1. Introduction
Cyclodextrins (CDs), the cyclic oligomers of glucopyranose, are by far the most commonly used organic host compounds for the inclusion of organic guest molecules [
1,
2,
3]. The inclusion of guest molecules into cyclodextrin host cavities results in significant changes to the guest properties, including solubility [
4], spectroscopic properties [
5] and stability [
6]. These effects of cyclodextrin inclusion have been utilized in a wide variety of research and commercial applications [
1,
2,
3], including in chromatography [
7], trace analysis [
8], drug delivery [
9], guest stabilization [
6], food science [
6] and industrial applications [
10].
However, it remains challenging to predict the stoichiometry of cyclodextrin host:guest inclusion complexes. In a broad sense, consideration of the size of the guest will to some extent help to predict this stoichiometry, as guests that are small relative to the native cyclodextrin host cavity diameter will tend to form 1:2 CD:guest complexes [
11]; those that are similar in size to the CD cavity will form 1:1 complexes [
12]; and those that are significantly larger (or in particular significantly longer) than the host cavity will tend to form 2:1 cyclodextrin:guest complexes [
13,
14]. However, small changes in guest shape can have a significant effect on the observed stoichiometry. In addition, higher order complexes have also been reported, including 2:2 [
13] and even 2:3 [
14], and in the solid state, even higher order cyclodextrin complexes have been reported, involving 3 CD hosts [
