The Chemistry and Pharmacology of Citrus Limonoids
Abstract
:1. Citrus Limonoids: An Introduction
1.1. Complexity and Evolution of Citrus Limonoid Research
1.2. Chemistry and Classifications
1.3. Biosynthesis and Enzymatic Modifications
2. Limonin Congeners: A Medicinal Chemistry Perspective
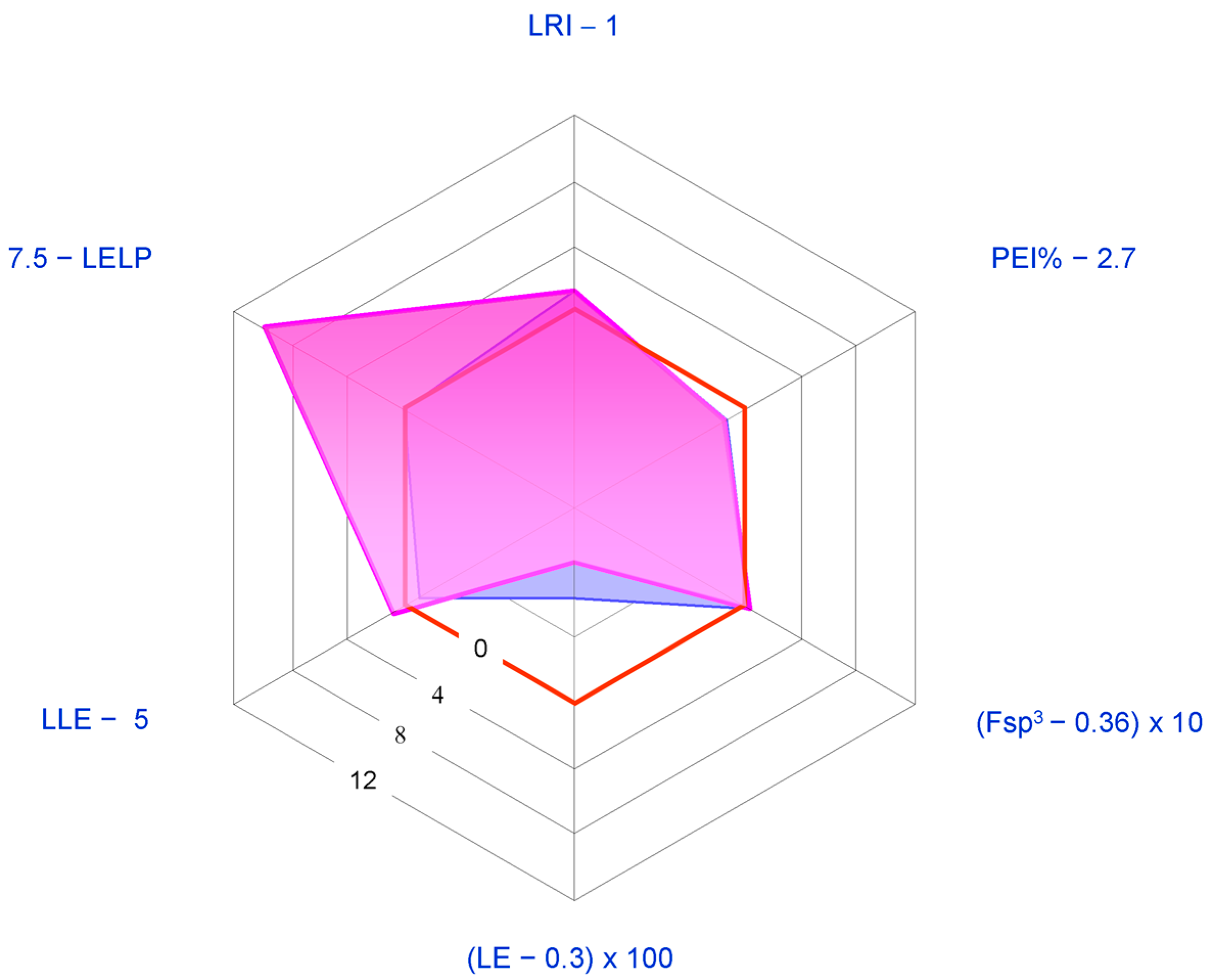
2.1. Medicinal Chemistry Tools
- Lean ring index (LRI) = heterocycles/carbocycles ratio. This index was introduced since the number of aromatic and heteroaromatic rings has been found to correlate with an increased risk of attrition in development [106]. In particular, the following scale in the detrimental effects of rings has been observed [107]: carboaromatics >> heteroaromatics > carboaliphatics > heteroaliphatics, with the latter generally being beneficial. Thus, the higher LRI, the better the developability.
