Constituents of Saffron (Crocus sativus L.) as Potential Candidates for the Treatment of Anxiety Disorders and Schizophrenia
Abstract
:1. Introduction
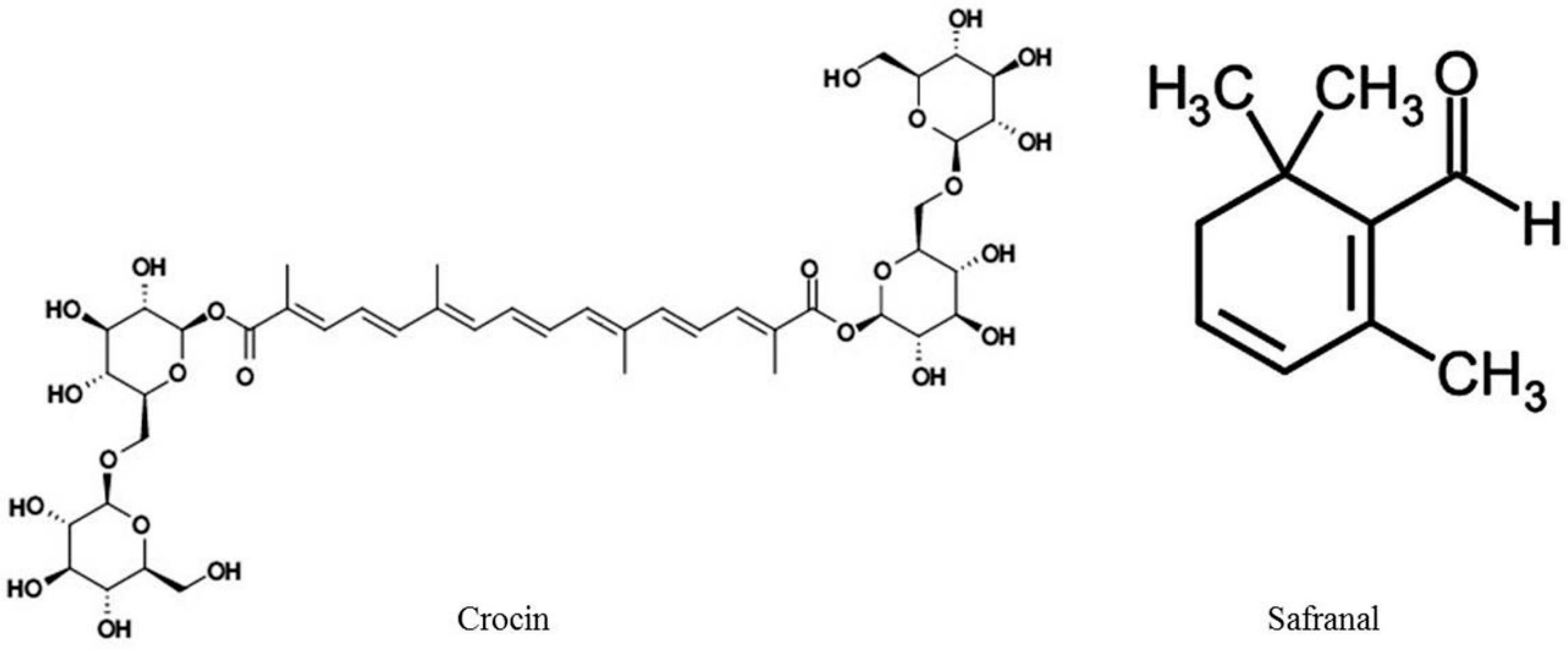
2. Chemistry of C. sativus
3. Effects of C. sativus and Its Constituents on Anxiety
3.1. Preclinical Studies
3.2. Mechanism of Action of C. sativus and Its Constituents in Anxiety Disorders
4. Effects of C. sativus and Its Constituents in Schizophrenia
4.1. Preclinical Studies
4.2. Clinical Studies
4.3. Mechanism of Action of C. sativus and Its Constituents in Schizophrenia
5. Effects of C. sativus and Its Constituents on Other Neurological/Neuropsychiatric Diseases
5.1. Anticonvulsant Activity, Neurodegeneration
5.2. Memory
5.3. Depression
6. Effects of C. sativus and Its Constituents on Other Non-Neurological/Neuropsychiatric Diseases
7. Safety Evaluation of C. sativus and Its Constituents
8. Conclusions
Conflicts of Interest
References
- International Standard. Saffron-Specification ISO 3632:1980; International Organization for Standardization: Geneva, Switzerland, 1980.