15], and even 4:3 [
16] and 4:5 [
17] overall CD:guest stoichiometries! Moreover, in some cases, a mixture of complexes with different stoichiometries can be observed for a single host-guest pair [
13,
18,
19], resulting in non-integer average stoichiometries (e.g., 1.3:1) [
19]. In this paper, we report an example of a cyclodextrin:guest system in which varying the specific substituent on the guest, in this case 1,4-naphthoquinoline, results in a change in stoichiometry from 1:1 to 2:1. These results will be analyzed and discussed with the aim of furthering the current understanding of the host:guest inclusion phenomenon, and the prediction of host:guest stoichiometry, for cyclodextrin hosts.
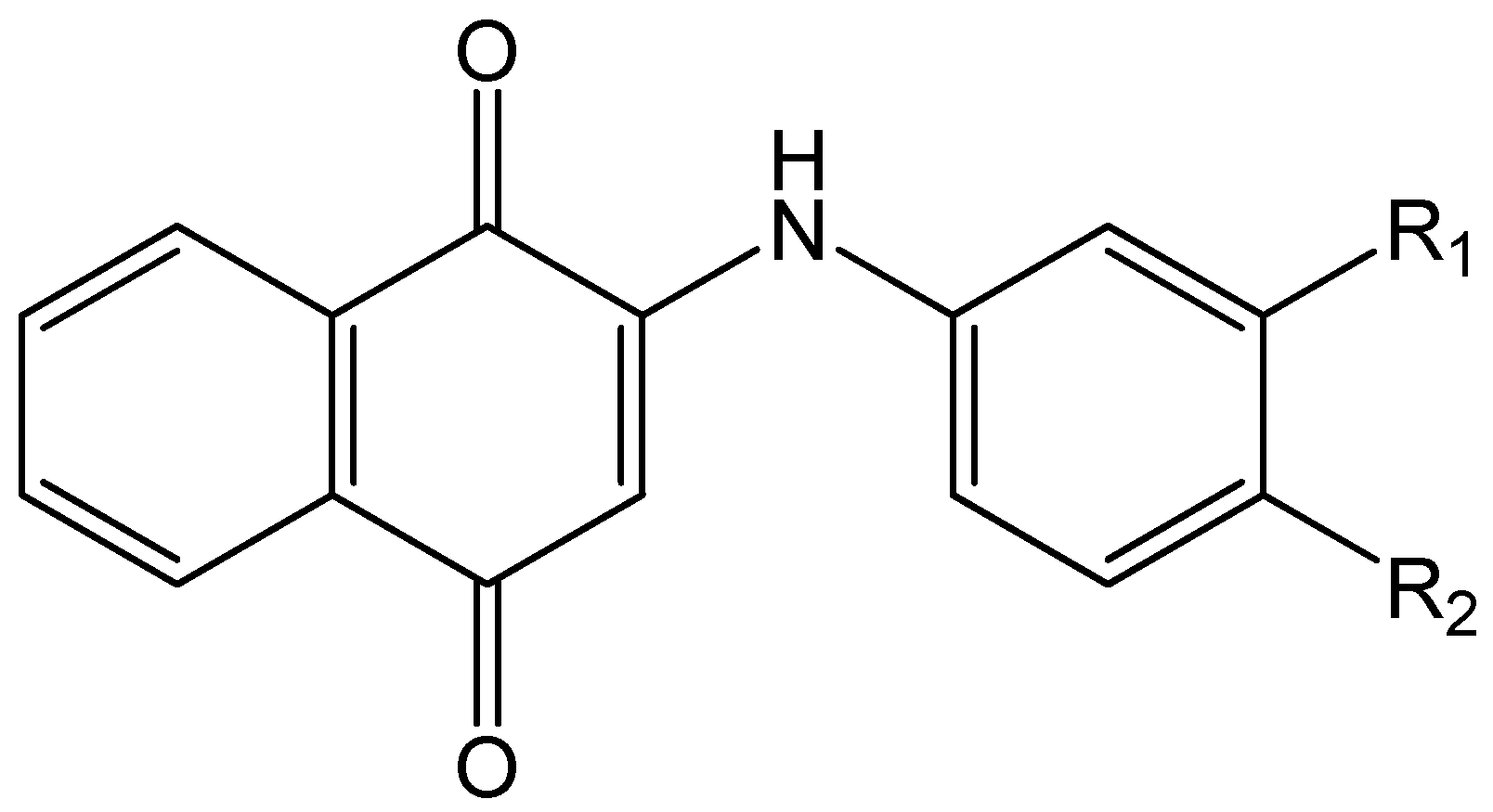
The host:guest inclusion complexes of 1,4-naphthoquinolines have been extensively studied because of their applications in medicine and biology [
20]. The
p-quinone system is quite popular as a part of many natural products [
18,
21] Furthermore, the electrochemistry of these and related systems has been well studied [
22,
23,
24,
25,
26]. For example, this system allows for electron transfer to occur in bacteria [
20,
22], mitochondria [
20,
23] and chloroplasts [
20,
24]. In addition, 1,4-naphthoquinolines have found many practical applications, including as fungicides [
27] and antimalarial agents [
28]. Recently, some newer 1,4-quinone structures have been prepared and studied by one of our co-authors and his co-workers, most interestingly those having an N-mono substituted amine group in position 2 of the quinone ring [
