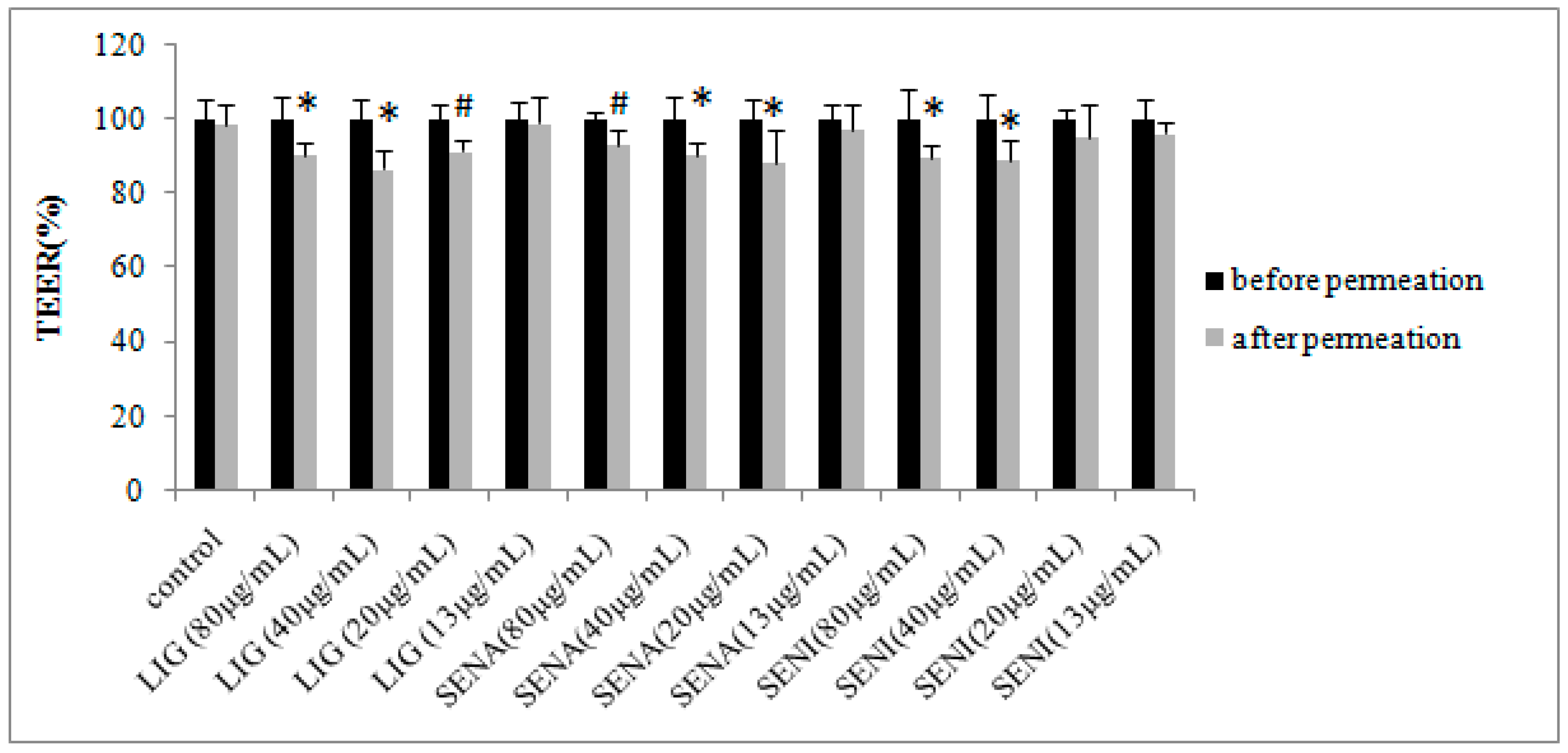
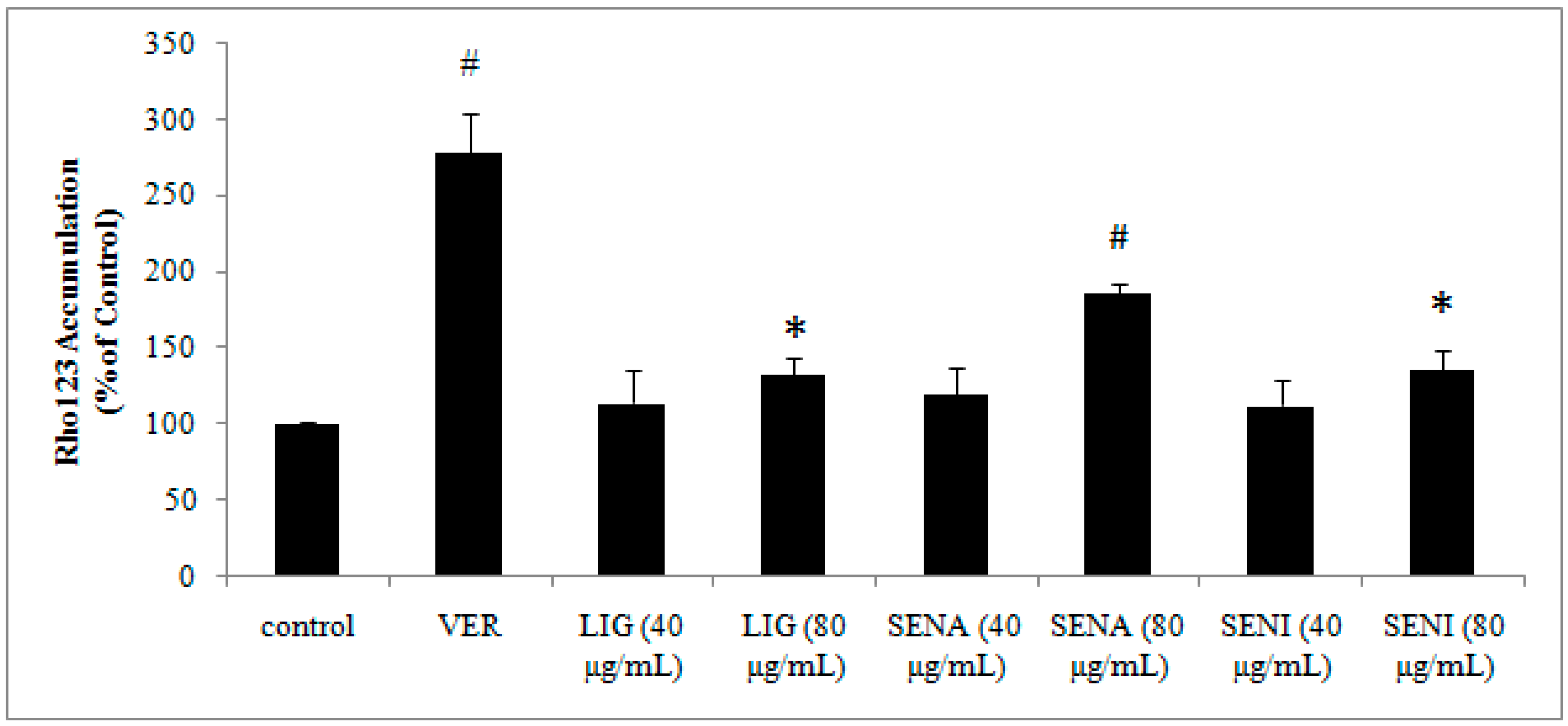
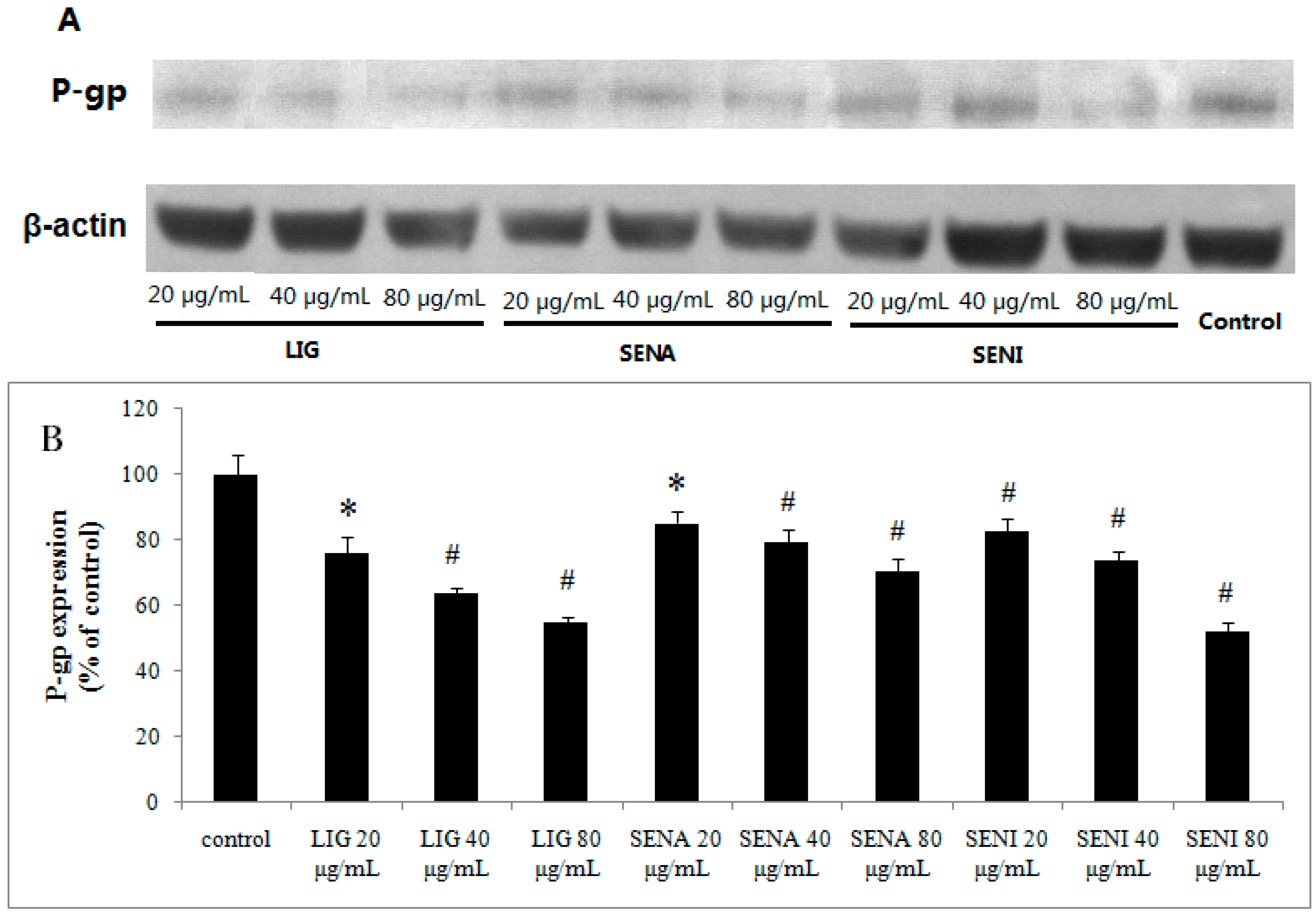
2. Results
3. Materials and Methods
3.1. Materials
3.2. Cell Culture
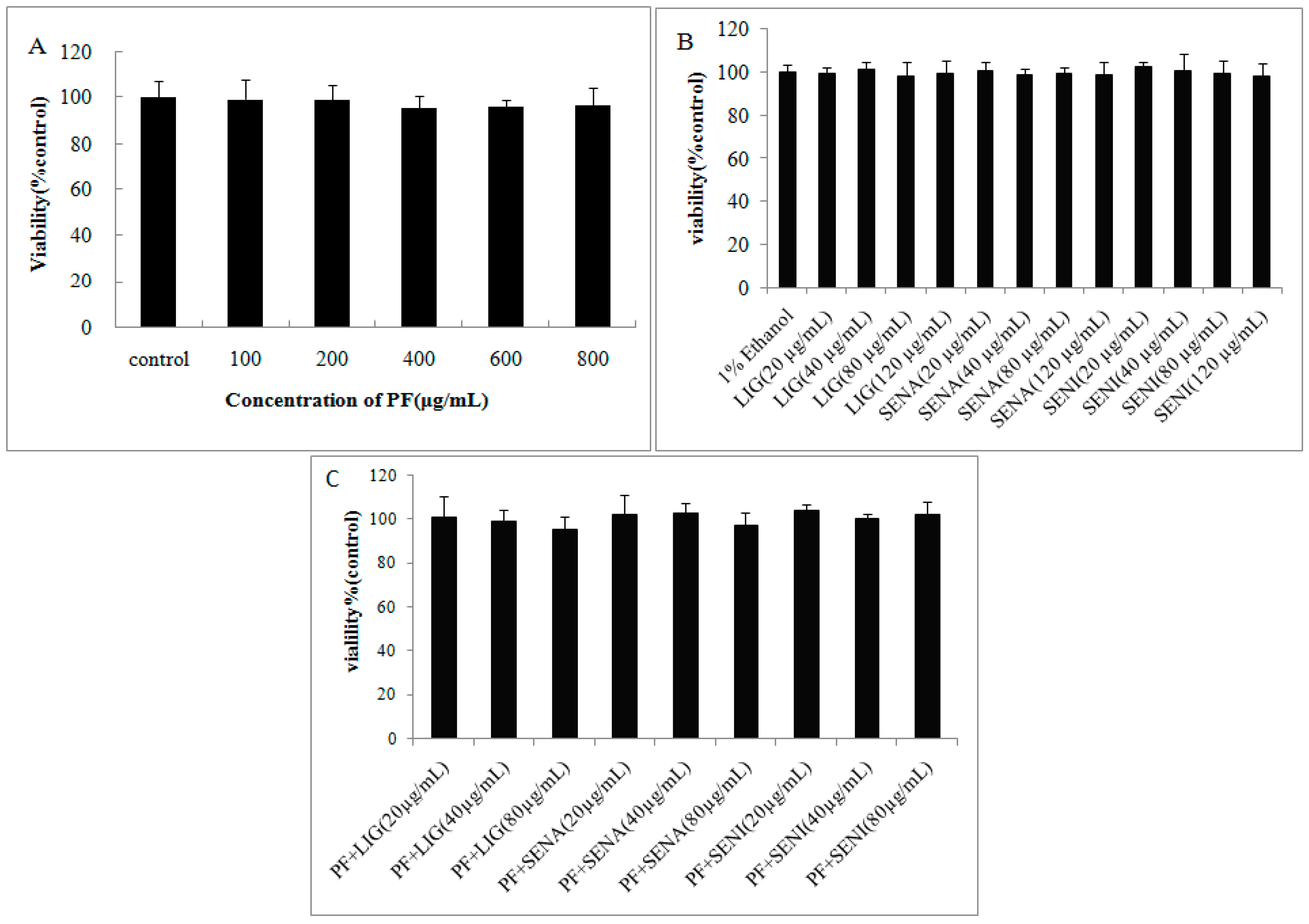
3.3. Cytotoxicity Assays
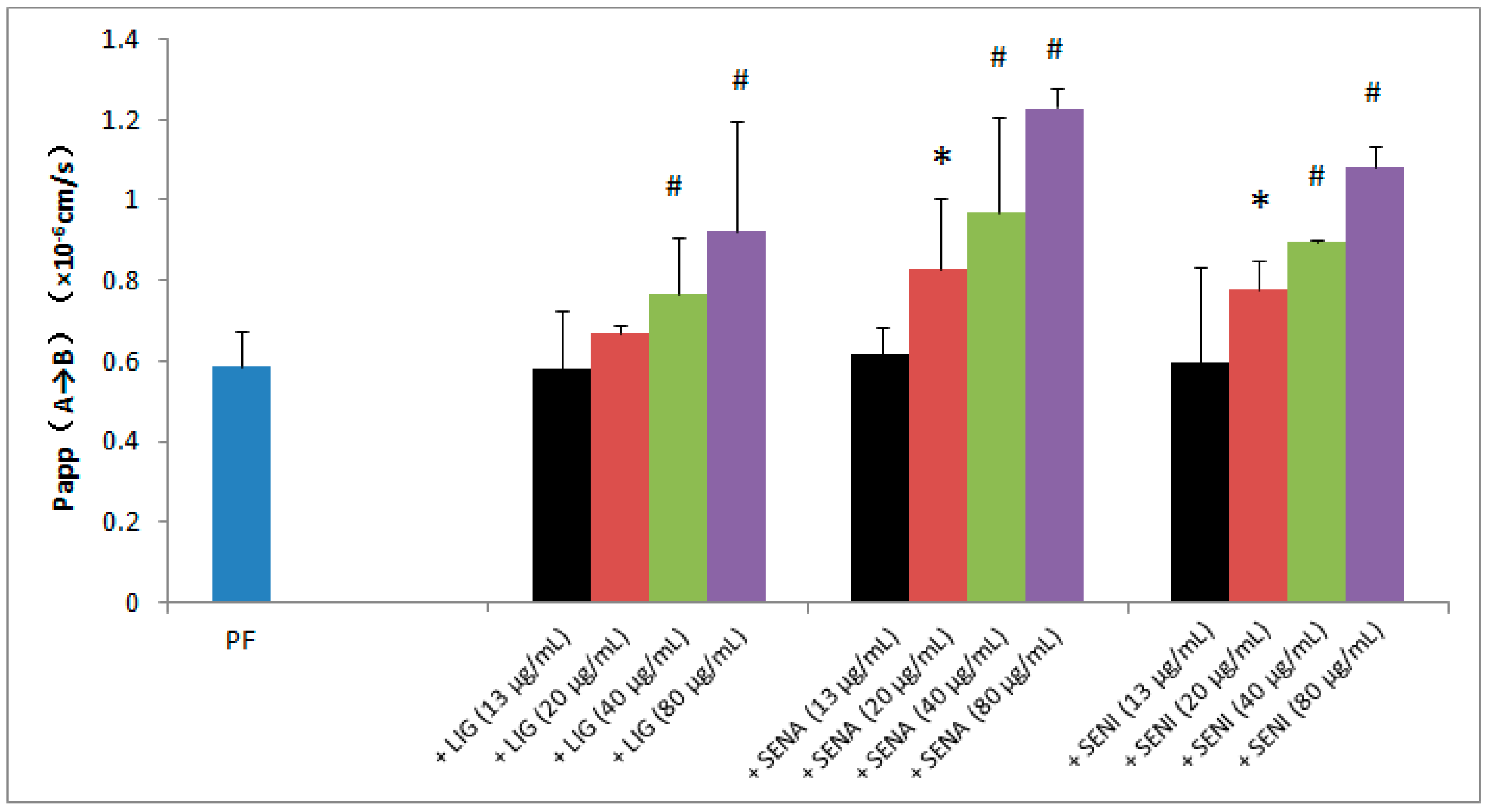
3.4. Transport Studies Across MDCK-MDR1
3.5. LC-MS/MS Measurement of PF.
3.6. Data Analysis and Statistics
3.7. TEER Measurement
3.8. The Effects of LIG, SENI and SENA on Rhodamine 123 Accumulation
3.9. Western-Blot Analysis
3.10. Molecular Docking
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ji, Q.; Yang, L.; Zhou, J.; Lin, R.; Zhang, J.; Lin, Q.; Wang, W.; Zhang, K. Protective effects of paeoniflorin against cobalt chloride induced apoptosis of endothelial cells via HIF-1alpha pathway. Toxicol. Vitro 2012, 26, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Bian, D.; Jiao, X.; Wei, Z.; Zhang, H.; Xia, Y.; He, Y.; Dai, Y. Paeoniflorin protects against lipopolysaccharide-induced acute lung injury in mice by alleviating inflammatory cell infiltration and microvascular permeability. Inflamm. Res. 2011, 60, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Y.; Xu, L.; Yan, R.; Huang, Y.; Liu, G.; Zhou, W.X.; Zhang, Y.X. Advance in studies on effect of paeoniflorin on nervous system. China J. Chin. Mater. Med. 2013, 38, 297–301. [Google Scholar]
- Zhong, S.Z.; Ge, Q.H.; Li, Q.; Qu, R.; Ma, S.P. Peoniflorin attenuates Aβ (1-42)-mediated neurotoxicity by regulating calcium homeostasis and ameliorating oxidative stress in hippocampus of rats. J. Neurol. Sci. 2009, 280, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Z.; Zhu, J.; Jin, D.Z.; Zhang, L.M.; Ji, X.Q. Behavioral recovery following subchronic paeoniflorin administration in the striatal6-OHDA lesion rodent model of Parkinson’s disease. J. Ethnopharmacol. 2007, 112, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W.; Guo, J.; Xu, W. Absorption and transport characteristic of paeoniflorin and its derivatives in model of Caco-2 cell monolayers. Chin. Tradit. Herbal Drugs 2013, 45, 2097–2104. [Google Scholar]