- Rios, J.L.; Recio, M.C.; Ginger, R.M.; Manz, S. An update review of saffron and its active constituents. Phytother. Res. 1996, 10, 189–193. [Google Scholar] [CrossRef]
- Akhondzabeh, S. Herbal medicine in the treatment of psychiatric and neurological disorders. In Low-Cost Approaches to Promote Physical and Mental Health: Theory, Research and Practice; Abate, L., Ed.; Springer: New York, NY, USA, 2007; pp. 119–138. [Google Scholar]
- Winterhalter, P.; Straubinger, M. Saffron-renewed interest in an ancient spice. Food Rev. Int. 2000, 16, 39–59. [Google Scholar] [CrossRef]
- Liakopoulou-Kyriakides, M.; Kyriakidis, D. Crocus sativus-biological active constituents. Stud. Nat. Prod. Chem. 2002, 16, 293–312. [Google Scholar]
- Kanakis, C.D.; Daferera, D.J.; Tarantilis, P.A.; Polissiou, M.G. Qualitative determination of volatile compounds and quantitative evaluation of safranal and 4-hydroxy-2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde. J. Agric. Food Chem. 2004, 52, 4515–4521. [Google Scholar] [CrossRef] [PubMed]
- Tarantilis, P.A.; Tsoupras, G.; Polissiou, M. Determination of saffron (Crocus sativus L.) components in crude plant extract using high-performance liquid chromatography-UV/Visible photodiode-array detection-mass spectrometry. J. Chromatogr. 1995, 699, 107–118. [Google Scholar] [CrossRef]
- Hammer, M.B.; Robert, S.; Fruech, B.S. Treatment-resistant posttraumatic stress disorder: Strategies for intervention. CNS Spectr. 2004, 9, 740–752. [Google Scholar]
- Van Ameringen, M.; Mancini, C.; Pipe, B.; Bennett, M. Optimizing treatment in social phobia: A review of treatment resistance. CNS Spectr. 2004, 9, 753–762. [Google Scholar] [PubMed]
- Cryan, J.F.; Sweeney, F.F. The age of anxiety: Role of animal models of anxiolytic action in drug discovery. Br. J. Pharmacol. 2011, 164, 1129–1161. [Google Scholar] [CrossRef] [PubMed]
- Gorman, J.M. New molecule targets for antianxiety interventions. J. Clin. Psychiatry 2003, 64, 28–35. [Google Scholar] [PubMed]
- Pitsikas, N.; Boultadakis, A.; Georgiadou, G.; Tarantilis, P.A.; Sakellaridis, N. Effects of the active constituents of Crocus sativus L.; crocins, in an animal model of anxiety. Phytomedicine 2008, 15, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Georgiadou, G.; Tarantilis, P.A.; Pitsikas, N. Effects of the active constituents of Crocus sativus L.; crocins in an animal model of obsessive-compulsive disorder. Neurosci. Lett. 2012, 528, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Noraei, N.B. Anxiolytic and hypnotic effect of Crocus sativus aqueous extract and its constituent, crocins and safranal in mice. Phytother. Res. 2009, 23, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Halatei, B.S.; Khosravi, M.; Sahrei, H.; Golmanesch, L.; Zardooz, H.; Jalili, C.; Ghoshoomi, H. Saffron (Crocus sativus) acueous extract and its constituent crocin reduces stress-induced anorexia in mice. Phytother. Res. 2011, 25, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- Georgiadou, G.; Grivas, V.; Tarantilis, P.A.; Pitsikas, N. Crocins the active constituents of Crocus sativus L.; counteracted ketamine-induced behavioural deficits in rats. Psychopharmacology 2014, 231, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Dekermendjian, K.; Wang, X.; Nielsen, M.; Witt, M.R. 6-Methylflavone, a benzodiazepine receptor ligand with antagonistic properties on rat brain and human recombinant GABAA receptors in vitro. Drug Dev. Res. 1997, 41, 99–106. [Google Scholar] [CrossRef]
- Marder, M.; Estiu, G.; Blanch, L.B.; Viola, H.; Wasowski, C.; Medina, J.H.; Paladini, A.C. Molecular modelling and QSAR analysis of the interaction of flavone derivatives with the benzodiazepine binding site of the GABAA receptor complex. Bioorg. Med. Chem. 2001, 9, 323–335. [Google Scholar] [CrossRef]