29,
30,
31]. These compounds, in particular the 2-[(R-phenyl)amine]-1,4-naphthoquinone (PAN) (R = substituent) (
1) and its eighteen derivatives were synthesised by a member of our group [
29,
30] and studied as dyes for their effects on various systems [
29,
30,
31]. These syntheses were designed in order to vary the size of the substituent on the benzene ring and to assure different electronic effects of these substituents (
Figure 1).
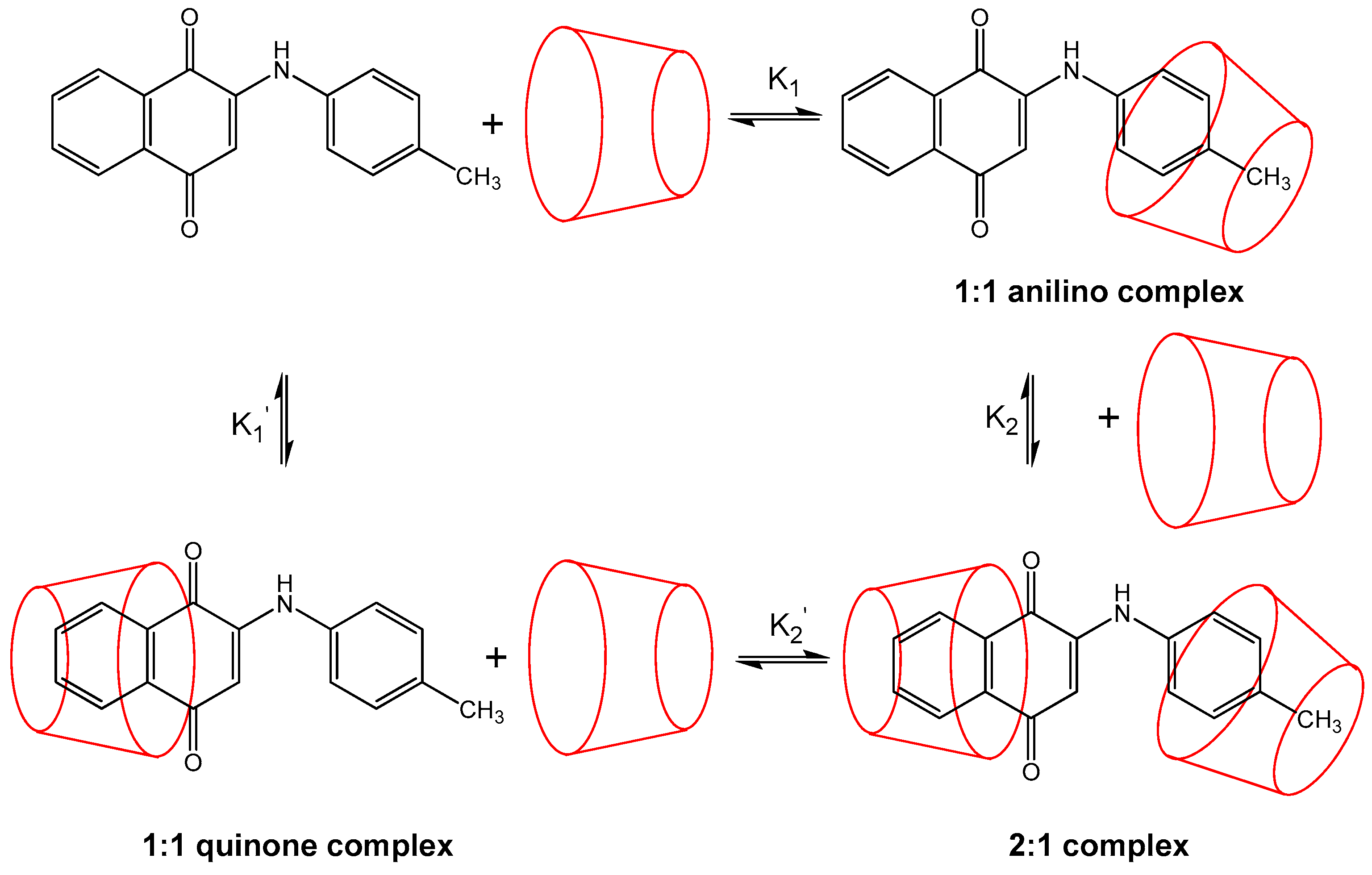
We decided to undertake further study of this family of compounds using a new approach, namely supramolecular host-guest inclusion, whereby the PAN derivatives will be the guest molecules and enter the internal cavity of cyclodextrin (CD) hosts, in particular β- and γ-CD (α-CD was not used due to its small cavity size relative to PAN). The transport of PAN encapsulated in the CD hosts in biological systems is of significant interest. In their recent studies Gomez-Sandoval [
29], Wen [
32] and Kobetic [
33] studied the host-guest inclusion and the stoichiometry of the resulting complexes of various biologically active compounds. Interestingly, it was found that such complexes often involved a 2:1 host:guest ratio, for example with the antiviral bioactive agent acyclovir [
34]. This observation was subsequently related to central issues in molecular recognition and inclusion phenomenon, namely the size-fit and geometric complementarity factors involved in determining the inclusion complex stoichiometry for a specific host-guest pair [
35].