- Wu, J.; Yao, N.; Wang, D.W. Determination of paeoniflorin in rat plasma by HPLC-MS/MS and its pharmacokinetics. China J. Chin. Mater. Med. 2008, 33, 2369–2373. [Google Scholar]
- Lai, L.L.; Fan, H.J.; Zhang, Y.Y.; Yang, J.H.; Wang, H.T. Analysis of compatibility regularity of ligustrazine. Chin. Arch. Tradit. Chin. Med. 2015, 33, 2335–2337. [Google Scholar]
- Su, Z.T.; Xu, J.L.; Liu, Y.; Yang, S.; Yang, M. The study on promotion of absorption and synergistic effect of Chuanxiong Rhizoma with helicid. Chin. J. Exp. Tradit. Med. Formulae 2012, 18, 176–178. [Google Scholar]
- Wang, Q.; Shen, L.; Liang, S.; Ma, S.Y.; Feng, Y.; Ruan, K.F. Effects of Ligusticum chuanxiong on plasma and brain pharmacokinetics of active ingredients from Gastrodia elata in migraine rats. Chin. Tradit. Pat. Med. 2015, 37, 62–69. [Google Scholar]
- Zhen, Q.; Liu, J.Y.; Fu, S.L.; Liu, Y.J.; Qian, J.; Yang, M. Effects of chuanxiong rhizoma on brain pharmacokinetics of gastrodigenin in rats. Chin. J. New Drugs Clin. Rem. 2012, 31, 263–266. [Google Scholar]
- Wang, P.; Wang, C.; Lou, Y.C. Borneol and Rhizome of Chuanxiong on facilitating permeation for compound shuyu jiangnao decoction through blood brain barrier. J. Hubei Univ. Chin. Med. 2011, 13, 21–23. [Google Scholar]
- Ren, C.; Wang, B.; Li, N.; Jin, K.; Ji, X. Herbal formula Danggui-Shaoyao-San promotes neurogenesis and angiogenesis in rat following middle cerebral artery occlusion. Aging Dis. 2015, 6, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Hu, Z.Y.; Wang, T.; Yang, S.; Zhou, W.X.; Zhang, Y.X. Preliminary study of Danggui Shaoyao San active ingredient paeoniflorinresisting nerve inflammatory reaction. Chin. J. Pharmacol. Toxicol. 2011, 25, 94. [Google Scholar]