- Miller, D.B.; O’Callaghan, J.P. Neuroendocrine aspects of the response to stress. Metabolism 2002, 51, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Lechtenberg, M.; Schepmann, M.; Niehues, M.; Hellenbrand, N.; Wunsch, B.; Hensel, A. Quality and functionality of saffron: Quality control species assortment and affinity of extract and isolated saffron compounds to NMDA and σ1 (sigma-1) receptors. Planta Med. 2008, 74, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, S.; Mick, S.; Dilworth, V.; Michel, J.; Rao, T.S.; Farah, J.M.; Wood, P.L. Sigma receptors modulate the hypothalamic-pituitary-adrenal (HPA) axis centrally: Evidence for a functional interaction with NMDA receptors in vivo. Neuropharmacology 1990, 29, 299–303. [Google Scholar] [CrossRef]
- Freedman, R. Schizophrenia. N. Engl. J. Med. 2003, 349, 1738–1749. [Google Scholar] [PubMed]
- Steeds, H.; Carhart-Harris, R.L.; Stone, J.M. Drug models of schizophrenia. Ther. Adv. Psychopharmacol. 2015, 5, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Javitt, D.C. Glutamate and schizophrenia: Phencyclidine, N-methyl-d-aspartate receptors, and dopamine-glutamate interactions. Int. Rev. Neurobiol. 2007, 78, 69–108. [Google Scholar] [PubMed]
- Pratt, J.; Winchester, C.; Dawson, N.; Morris, B. Advancing schizophrenia drug discovery: Optimizing rodent models to bridge the translational gap. Nat. Rev. Drug Discov. 2012, 11, 560–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, D.; Pierri, J.; Volk, D.; Melchitzky, D.; Woo, T. Altered GABA neurotransmission and prefrontal cortical dysfunction in schizophrenia. Biol. Psychiatry 1999, 46, 616–626. [Google Scholar] [CrossRef]
- Field, J.R.; Walker, A.G.; Conn, P.J. Targeting glutamate synapses in schizophrenia. Trends Mol. Med. 2011, 17, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Akhondzadeh, S.; Shafiee-Sabet, M.; Harirchian, M.H.; Togha, M.; Cheraghmakani, H.; Razeghi, S.; Hejazi, S.S.; Yousefi, M.H.; Alimardani, R.; Jamshidi, A.; et al. Saffron in the treatment of patients with mild to moderate Alzheimer’s disease: A 16-week, randomized and placebo-controlled study. J. Clin. Pharm. Ther. 2010, 35, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Akhondzadeh, S.; Shafiee-Sabet, M.; Harirchian, M.H.; Togha, M.; Cheraghmakani, H.; Razeghi, S.; Hejazi, S.S.; Yousefi, M.H.; Alimardani, R.; Jamshidi, A.; et al. A 22-week, multicentre randomized, double-blind controlled trial of Crocus sativus L.; in the treatment of mild-to-moderate Alzheimer’s disease. Psychopharmacology 2010, 207, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Farokhnia, M.; Shafiee-Sabet, M.; Iranpour, N.; Gougol, A.; Yekehtaz, H.; Alimardani, R.; Farsad, F.; Akhondzadeh, S. Comparing the efficacy and safety of Crocus sativus L. with memantine in patients with moderate to severe Alzheimer’s disease: A double-blind randomized clinical trial. Hum. Psychopharmacol. Clin. Exp. 2014, 29, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, B.; Bathaie, S.Z.; Fadai, F.; Ashtari, Z.; Ali beigi, N.; Farhang, S.; Hashempour, S.; Shahhamzei, N.; Heidarzadeh, H. Safety evaluation of saffron stigma (Crocus sativus L.) aqueous extract and crocin in patients with schizophrenia. Avicenna J. Phytomed. 2015, 5, 413–419. [Google Scholar] [PubMed]
- Moghaddam, B.; Adams, B.; Verma, A.; Daly, D. Activation of glutamatergic transmission by ketamine: A novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J. Neurosci. 1997, 17, 2921–2927. [Google Scholar] [PubMed]
- Hosseinzadeh, H.; Sadeghnia, H.R.; Rahimi, A. Effects of safranal on extracellular hippocampal levels of glutamate and aspartate during kainic acid treatment in anesthetized rats. Planta Med. 2008, 74, 1441–1445. [Google Scholar] [CrossRef] [PubMed]