- Potency efficiency index percentage (PEI%) = (potency/MW Da) × 100, where potency is generally given in the form of –Log (half maximal inhibitory concentration) (pIC50) or –Log (half maximal effective concentration) (pEC50) for the activity actually concerned. We adopted this index as a slight modification of the well-known binding efficiency index (BEI) = pIC50/MW kDa [104] since PEI% values allow clearer graphical representations than the former. Since an idealized compound should have a BEI value of 27, the corresponding PEI% value would be 2.7.
- Ligand efficiency index (LEI) = potency/number of non-hydrogen atoms (“heavy atoms”, HA). For the sake of graphical clarity, we adopted the descriptor LE (ligand efficiency) corresponding to the product LEI × 1.37 (LE = ∆G°/HA = −2.303 RT/HA ≈ 1.37 potency/HA). An ideal lead compound should have LE > ~0.3 kcal per mole per HA [102].Fraction sp3 (Fsp3 = number of sp3 hybridized carbons/total carbon count). Lovering [108] suggested this descriptor as a measure of complexity since he noted that drug candidates nearest to clinical use generally present high Fsp3 and chirality centers. 0.36 is the suggested lowest allowed limit value [109].
- Lipophilic ligand efficiency (LLE = potency − cLog P, also referred to as lipophilic efficiency, LipE). LLE is simply the difference between potency and the most popular lipophilicity descriptor (cLog P), and may be considered as a measure of the specificity of binding. Proposed acceptable values of LLE for drug candidates are >~5 [102].
2.2. Citrus Limonoids: Sources, Main Structural Features, and Developability As Therapeutic Agents
2.2.1. Limonin (1, Figure 1)
2.2.2. Nomilin (2, Figure 1)
2.2.3. Obacunone (3, Figure 1)
2.2.4. Deacetylnomilin (4)
2.2.5. Pseudoacids: Limonexic Acid (5), Isolimonexic Acid (6), and Citrusin (7)
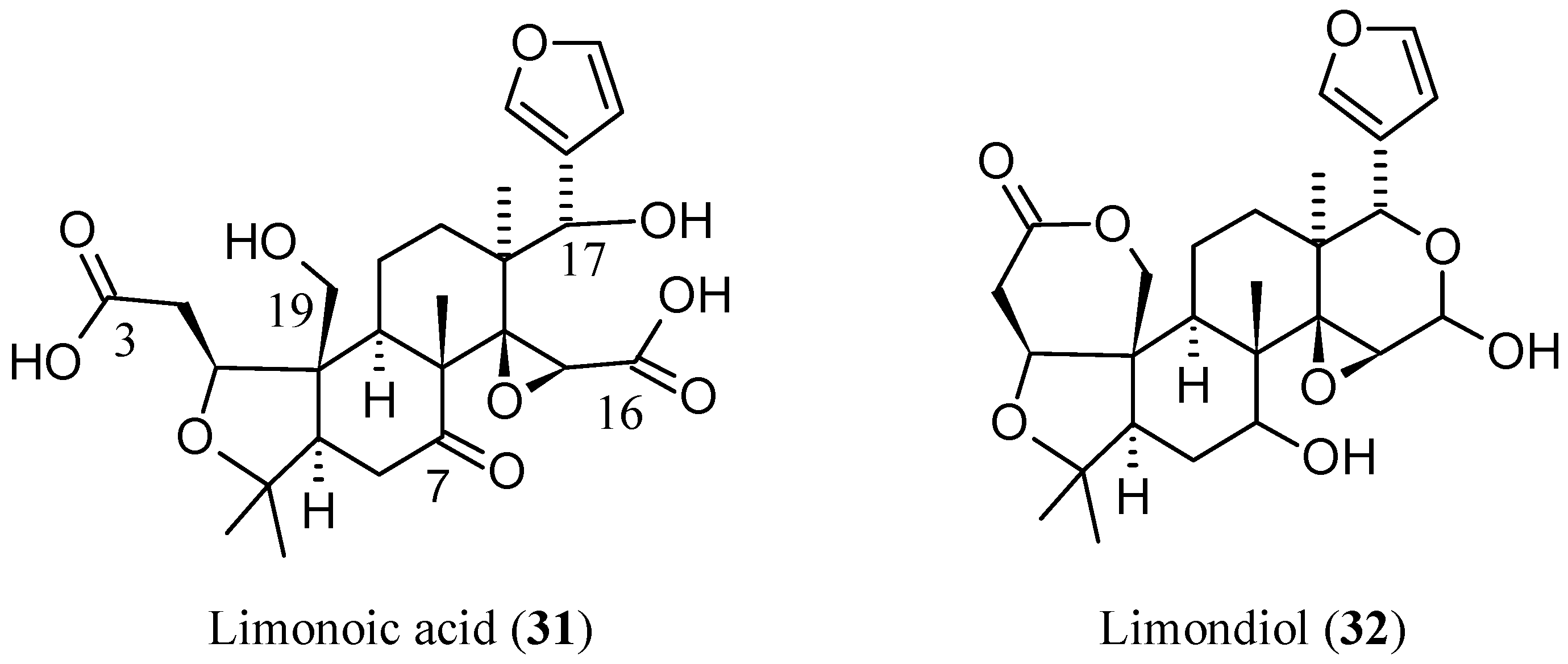
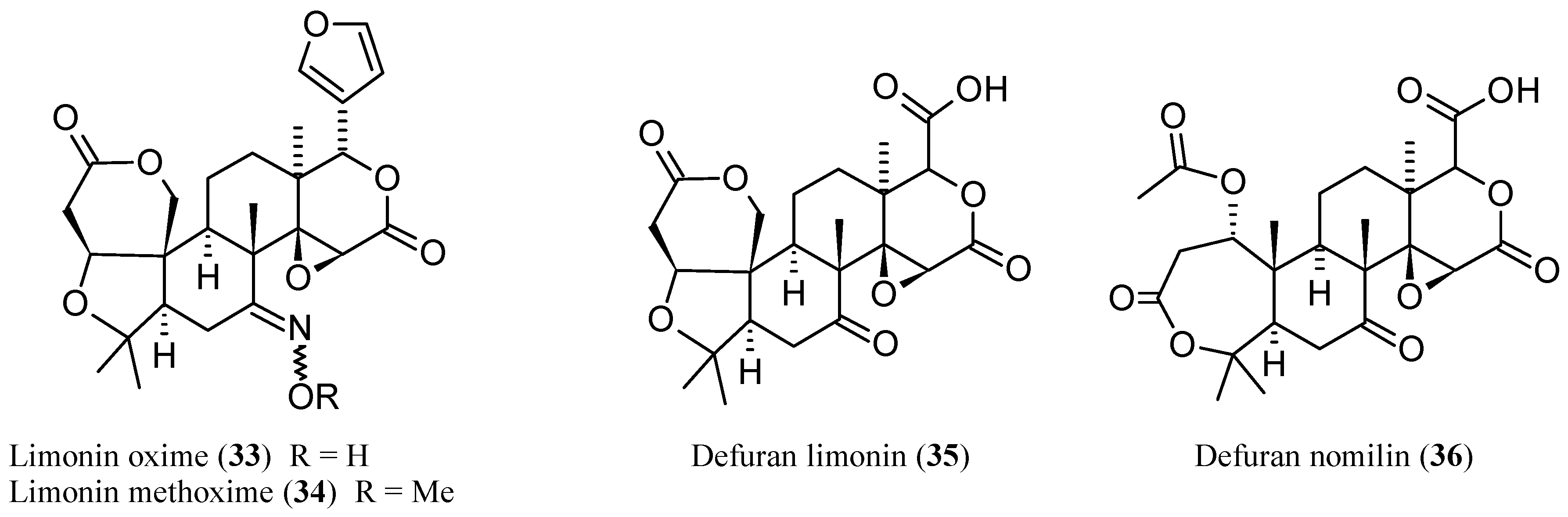
2.2.6. Newly Identified Limonin Related Limonoids
3. Isolation and Identification of Limonoids from the Citrus Genus
3.1. Limonoid Aglycones
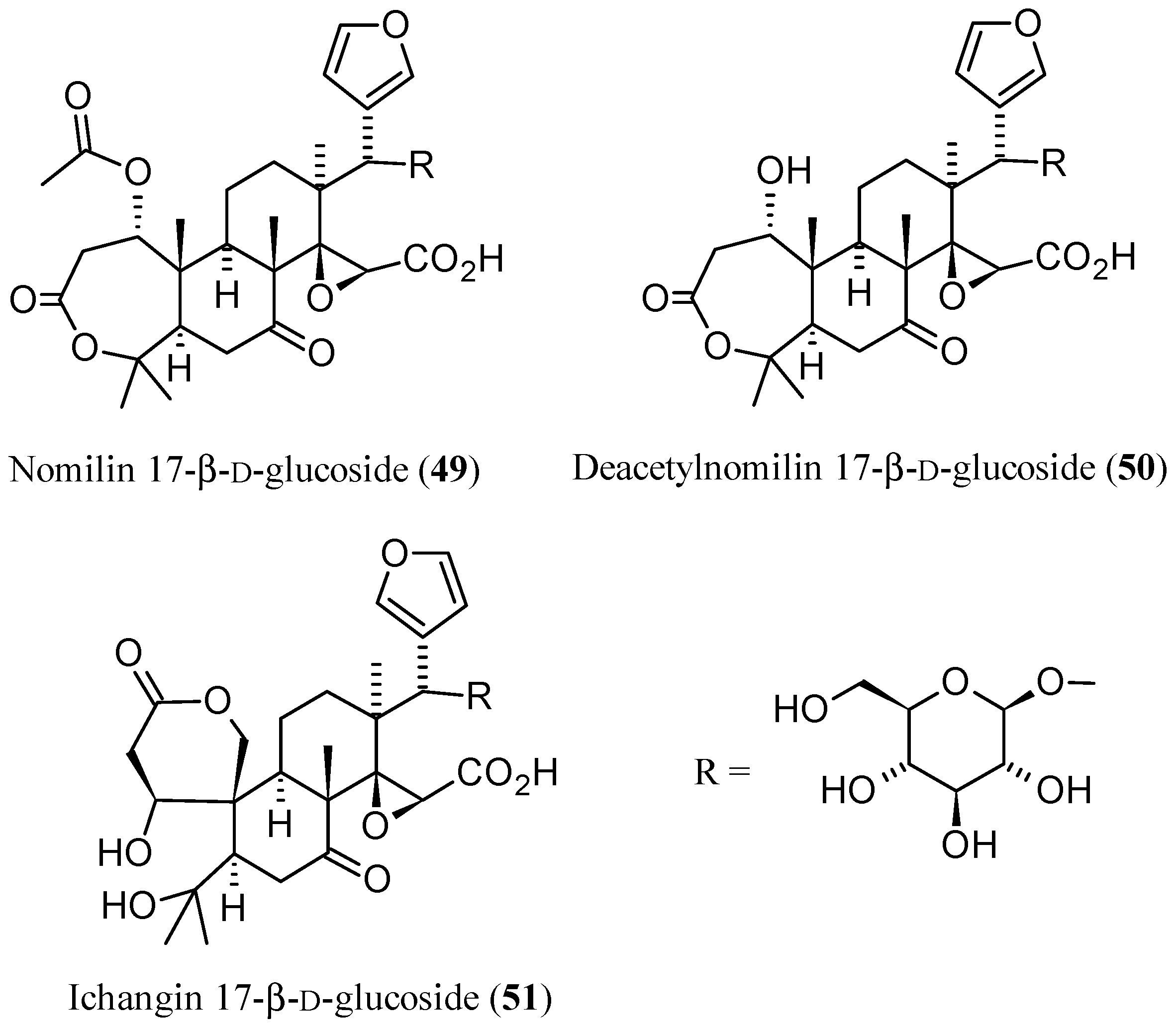
3.2. Limonoid Glucosides
3.3. Limonoid Aglycones and Glucosides