- Mu, Q.; Liu, P.; Hu, X.; Gao, H.; Zheng, X.; Huang, H. Neuroprotective effects of Buyang Huanwu decoction on cerebral ischemia-induced neuronal damage. Neural Regen. Res. 2014, 9, 1621–1627. [Google Scholar] [PubMed]
- Li, Q.Y.; Gan, G.P.; Liu, Y.W. The chemical composition and pharmacological research progress of rhizoma Ligusticum chuanxiong. Lishizhen Med. Mater. Med. Res. 2006, 17, 1298–1299. [Google Scholar]
- Wang, H.J.; Zhang, L.C.; Zhang, Y.J.; Gu, W.Y.; Wu, Z.H.; Hu, J.H. Mechanism of enhancing effect of essential oils from Ligusticum chuanxiong Hort. through rabbit skin by increased skin blood flow. Chin. Pharm. J. 2010, 45, 1925–1929. [Google Scholar]
- Sheng, Y.M.; Meng, X.L.; Chun, Y.; Wang, Z.; Zhang, Y. Effects of aetherolea from chuanxiong on ischemical reperfusion injury in rats and the survival of cerebral cortex neurons in vitro. Lishizhen Med. Mater. Med. Res. 2012, 23, 536–538. [Google Scholar]
- Wang, J.; Du, J.R.; Wang, Y.; Wan, F.C. Z-ligustilide attenuates lipopolysaccharide-induced proinfammatory response via inhibiting TNF-κB pathway in primary rat microglia. Acta Pharmacol. Sin. 2010, 31, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.K.; Cheng, T.Y.; Lin, G. Relaxation effects of ligustilide and senkyunolide A, two main constituents of Ligusticum chuanxiong, in rat isolated aorta. J. Ethnopharmacol. 2007, 111, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Liang, S.; Xu, D.S.; Lin, X.; He, C.Y.; Feng, Y.; Hong, Y.L. Effect and mechanism of senkyunolideI as an anti-migraine compound from Liguchuanxiong. J. Pharm. Pharmacol. 2011, 63, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Zuo, A.H.; Cheng, M.C.; Wang, L.; Xiao, H.B. Analysis of chemical constituents of Chuanxiong Rhizoma absorbed into rat brain tissues by UPLC-Q-TOF-MS. China J. Chin. Mater. Med. 2012, 37, 3647–3650. [Google Scholar]