- Berger, F.; Hensel, A.; Nieber, K. Saffron extracts and trans-crocetin inhibit glutamatergic synaptic transmission in rat cortical brain slices. Neuroscience 2011, 180, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Naghizadeh, B.; Mansouri, M.T.; Ghorbanzadeh, B.; Farbood, Y.; Sarkaki, A. Protective effects of oral crocin against intracerebroventricular streptozotocin-induced spatial memory deficit and oxidative stress in rats. Phytomedicine 2013, 20, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, T.; Soeda, S.; Ohno, S.; Tanaka, H.; Shoyama, Y.; Shimeno, H. Crocin prevent the death of PC-12 cells through sphingomyelinase-ceramide signaling by increasing glutathione synthesis. Neurochem. Int. 2004, 44, 321–330. [Google Scholar] [CrossRef]
- Papandreou, M.A.; Tsachaki, M.; Efthimiopoulos, S.; Cordopatis, P.; Lamari, F.N.; Margarity, M. Memory enhancing effects of saffron in aged mice are correlated with antioxidant protection. Behav. Brain Res. 2011, 219, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Q.; Liu, J.X.; Wang, J.N.; Xu, L. Effects of crocin on reperfusion induced oxidative/nitrative injury to cerebral microvessels after global cerebral ischemia. Brain Res. 2007, 1138, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Ghadrdoost, B.; Vafaei, A.; Rashidy-Pour, A.; Hajisoltani, R.; Bandegi, A.R.; Motamedi, F.; Haghighi, S.; Sameni, H.R.; Pahlvan, S. Protective effect of saffron extracts and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur. J. Pharmacol. 2011, 667, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Bitanihirwe, B.K.; Woo, T.U. Oxidative stress in schizophrenia: An integrated approach. Neurosci. Biobehav. Rev. 2011, 35, 878–893. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.; Spiazzi, C.M.; Bortolin, T.; Canever, L.; Petronilho, F.; Mina, F.G.; Dal-Pizzol, F.; Quevedoa, J.; Zugno, A.I. Different sub-anesthetic doses of ketamine increase oxidative stress in brain of rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Khosravan, V. Anticonvulsant effects of aqueous and ethanolic extracts of Crocus sativus L.; stigmas in mice. Arch. Iran Med. 2005, 5, 44–47. [Google Scholar]
- Hosseinzadeh, H.; Sadeghnia, H.R. Protective effect of safranal on pentylenetetrazol-induced seizures in the rat: Involvement of the GABAergic and opioids systems. Phytomedicine 2007, 14, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.S.; Ansari, M.A.; Ahmad, M.; Saleem, S.; Yousuf, S.; Hoda, M.N.; Islam, F. Neuroprotection by crocetin in a hemi-parkinsonian rat model. Pharmacol. Biochem. Behav. 2005, 81, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Sadeghnia, H.R. Safranal a constituent of Crocus sativus (saffron) attenuated cerebral ischemia-induced oxidative damage in rat hippocampus. J. Pharm. Pharm. Sci. 2005, 8, 394–399. [Google Scholar] [PubMed]
- Hosseinzadeh, H.; Sadeghnia, H.R.; Ghaeni, F.A.; Motamedshariaty, V.S.; Mohajeri, S.A. Effects of saffron (Crocus sativus L.) and its active constituent crocin, on recognition and spatial memory after chronic cerebral hypofunction in rats. Phytother. Res. 2012, 26, 381–386. [Google Scholar] [PubMed]
- Saleem, S.; Ahmad, M.; Ahmad, A.S.; Yousuf, F.; Ansari, M.A.; Khan, M.B.; Ishrat, T.; Islam, F. Effect of Saffron (Crocus sativus) on neurobehavioral and neurochemical changes in cerebral ischemia in rats. J. Med. Food 2006, 9, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Pitsikas, N. The effects of Crocus sativus L. and its constituents on memory: Basic studies and clinical applications. Evid. Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Karimi, G.; Niapoor, M. Antidepressant effects of Crocus sativus stigma extracts and its constituents, crocins and safranal in mice. J. Med. Plants 2004, 3, 48–58. [Google Scholar]