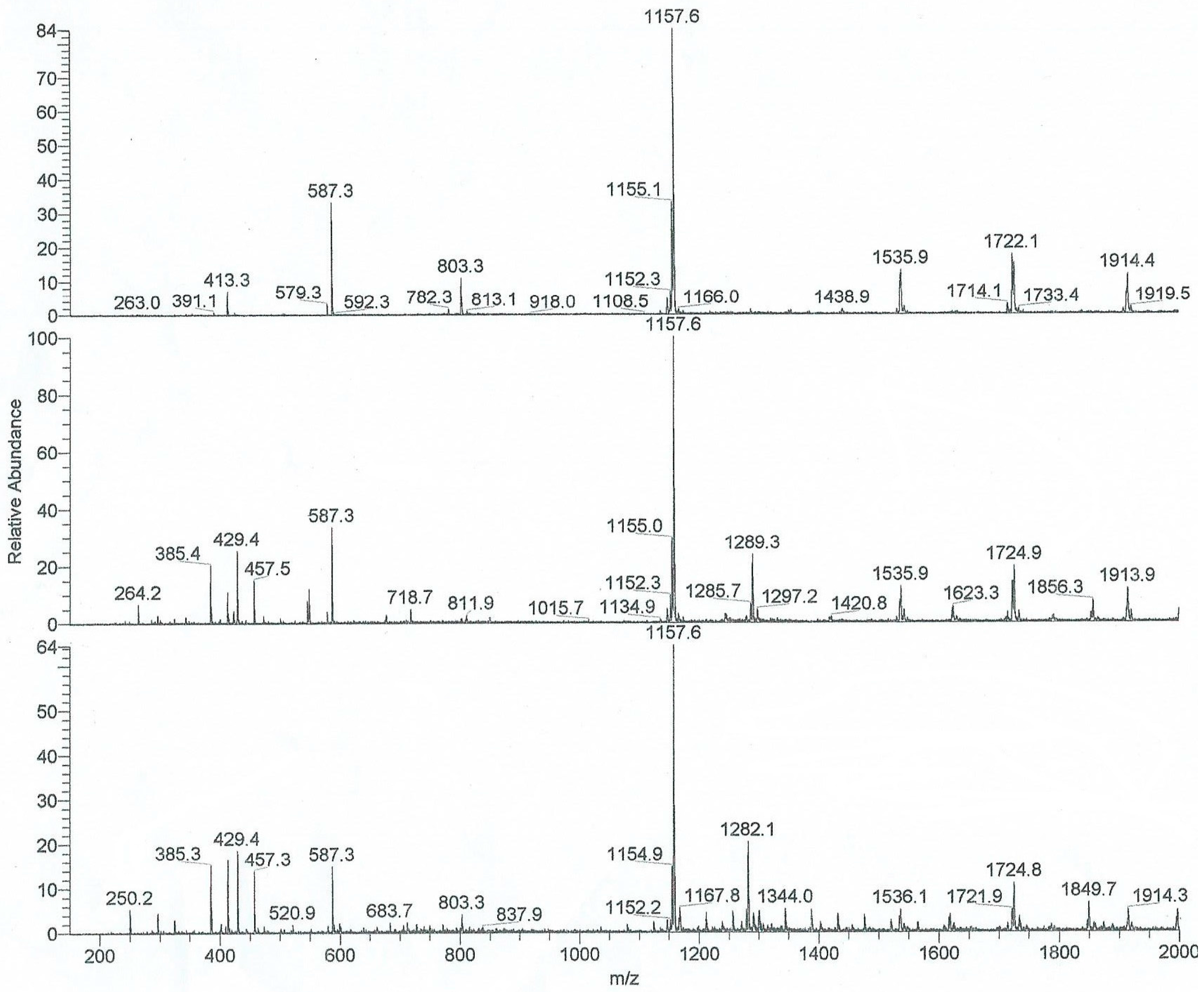
For this investigation the most important technique for studying the inclusion phenomena was ESI mass spectroscopy [
36,
37,
38], which is a relatively straightforward and rapid technique, which directly and rapidly determines the CD:guest stoichiometry (from the observed mass of the complexes), without using complex analysis and fitting techniques, as are required for example for microcalorimetry or fluorescence spectroscopy. We [
39] and others [
40] have recently studied, using ESI-MS, the use of various CD hosts, of different sizes, in the context of the transport of drugs through the digestive system [
39] or to encapsulate drugs such as analgesics [
40]. We have also previously used ESI mass spectroscopy, in combination with fluorescence spectroscopy, to study and characterize the binding of the fluorescent probe Nile Red in various cyclodextrins [
41]. In this case the formation in solution of CD: guest complexes at 1:1 or 2:1 could easily be detected. In parallel, we use molecular modelling, which has been shown to produce quite reliable data for the stability of such complexes [
32,
39,
41,
42,
43,
44], and also allows for the calculation of the energies of potential 1:1 or 2:1 host:guest complexes.