- Ueda, K. ABC proteins protect the human body and maintain optimal health. Biosci. Biotechnol. Biochem. 2011, 75, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Shityakov, S.; Forster, C. In silico structure-based screening of versatile P-glycoprotein inhibitors using polynomial empirical scoring functions. Adv. Appl. Bioinform. Chem. 2014, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Rager, J.D.; Weinstein, K.; Kardos, P.S.; Dobson, G.L.; Li, J.; Hidalgo, I.J. Evaluation of the MDR-MDCK cell line as a permeability screen for the blood–brain barrier. Int. J. Pharm. 2005, 288, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Navarroa, C.; Gonzalez-Alvarez, I.; Gonzalez-Alvarez, M. Influence of polyunsaturated fatty acids on cortisol transport through MDCK and MDCK-MDR1 cells as blood–brain barrier in vitro model. Eur. J. Pharm. Sci. 2011, 42, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Xu, C.; O’Neal, S.; Bi, H.C.; Huang, M.; Zheng, W.; Zeng, S. Roles of P-glycoprotein andmultidrugresistance protein in transporting para-aminosalicylic acid and its N-acetylated metabolite in mice brain. Acta Pharmacol. Sin. 2014, 35, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Hellinger, E.; Veszelka, S.; Tóth, A.E.; Walter, F.; Kittel, A.; Bakk, M.L.; Tihanyi, K.; Háda, V.; Nakagawa, S.; Duy, T.D.; et al. Comparison of brain capillary endothelial cell-based and epithelial (MDCK-MDR1, Caco-2, and VB-Caco-2) cell-based surrogate blood–brain barrier penetration models. Eur. J. Pharm. Biopharm. 2012, 82, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jin, H.E.; Kim, W.; Han, Y.H.; Kim, D.D.; Chung, S.J.; Shim, C.K. Involvementof P-glycoprotein, multidrug resistance protein 2 and breast cancer resistanceprotein in the transport of belotecan and topotecan in Caco-2 and MDCKII cells. Pharm. Res. 2008, 25, 2601–2612. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.Y.; Xin, R.; Tan, X.B. Impact of saikosaponin on absorption and transport of paeoniflorin in Caco-2 cell model. China J. Chin. Mater. Med. 2012, 37, 1850–1854. [Google Scholar]