- Akhondzadeh, S.; Fallah-Pour, H.; Afkham, K.; Jamshidi, A.H.; Khalighi-Cigaroudi, F. Comparison of Crocus sativus L.; and imipramine in the treatment of mild to moderate depression: A pilot double-blind, randomized trial. BMC Complement. Altern. Med. 2004, 4, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Noorbala, A.A.; Akhondzadeh, S.; Tahmacebi-Pour, N.; Jamshidi, A.H. Hydro-alcoholic extract of Crocus sativus L.; versus fluoxetine in the treatment of mild to moderate depression: A double-blind, randomized trial. J. Ethnopharmacol. 2005, 97, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Kashani, L.; Raisi, F.; Saroukhani, S.; Sohrabi, H.; Modabbernia, A.; Nasehi, A.-A.; Jamshidi, A.; Ashrafi, M.; Mansouri, P.; Ghaeli, P.; et al. Saffron for treatment of fluoxetine-induced sexual dysfunction in women: Randomized double-blind placebo-controlled study. Hum. Psychopharmacol. 2012, 28, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Modabbernia, A.; Sohrabi, H.; Nasehi, A.A.; Raisi, F.; Saroukhani, S.; Jamshidi, A.; Tabrizi, M.; Ashrafi, M.; Akhondzadeh, S. Effect of saffron on fluoxetine-induced sexual impairment in men: Randomized double-blind placebo-controlled trial. Psychopharmacology 2013, 223, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.C.; Panikkar, B.; Panikkar, K.R. Antitumor activity of saffron. Cancer Lett. 1991, 57, 109–114. [Google Scholar] [CrossRef]
- Salomi, M.J.; Nair, S.C.; Panikkar, K.R. Inhibitory effects of nigella sativa and saffron on chemical carcinogenesis in mice. Nutr. Cancer 1991, 16, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Tarantilis, P.A.; Morjani, H.; Manfait, M.; Polissiou, M. Inhibition of growth and induction of differentiation of promyelocytic leukemia (HL-60) by carotenoids from Crocus sativus L. Anticancer Res. 1994, 14, 1913–1918. [Google Scholar] [PubMed]
- Hosseinzadeh, H.; Younesi, H.M. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002, 15, 2–7. [Google Scholar]
- Gainer, J.L.; Jones, J.R. The use of crocetin in experimental atherosclerosis. Experientia 1975, 31, 548–549. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Shiow, S.J.; Lin, J.K. Effects of crocetin on the hepatotoxicity and hepatic DNA binding of aflatoxin B1 in rats. Carcinogenesis 1991, 12, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.A.; Lee, J.H.; Baek, N.I.; Kim, D.H. Antihyperilipidemic effect of crocin isolated from the fructus of Gardenia jasminoides. J. Microbiol. Biotechnol. 2005, 16, 1084–1089. [Google Scholar]
- Du, P.; Qian, Z.; Shen, X.; Rao, S.; Wen, N. Effectiveness of crocin against myocardial injury. Chin. J. New Drugs 2005, 14, 1424–1427. [Google Scholar]
- Fatehi, M.; Rashidabady, T.; Fatehi-Hassanabad, Z. Effects of Crocus sativus petal’s extract on rat blood pressure and on responses induced by electrical field stimulation in the rat isolated vas deferens and guinea-pig-ileum. J. Ethnopharmacol. 2003, 84, 199–203. [Google Scholar] [CrossRef]
- Imenshahidi, M.; Hosseinzadeh, H.; Javadpour, Y. Hypotensive effect of aqueous saffron extract (Crocus sativus L.) and its constituents, safranal and crocin, in normotensive and hypertensive rats. Phytother. Res. 2010, 24, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Abdullaev, F.I.; Espinosa-Aguirre, J.J. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect. Prev. 2004, 28, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Alavizadeh, S.H.; Hosseinzadeh, H. Bioactivity assessment and toxicity of crocin: A comprehensive review. Food Chem. Toxicol. 2014, 64, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Bathaie, S.Z.; Mousavi, S.Z. New applications and mechanisms of action of saffron and its important ingredients. Crit. Rev. Food Sci. Nutr. 2010, 50, 761–786. [Google Scholar] [CrossRef] [PubMed]
- Abdullaev, F.I. Cancer chemoprotective and tumoricidial properties of saffron (Crocus sativus L.). Exp. Biol. Med. 2002, 227, 20–25. [Google Scholar]