3.4. Metabolomic Analysis
3.5. Metabolic Transformations of Secondary Metabolites
4. Pharmacological Properties
4.1. Anticancer and Antioxidant Activities
4.1.1. In Vitro Tests
4.1.2. In Vivo Tests
- While there is no doubt that antioxidant activity has been demonstrated for CLs, the reported higher micromolar concentration range is not making these group of compounds as optimal leads for this biological activity. For example, our decades or research in this field has shown that promising antioxidants should act at micro/submicromlar ranges and numerous polyphenolic compounds such as gallic acid and flavonoid derivatives fit into this category, e.g., [177,178,179,180,181,182,183,184,185,186].
- Similarly, there is no doubt that anticancer activity has been demonstrated for the numerous CLs which generally ranges from weak to good hits. This moderate activity is in line with the general anticancer profile of various natural terpenoids that bear α,β-unsaturated functional moieties, e.g., [183,187,188,189]. As there are numerous examples of other natural products that act in nanomolar ranges, e.g., [190], further medicinal chemistry studies, in addition to the one presented in Section 2, focusing on lead optimisation is necessary.
- Irrespective of drug lead identification and rather on the basis that CLs are consumed in large amount on daily basis, however, the reported cytotoxic and antioxidant activity have significant implication on the potential health/medical benefit of citrus juices. In this regard, some in vivo data already substantiated the potential benefit of CLs as anticancer agents. On this basis, there should be an urge to enhance the potency and/or increased multifunctionality (e.g., through increased antioxidant effects) of CLs.
4.2. Selective Toxicity against Pathogens
4.2.1. Antiviral Activities
4.2.2. Antibacterial Activities
4.2.3. Larvicidal and Insecticidal Activities
4.3. Other Bioactivities of Citrus Limonoids Studied in the Last Decade
4.3.1. Analgesic and Anti-Inflammatory Activities
4.3.2. Anti-HyperGlycemic Properties
4.3.3. Inhibition of Osteoclastogenesis
5. General Summary and Conclusions
Acknowledgments