The relative abundance of complexes with varying stoichiometries in the ESI-MS experiments is directly related to the energy and thus stability of these complexes. Wen et al. [
32] calculated the energies of such complexes in the case of the malarial drug chinchonine using molecular modeling, to explain their ESI-MS results. We will use a similar approach in this work; however, we were also fascinated by the observed persistence of 2:1 complexes in the case of certain derivative guests, and their rather unusual behaviour under ESI conditions, and the reasons why some substituted PAN derivatives showed 2:1 complexation in the ESI-MS results, but the majority did not. In order to follow this trend we used in our ESI experiments the condition of excess of cyclodextrin in order to assure the formation and observation of only the most stable multi-cyclodextrin adduct ions. When the Wen group used protected CDs [
32], many unexpected reactions took place, for example dealkylation or sharing of guests between cyclodextrin hosts. For this reason we preferred, as suggested by Kobetic [
33], using the “native” CDs of differing size, namely β- and γ-CD, which have an appropriate cavity size for these guests. Furthermore, we decided to perform molecular modelling studies of the 1:1 and 2:1 host:guest complexes, with the goal of observing some significant differences justifying the stability of some of these complexes and the absence of others. Overall, our interest in unusual, uncommon or simply strange structures, proposed for ion structures observed under ESI conditions, was undertaken to rationalise the observed complexes using the thermodynamic calculations via molecular modelling (MM+ in particular as a first step) to develop a hypothesis for their formation and in particular to explain the unusual stability in solution of some of these complexes.
We present herein the comparison of the data obtained from the mass spectrometry of the 19 PAN dyes (shown in
Figure 1 and
Table 1) and two CDs, namely β- and γ-CD, and the corresponding data of calculated energies for 1:1 and 2:1 complexes obtained via molecular modelling. The experimental and calculated data and results will be discussed in the following sections. Surprisingly, the 1:1 complexes between PAN and CD are not easily formed under the standard experimental conditions for ESI/PI (methanol, drop of formic acid, optimized ionization energy to observed the complex ions), and were only observed at a relatively high excess of CD. As will be discussed below, under such excess CD conditions, 1:1 and in the case of some host-guest pairs 2:1 host:guest complexes were observed.
3. Discussion
From the molecular modeling studies described above, it is clear that the electron withdrawing/donating abilities of the various substituents on this set of guests plays a significant role in the nature of the inclusion complexes obtained. This effect can of course be investigated quantitatively using the well-known set of Hammett σ
m and σ
p substituent constants, which have been determined and tabulated based on the effect of
meta or
para substitution of a substituent the acid equilibrium constant of benzoic acid [
45,
46]. These parameters take into account the inductive and resonance effect of the substituent on the electron distribution of the aromatic ring(s). In general, substituents can be considered as either electron-donating groups (increased aromatic electron density) or electron-withdrawing groups (decreased aromatic electron density). Electron-donating groups have negative σ
m and σ
p substituent constant values, whereas electron-withdrawing groups have positive σ
m and σ
p substituent constant values.
It is clear from
Table 3 that, other than the unsubstituted PAN itself, only PAN derivatives with substituents with negative σ
m values, i.e., with electron donating groups, exhibit 2:1 CD:PAN complexes with β-CD in the ESI-MS spectrum. No PAN derivative with a
meta substituent with a positive σ
m forms 2:1 complexes with β-CD. A similar result is observed for
para-substituted PAN derivatives with β-CD, as shown in
Table 4: the only ones that form 2:1 complexes are those with substituents with negative σ
p values, i.e., with electron donating groups. Once again, no PAN derivative with a
para substituent with a positive σ
p forms 2:1 complexes with β-CD. Thus, a relatively high aromatic electron density is required for the formation of 2:1 complexes, which is supported by the presence of electron-donating substituents. However, not all derivatives with negative σ
m meta substituents show 2:1 complexes: ethyl, n-butyl, and n-hexyl all have negative values but do not form 2:1 complexes. In the latter two cases, the steric effect of these larger alkyl substituents must play a role. In the case of ethyl substitution, the steric effect is seen to be intermediate between the small methyl group and the larger n-butyl, and n-hexyl groups: ethyl does form 2:1 complexes with β-CD when substituted in the
meta position, but not in the
para. It is interesting and informative to note that the only substituent which gave 2:1 complexes in all four cases (
meta and
para substitution, β- and γ-CD) was the smallest alkyl substituent, the methyl group.