- Liang, X.L.; Zhao, L.J.; Liao, Z.G.; Zhao, G.W.; Zhang, J.; Chao, Y.C.; Yang, M.; Yin, R.L. Transport properties of puerarin and effect of Radix Angelicae Dahuricae extract on the transport of puerarin in Caco-2 cell model. J. Ethnopharmacol. 2012, 144, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, S.; Stavchansky, S. Effects of citicholine and dimethylsulfoxide on transepithelialtransport of passively diffused drugs in the Caco-2 cell culturemodel. Int. J. Pharm. 2003, 251, 107–112. [Google Scholar] [CrossRef]
- Fan, X.; Chai, L.J.; Zhang, H.; Wang, Y.F.; Zhang, B.L.; Gao, X.M. Borneol depresses P-glycoprotein function by a NF-κB signaling mediated mechanism in a Blood Brain Barrier in vitro model. Int. J. Mol. Sci. 2015, 16, 27576–27588. [Google Scholar] [CrossRef] [PubMed]
- Sarvagalla, S.; Singh, V.K.; Ke, Y.Y.; Shiao, H.Y.; Lin, W.H.; Hsu, T.A.; Coumar, M.S. Identification of ligand efficient, fragment-like hits from an HTS library: Structure-based virtual screening and docking investigations of 2H- and 3H-pyrazolo tautomers for Aurora kinase A selectivity. J. Comput. Aided Mol. Des. 2015, 29, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Usha, T.; Middha, S.K.; Goyal, A.K.; Karthik, M.; Manoj, D.; Faizan, S.; Goyal, P.; Prashanth, H.; Pandeb, V. Molecular docking studies of anti-cancerous candidates in Hippophae rhamnoides and Hippophae salicifolia. J. Biomed. Res. 2014, 28, 406–415. [Google Scholar] [PubMed]
- Shityakov, S.; Neuhaus, W.; Dandekar, T.; Forster, C. Analysing molecular polar surface descriptors to predict blood–brain barrier permeation. Int. J. Comput. Biol. Drug Des. 2013, 6, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Portnoy, E.; Gurina, M.; Magdassi, S.; Eyal, S. Evaluation of the near infrared compound Indocyanine Green as a probe substrate of P-glycoprotein. Mol. Pharm. 2012, 12, 3595–3601. [Google Scholar] [CrossRef] [PubMed]