Conflicts of Interest
References
- Manners, G.D. Citrus limonoids: Analysis, bioactivity, and biomedical prospects. J. Agric. Food Chem. 2007, 55, 8285–8294. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tu, H.; Wan, J.; Chen, W.; Liu, X.; Luo, J.; Xu, J.; Zhang, H. Spatio-temporal distribution and natural variation of metabolites in Citrus fruits. Food Chem. 2016, 199, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, D.; El-Readi, M.Z.; Tahrani, A.; Herrmann, F.; Kaufmann, D.; Farrag, N.; El-Shazly, A.; Wink, M. Secondary metabolites of ponderosa lemon (Citrus pyriformis) and their antioxidant, anti-inflammatory, and cytotoxic activities. Z. Naturforsch. 2011, 66, 385–393. [Google Scholar] [CrossRef]
- Hamdan, D.; El-Readi, M.Z.; Tahrani, A.; Herrmann, F.; Kaufmann, D.; Farrag, N.; El-Shazly, A.; Wink, M. Chemical composition and biological activity of Citrus jambhiri Lush. Food Chem. 2011, 127, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Arigò, A.; Calabrò, M.L.; Farnetti, S.; Mondello, L.; Dugo, P. Bergamot (Citrus bergamia Risso) as a source of nutraceuticals: Limonoids and flavonoids. J. Funct. Food 2016, 20, 10–19. [Google Scholar] [CrossRef]
- Nakagawa, H.; Takaishi, Y.; Tanaka, N.; Tsuchiya, K.; Shibata, H.; Higuti, T. Chemical constituents from the peels of Citrus sudachi. J. Nat. Prod. 2006, 69, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, X.J.; Pan, Y.; Zhou, Z. Identification of the chemical compositions of Ponkan peel by ultra performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. Anal. Methods 2016, 8, 893–903. [Google Scholar] [CrossRef]
- Hasegawa, S. Biochemistry of Limonoids. In Citrus Limonoids; ACS Symposium Series, Berhow, M.A., Hasegawa, S., Manners, G.D., Eds.; American Chemical Society: Washington, DC, USA, 2000; Volume 758, pp. 9–30. [Google Scholar]
- Hasegawa, S.; Miyake, M. Biochemistry and biological functions of Citrus limonoids. Food Rev. Int. 1996, 12, 413–435. [Google Scholar] [CrossRef]