- Hosseinzadeh, H.; Motamedshariaty, V.S.; Sameni, A.K.; Vahabzadeh, M. Acute and sub-acute toxicity of crocin, a constituent of Crocus sativus L.; (saffron), in mice and rats. Pharmacologyonline 2010, 2, 943–951. [Google Scholar]
- Modagheghi, M.H.; Shahabian, M.; Esmaeli, H.A.; Rajbai, O.; Hosseinzadeh, H. Safety evaluation of saffron (Crocus sativus) tablets in healthy volunteers. Phytomedicine 2008, 15, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Mohamadpour, A.H.; Ayati, Z.; Parizadeh, M.R.; Rajbai, O.; Hosseinzadeh, H. Safety evaluation of crocin (a constituent of saffron) tablets in healthy volunteers. Iran J. Basic Med. Sci. 2013, 16, 39–46. [Google Scholar] [PubMed]
| Species | Agent | Dose Range | Route | Behavioural Test | Effect | Ref. |
|---|---|---|---|---|---|---|
| Rat | Crocins | 15, 30, 50 mg/kg | i.p. acute | Light/dark | Anxiolytic-like (50 mg/kg) effect | [12] |
| Motor activity | No effect | |||||
| Mouse | CsAE | 56, 80, 320, 560 mg/kg | i.p. acute | Elevated plus maze | CsAE (56, 80 mg/kg), safranal (0.15, 0.35 mL/kg) anxiolytic effect. Crocin was ineffective. | [14] |
| Crocin | 50, 200, 600 mg/kg | i.p. acute | Open field | CsAE (dose-dependently), crocin (200–600 mg/kg), reduced motility, grooming, rearing, leaning. | ||
| Safranal | 0.05, 0.15, 0.35 mL/kg | i.p. acute | Rotarod | CsAE (dose-dependently), crocin (200–600 mg/kg), reduced motility, grooming, rearing, leaning. | ||
| Safranal (0.05, 0.15 mL/kg) reduced motility; (0.15, 0.35 mL/kg) increased grooming, leaning, rearing. | ||||||
| CsAE (dose-dependently) decreased motor coordination. Crocin and safranal were ineffective. | ||||||
| Mouse | CsAE | 1, 5, 10 mg/kg | i.p. acute | Food intake | CsAE and crocin decreased stress-induced anorexia. | [15] |
| Plasma corticosterone levels were not increased in CsAE and crocin-treated mice. | ||||||
| CsEE | 1, 5, 10 mg/kg | i.p. acute | CsEE and safranal were ineffective. | |||
| Crocin | 1, 5, 10 mg/kg | i.p. acute | ||||
| Safranal | 1, 5, 10 mg/kg | i.p. acute | ||||
| Rat | Crocins | 15, 30 mg/kg | i.p. acute | Measurement of grooming behaviour | Attenuated mCPP-induced excessive grooming (anxiolytic effect). | [13] |
| m-CPP | 0.6 mg/kg | i.p. acute. | Motor activity | No effect | ||
| Rat | Crocins | 15, 30, 50 mg/kg | i.p. acute | NORT | Crocins (15, 30 mg/kg) counteracted ketamine-induced recognition memory deficits. | [16] |
| Ketamine | 3 mg/kg (NORT) | i.p. acute | NORT | Crocins (15, 30 mg/kg) counteracted ketamine-induced recognition memory deficits. | ||
| SI | Crocins (50 mg/kg) attenuated ketamine-induced social isolation. | |||||
| Ketamine | 8 mg/kg (SI) | i.p. sub-chronic | SI | Crocins (50 mg/kg) attenuated ketamine-induced social isolation. | ||
| Motor activity, stereotypies, ataxia | Crocins (50 mg/kg) attenuated ketamine-induced hypermotility, stereotypies and ataxia. | |||||
| Ketamine | 25 mg/kg (motor activity) | i.p. acute | Motor activity, stereotypies, ataxia | Crocins (50 mg/kg) attenuated ketamine-induced hypermotility, stereotypies and ataxia. |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitsikas, N. Constituents of Saffron (Crocus sativus L.) as Potential Candidates for the Treatment of Anxiety Disorders and Schizophrenia. Molecules 2016, 21, 303. https://doi.org/10.3390/molecules21030303
Pitsikas N. Constituents of Saffron (Crocus sativus L.) as Potential Candidates for the Treatment of Anxiety Disorders and Schizophrenia. Molecules. 2016; 21(3):303. https://doi.org/10.3390/molecules21030303
Chicago/Turabian StylePitsikas, Nikolaos. 2016. "Constituents of Saffron (Crocus sativus L.) as Potential Candidates for the Treatment of Anxiety Disorders and Schizophrenia" Molecules 21, no. 3: 303. https://doi.org/10.3390/molecules21030303