- Vadlapudi, A.D.; Vadlapatla, R.K.; Kwatra, D.; Earla, R.; Samanta, S.K.; Pal, D.; Mitra, A.K. Targeted lipid based drug conjugates: A novel strategy for drug delivery. Int. J. Pharm. 2012, 434, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.L.; Zhao, L.J.; Liao, Z.G.; Yin, R.L.; Zhao, G.W.; Cao, Y.C. Effects of radix angelicae dahuricae extract on p-glycoprotein activity in Caco-2 cells. Chin. Hosp. Pharm. J. 2012, 32, 1781–1785. [Google Scholar]
- The Molecular Structure of the Ligands was Found from NCBI. Available online: http://www.ncbi.nlm.nih.gov/pubmed (accessed on 31 July 2015).
- Mei, M.; Zhang, Y.; Ren, J.H.; Xie, D.; Jia, Y.F.; Hu, J.P.; Li, Y.; Dai, J.G.; Chen, X.G. Resistance reversal effect of a novel taxane compound NPB304 and its collaboration with verapamil. Acta Pharm. Sin. 2014, 49, 1279–1288. [Google Scholar]
- Aller, S.G.; Yu, J.; Ward, A.; Weng, Y.; Chittaboina, S.; Zhuo, R.P.; Harrell, P.M.; Trinh, Y.T.; Zhang, Q.H.; Urbatsch, I.L.; et al. Structure of p-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 2009, 323, 1718–1722. [Google Scholar] [CrossRef] [PubMed]
- Shityakov, S.; Forster, C. Multidrug resistance protein P-gp interaction with nanoparticles (fullerenes and carbon nanotube) to assess their drug delivery potential: A theoretical molecular docking study. Int. J. Comput. Biol. Drug Des. 2013, 6, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Shityakov, S.; Forster, C. In silico predictive model to determine vector-mediated transport properties for the blood–brain barrier choline transporter. Adv. Appl. Bioinform. Chem. 2014, 7, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not Available.