- Glabasnia, A.; Hofmann, T. On the non-enzymatic liberation of limonin and C17-epilimonin from limonin-17-β-d-glucopyranoside in orange juice. Eur. Food Res. Technol. 2008, 228, 55–63. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Ding, F.; Sun, D.; Ma, Z.; Cheng, Y.; Xu, J. Content changes of bitter compounds in “Guoqing No. 1” Satsuma mandarin (Citrus unshiu Marc.) during fruit development of consecutive 3 seasons. Food Chem. 2014, 145, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, E.; Plotto, A.; Manthey, J.; McCollum, G.; Bai, J.; Irey, M.; Cameron, R.; Luzio, G. Effect of Liberibacter infection (Huanglongbing disease) of citrus on orange fruit physiology and fruit/fruit juice quality: Chemical and physical analyses. J. Agric. Food Chem. 2010, 58, 1247–1262. [Google Scholar] [CrossRef] [PubMed]
- Raithore, S.; Dea, S.; McCollum, G.; Manthey, J.A.; Bai, J.; Leclair, C.; Hijaz, F.; Narciso, J.A.; Baldwin, E.A.; Plotto, A. Development of delayed bitterness and effect of harvest date in stored juice from two complex citrus hybrids. J. Sci. Food Agric. 2016, 96, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.R.; Hashinaga, F. Possible role of carboxyl and imidazole groups in the catalysis of pummelo limonoid glucosyltransferase. Chin. J. Catal. 2010, 31, 1445–1451. [Google Scholar] [CrossRef]
- Roy, A.; Saraf, S. Limonoids: Overview of significant bioactive triterpenes distributed in plants kingdom. Biol. Pharm. Bull. 2006, 29, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Todaro, A.; Palmeri, R.; Scalone, D.; Alberio, G.R.A.; Serafini, M.; Spagna, G. Removal of bitter compounds from citrus byproducts. Ital. J. Food Sci. 2013, 25, 465–469. [Google Scholar]
- Verma, J.P.; Singh, S.; Ghosh, M.; Srivastava, P.K. Identification and characterization of cellular locus of limonin biotransforming enzyme in Pseudomonas putida. Int. J. Food Sci. Technol. 2010, 45, 319–326. [Google Scholar] [CrossRef]
- Patil, B.S.; Yu, J.; Dandekar, D.V.; Toledo, R.T.; Singh, R.K.; Pike, L.M. Citrus bioactive limonoids and flavonoids extraction by supercritical fluids. In Potential Health Benefits of Citrus; ACS Symposium Series; Patil, B.S., Turner, N.D., Miller, E.G., Brodbelt, J.S., Eds.; American Chemical Society: Washington, DC, USA, 2006; Volume 936, pp. 18–33. [Google Scholar]