Table 5 and
Table 6 show the analogous results in the case of the larger γ-CD; in this case, only methyl substituents show 2:1 complexes. As discussed in
Section 2.1 above, it is less likely that 2:1 host:guest complexes will form with the much larger γ-CD, as there would be much more steric interference between the two hosts encapsulating the same PAN guest. In this case, the electron donating requirement of the guest is more important, to help overcome the host steric effect, as evidenced by the fact that the unsubstituted PAN does not form 2:1 complexes (contrary to the result in β-CD). It is, however, surprising that the larger alkyl substituents do not encourage 2:1 complexation, as a larger guest would be more likely to be encapsulated by two hosts.
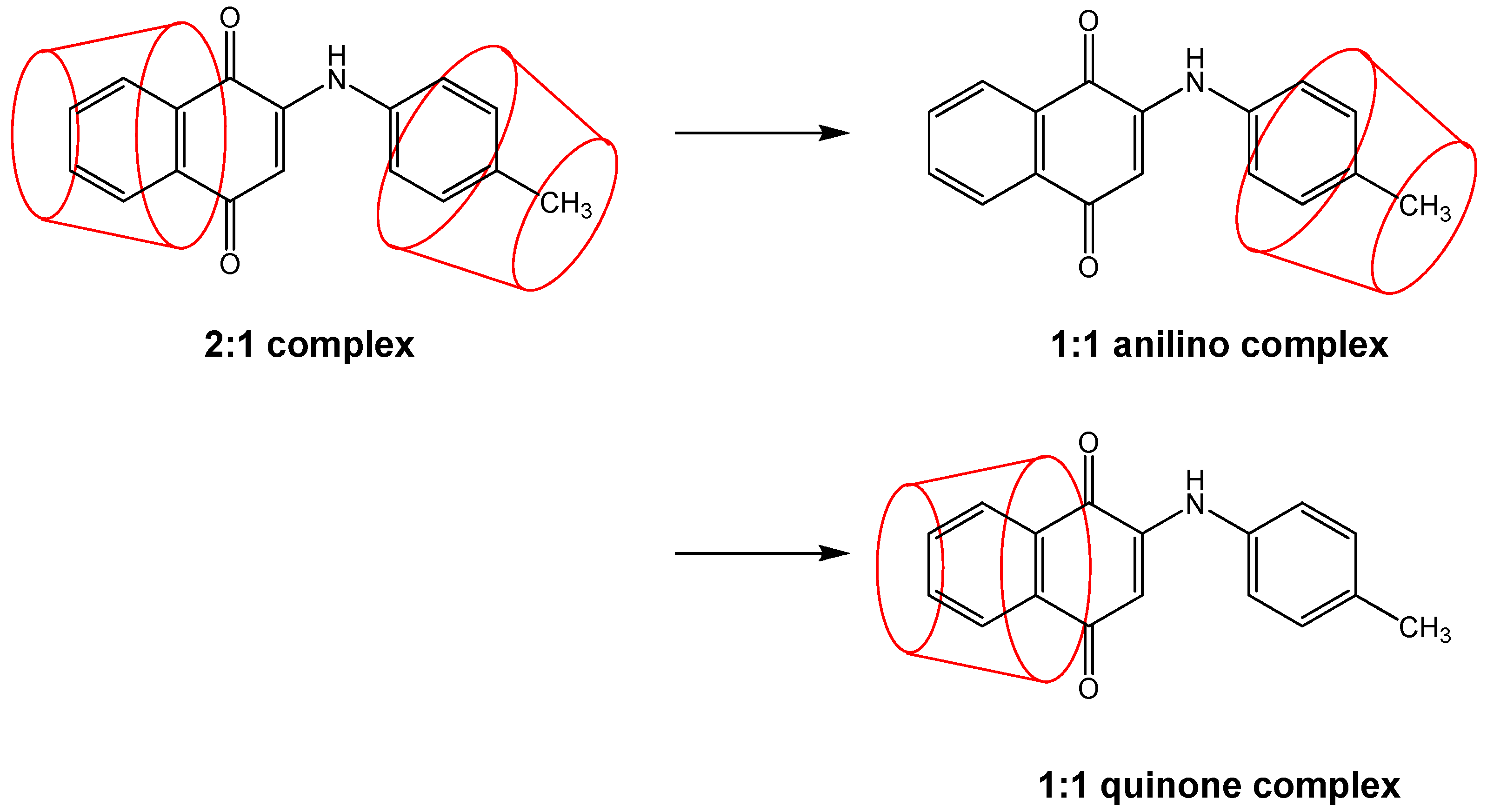
It should be mentioned that in the ESI-MS experiments, only complexes which survive the ionization process are observed. Thus, there is a small possibility that 2:1 complexes form in most cases (especially considering the experimental conditions of a large excess of cyclodextrin), but that such complexes fall apart under MS-ESI conditions for all but a few of the PAN derivatives, resulting in only 1:1 complexes, either anilino (most likely), quinone, or both, being observed in the ESI experiments. This possibility is depicted in
Figure 5.