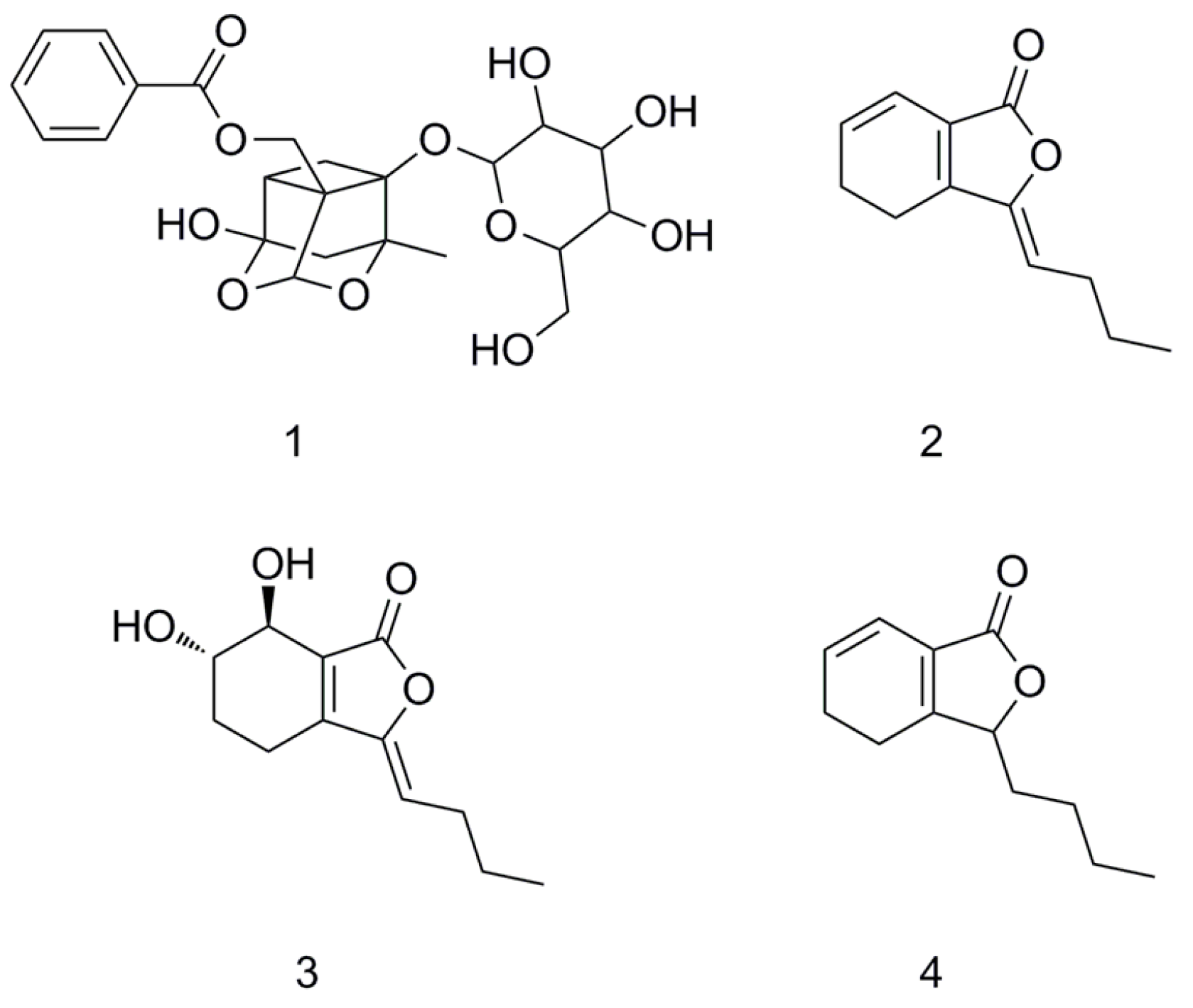
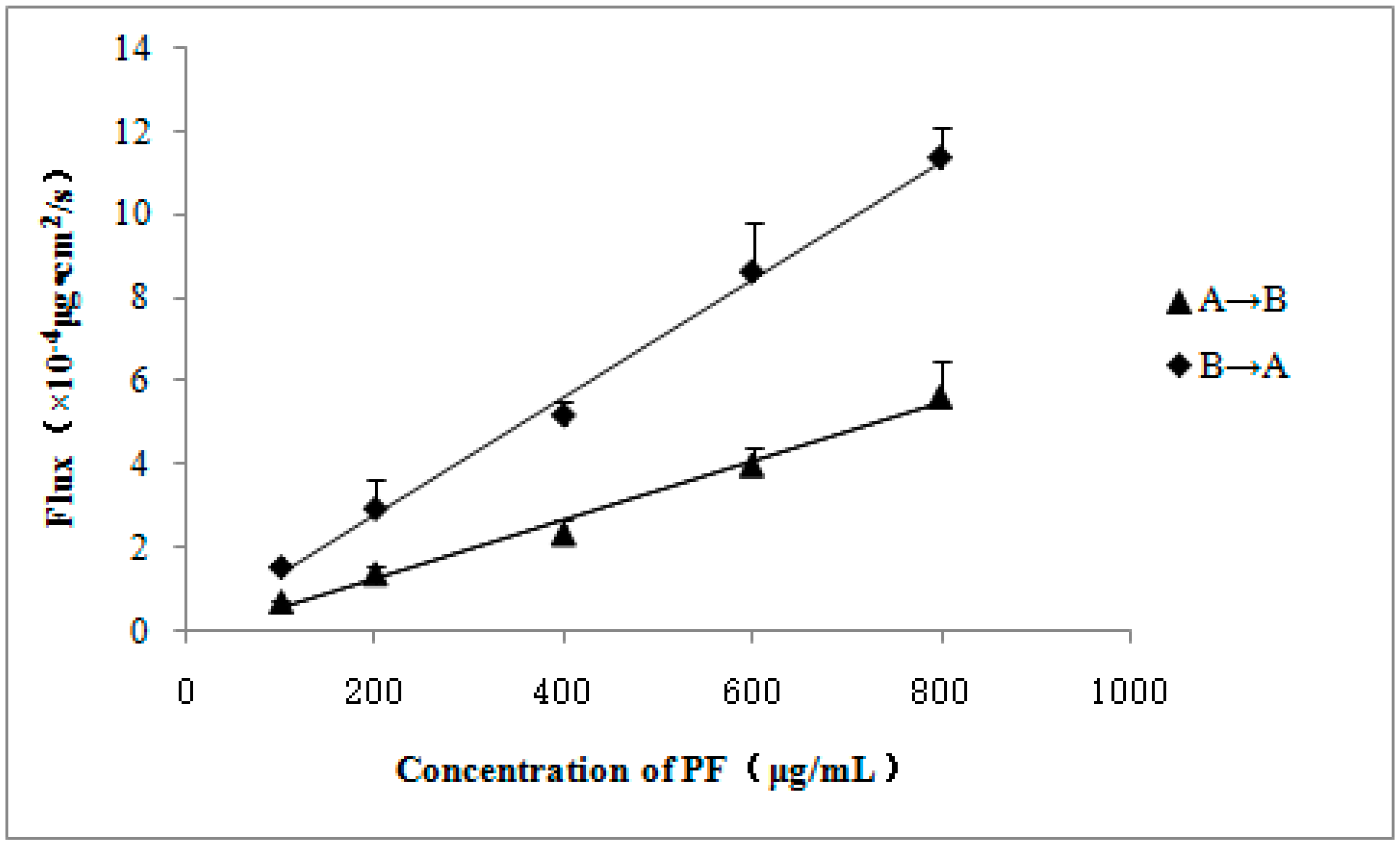
| Concentration of PF (μg/mL) | Papp (A→B) (×10−6 cm/s) | Papp (B→A) (×10−6 cm/s) | ER (B→A/A→B) |
|---|---|---|---|
| 100 | 0.697 ± 0.053 | 1.549 ± 0.092 | 2.222 |
| 200 | 0.691 ± 0.082 | 1.475 ± 0.363 | 2.134 |
| 400 | 0.587 ± 0.088 | 1.297 ± 0.084 | 2.210 |
| 600 | 0.666 ± 0.072 | 1.436 ± 0.201 | 2.156 |
| 800 | 0.705 ± 0.107 | 1.418 ± 0.097 | 2.011 |
| Group | Papp (A→B) (×10−6 cm/s) | Papp (B→A) (×10−6 cm/s) | ER (B→A/A→B) |
|---|---|---|---|
| 400 μg/mL PF | 0.587 ± 0.088 | 1.297 ± 0.084 | 2.210 |
| 400 μg/mL PF + 100 mmol/L verapamil | 0.707 ± 0.062 * | 0.488 ± 0.093 # | 0.690 |
| Group | Papp (A→B) (×10−6 cm/s) |
|---|---|
| 400 μg/mL PF | 0.587 ± 0.088 |
| 400 μg/mL PF + 2.5 mmol/LEDTA-Na2 | 2.736 ± 0.212 # |
| NO | English Name | The Type of Interaction | The Key Amino Acids | LibDock Score |
|---|---|---|---|---|
| 1 | PF | hydrogen-bonding interaction | gln721 and ser 725 | 139 |
| hydrophobic interaction | val978, leu971, and phe724 | |||
| 2 | LIG | hydrogen-bonding interaction | ser 975 | 86 |
| hydrophobic interaction | phe974, phe332, phe724, val978, and phe728 | |||
| 3 | SENI | hydrogen-bonding interaction | ser725 | 91 |
| hydrophobic interaction | leu971, phe728, phe332, phe724, and val978 | |||