- Chaudhary, P.R.; Jayaprakasha, G.K.; Patil, B.S.; Porat, R. Grapefruit degreening influence on health promoting limonoids and flavoniods. Acta Hortic. 2012, 939, 113–120. [Google Scholar] [CrossRef]
- Chaudhary, P.R.; Jayaprakasha, G.K.; Patil, B.S. Ethylene degreening modulates health promoting phytochemicals in Rio Red grapefruit. Food Chem. 2015, 188, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Jayaprakasha, G.K.; Porat, R.; Patil, B.S. Degreening and postharvest storage influences “Star Ruby” grapefruit (Citrus paradisi Macf.) bioactive compounds. Food Chem. 2012, 135, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Manthey, J.A.; Ford, B.L.; Luzio, G.; Cameron, R.G.; Narciso, J.; Baldwin, E.A. Effect of extraction, pasteurization and cold storage on flavonoids and other secondary metabolites in fresh orange juice. J. Sci. Food Agric. 2013, 93, 2771–2781. [Google Scholar] [CrossRef] [PubMed]
- Ram, L.; Dinesh, K. Effect of growth stages on the changes in bioactive compounds of Nagpur mandarin (Citrus reticulata) fruits of Ambia crops. Indian J. Agric. Sci. 2012, 82, 714–716. [Google Scholar]
- Arias, B.Á.; Ramón-Laca, L. Pharmacological properties of citrus and their ancient and medieval uses in the Mediterranean region. J. Ethnopharmacol. 2005, 97, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-Y.; Li, C.-H.; Shen, Y.-C.; Wu, T.-S. Anti-inflammatory principles from the stem and root barks of Citrus medica. Chem. Pharm. Bull. 2010, 58, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, Q.; Yin, Y.; Lv, C.; Sun, W.; He, B.; Liu, R.; Chen, X.; Bi, K. Simultaneous determination of three alkaloids, four ginsenosides and limonin in the plasma of normal and headache rats after oral administration of Wu-Zhu-Yu decoction by a novel ultra fast liquid chromatography-tandem mass spectrometry method: Application to a comparative pharmacokinetics and ethological study. J. Mass Spectrom. 2013, 48, 519–532. [Google Scholar] [PubMed]