4. Materials and Methods
PAN derivatives
1–
19 were synthesized as previously described [
28]. β- and γ-CD were obtained from Sigma-Aldrich (St. Louis, MO, USA), and used as received.
Electrospray ionization mass spectroscopy (ESI-MS) experiments were performed on a Quattro II Micromass (Waters, Elstree, Hertfordshire, UK) spectrometer (CEN de Saclay, Gif-sur-Yvette, France). Solutions contained 10−6 to 10−7 M of the PAN derivative, with a large (three- to ten-fold) excess of cyclodextrin). Samples were injected at 10 mL/min, with a source temperature of 80 °C and capillary voltage maintained at +3.35 KV. The cone voltage ranged from 10–70 V; at 20 V the skimmer voltage was 1.9 V. In a typical experimental setting, 100 μL of an ethanol solution of the CD and PAN (10:1 mole ratio; large excess of CD used to allow for formation of higher-order host:guest complexes ) was prepared, in some cases a drop of formic acid was added, and introduced through a Harvard Apparatus syringe pump. The ions were detected by scanning the first quadrupole and the mass range was monitored from m/z = 80–2000 in 7 s. At least 50 scans were averaged to obtain representative spectra.
Molecular modeling calculations were performed on HyperChem 6 MM+ (U de M) in gas phase and in solvent box (ethanol) modes. The minimization of energy was performed using the technique described in the HyperChem instruction manual, and in references [
39] and [
41]. Energies obtained were minimized potential energies as defined in the HyperChem 6 MM+ approach, and include conformational motions for the obtaining the lowest energy complex configuration. These calculations were done in the same way across the entire set of host:guest pairs, allowing for relatiave stability comparisons to be made. In a first phase, 1600 Polak Ribiere iterations were applied. A small incremental energy was applied in a second phase (0.001 ps for a total of 1 ps, 370 K) for selected insertion models only. All calculated complex structures (1:1, 2:1 and 1:2 stoichiometry) and energy data are available upon request from C.K. Jankowski (U de M).
5. Conclusions
2-[(R-phenyl)amine]-1,4-naphthoquinones (PAN) form host-guest inclusion complexes with both β- and γ-CD, as observed by ESI-MS experiments. For the majority of the 19 PAN derivatives studied in these two CDs, only 1:1 CD:PAN complexes were observed (32 out of 38 host:guest pairs). In six cases, 2:1 host:guest complexes were also observed: 4 cases for β-CD (parent PAN, m-methyl, m-ethyl, and p-methyl) and 2 cases for the larger γ-CD (m- and p-methyl). Thus, the specific nature of the PAN derivative, and the effect of the specific substituents, has a major effect on determining the resulting host-guest stoichiometry, as does the size of the host CD cavity. Molecular modeling studies showed that 1:1 complexation most likely involves encapsulation of the aniline rather than the quinone moiety, and indicated large stabilization upon 2:1 complexation. Consideration of the Hammett parameters of the substituents showed that 2:1 host-guest complexes formed only with PAN derivatives with small substituents with negative σm or σp values, i.e., for guests with electron donating substituents. This trend indicates that higher electron density on the aromatic ring encourages complexation by 2 host molecules. However, larger alkyl substituents with negative σm and σp parameters did not yield 2:1 complexes, even for the larger γ-CD host, showing that steric effects in terms of guest size are also highly important. It is clear that the prediction of host-guest stoichiometry for a specific host-guest pair is complicated, and involves a subtle interplay of both electronic and steric factors. However, as demonstrated in this paper, there are trends in terms of the electronic nature of the guest and the size of both the guest and host, which can be used to help predict the expected host:guest stoichiometry for a given host-guest pair.