| 4 | SENA | hydrogen-bonding interaction | ser729 | 89 |
| hydrophobic interaction | phe71, phe974, phe332, phe728, val978 | |||
| 5 | Verapamil | hydrogen-bonding interaction | gln721 | 138 |
| hydrophobic interaction | tyr303, tyr306, leu335, phe332, phe724, met 982, val978, and phe728 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, P.-Y.; Liu, D.; Zheng, Q.; Wu, Q.; Tang, Y.; Yang, M. Elucidation of Transport Mechanism of Paeoniflorin and the Influence of Ligustilide, Senkyunolide I and Senkyunolide A on Paeoniflorin Transport through Mdck-Mdr1 Cells as Blood–Brain Barrier in Vitro Model. Molecules 2016, 21, 300. https://doi.org/10.3390/molecules21030300
Hu P-Y, Liu D, Zheng Q, Wu Q, Tang Y, Yang M. Elucidation of Transport Mechanism of Paeoniflorin and the Influence of Ligustilide, Senkyunolide I and Senkyunolide A on Paeoniflorin Transport through Mdck-Mdr1 Cells as Blood–Brain Barrier in Vitro Model. Molecules. 2016; 21(3):300. https://doi.org/10.3390/molecules21030300
Chicago/Turabian StyleHu, Peng-Yi, Dan Liu, Qin Zheng, Qing Wu, Yu Tang, and Ming Yang. 2016. "Elucidation of Transport Mechanism of Paeoniflorin and the Influence of Ligustilide, Senkyunolide I and Senkyunolide A on Paeoniflorin Transport through Mdck-Mdr1 Cells as Blood–Brain Barrier in Vitro Model" Molecules 21, no. 3: 300. https://doi.org/10.3390/molecules21030300