- Lv, M.; Tian, Y.; Zhang, Z.; Liang, J.; Xu, F.; Sun, J. Plant metabolomics driven chemical and biological comparison of the root bark of Dictamnus dasycarpus and Dictamnus angustifolius. RSC Adv. 2015, 5, 15700–15708. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Yang, L.; Wei, J.; Huang, M.; Jiang, J.-G. Bioactivity evaluations of ingredients extracted from the flowers of Citrus aurantium L. var. amara Engl. Food Chem. 2012, 135, 2175–2181. [Google Scholar] [CrossRef] [PubMed]
- Patil, B.S.; Jayaprakasha, G.K.; Chidambara Murthy, K.N.; Vikram, A. Bioactive compounds: Historical perspectives, opportunities, and challenges. J. Agric. Food Chem. 2009, 57, 8142–8160. [Google Scholar] [CrossRef] [PubMed]
- Codoner-Franch, P.; Valls-Belles, V. Citrus as functional foods. Curr. Top. Nutraceutical Res. 2010, 8, 173–184. [Google Scholar]
- Zou, Z.; Xi, W.; Hu, Y.; Nie, C.; Zhou, Z. Antioxidant activity of citrus fruits. Food Chem. 2016, 196, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Kaur, G. An insight into the role of citrus bioactives in modulation of colon cancer. J. Funct. Food 2015, 13, 239–261. [Google Scholar] [CrossRef]
- Harris, E.D.; Poulose, S.M.; Patil, B. Citrus limonoids are unique andeffective anticancer agents. Acta Hortic. 2007, 744, 165–170. [Google Scholar] [CrossRef]
- Sato, R. Nomilin as an anti-obesity and anti-hyperglycemic agent. Vitam. Horm. 2013, 91, 425–439. [Google Scholar] [PubMed]
- Ejaz, S.; Ejaz, A.; Matsuda, K.; Lim, C.W. Limonoids as cancer chemopreventive agents. J. Sci. Food Agric. 2006, 86, 339–345. [Google Scholar] [CrossRef]
- Patil, B.S.; Brodbelt, J.S.; Miller, E.G.; Turner, N.D. Potential health benefits of citrus: An overview. In Potential Health Benefits of Citrus; ACS Symposium Series 936; Patil, B.S., Turner, N.D., Miller, E., Brodbelt, J., Eds.; Oxford University Press: Oxford, MS, USA, 2006; Volume 936, Chapter 1; pp. 1–16. [Google Scholar]
- Heasley, B. Synthesis of limonoid natural products. Eur. J. Org. Chem. 2011, 19–46. [Google Scholar] [CrossRef]
- Nikkon, A.; Silversmith, E.F. Appendix A. Brief etymology of some traditional chemical names. In The Name Game, 1st ed.; Pergamon Press: Oxford, UK, 1987; p. 317. [Google Scholar]
- Ruzicka, L. The isoprene rule and the biogenesis of terpenic compounds. Experientia 1953, 9, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Giles, P.M., Jr. Revised Section F: Natural products and related compounds. Pure Appl. Chem. 1999, 71, 587–643. [Google Scholar] [CrossRef]
- Behenna, D.C.; Corey, E.J. Simple enantioselective approach to synthetic limonoids. J. Am. Chem. Soc. 2008, 130, 6720–6721. [Google Scholar] [CrossRef] [PubMed]
- Okogun, J.I.; Fakunle, C.O.; Ekong, D.E.U. Chemistry of the meliacins (limonoids). The structure of melianin A, a new protomeliacin from Melia azedarach. J. Chem. Soc. Perkin I 1975, 1352–1356. [Google Scholar] [CrossRef]
- Lakshmi, V.; Gupta, P. An overview of the genus Xylocarpus. Nat. Prod. Res. 2008, 22, 1197–1224. [Google Scholar] [CrossRef] [PubMed]
- Fraser, L.A.; Mulholland, D.A.; Fraser, D.D. Classification of limonoids and protolimonoids using neural networks. Phytochem. Anal. 1997, 8, 301–311. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Zhu, Q.; Gong, G.; Luo, D.; Jiang, A.; Yang, L.; Xu, Y. Synthesis and pharmacological evaluation of novel limonin derivatives as anti-inflammatory and analgesic agents with high water solubility. Bioorg. Med. Chem. Lett. 2014, 24, 1851–1855. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jayaprakasha, G.K.; Patil, B.S. Limonoids and their anti-proliferative and anti-aromatase properties in human breast cancer cells. Food Funct. 2013, 4, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Poulose, S.M.; Jayaprakasha, G.K.; Mayer, R.T.; Girennavar, B.; Patil, B.S. Purification of citrus limonoids and their differential inhibitory effects on human cytochrome P450 enzymes. J. Sci. Food Agric. 2007, 87, 1699–1709. [Google Scholar] [CrossRef]
- Patil, J.R.; Chidambara Murthy, K.N.; Jayaprakasha, G.K.; Chetti, M.B.; Patil, B.S. Bioactive compounds from mexican lime (Citrus aurantifolia) juice induce apoptosis in human pancreatic cells. J. Agric. Food Chem. 2009, 57, 10933–10942. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.L.; Jayaprakasha, G.K.; Valdivia, V.; Munoz, D.; Dandekar, D.V.; Ahmad, H.; Patil, B.S. Limonin methoxylation influences the induction of glutathione S-transferase and quinone reductase. J. Agric. Food Chem. 2009, 57, 5279–5286. [Google Scholar] [CrossRef] [PubMed]
- Vanamala, J.; Leonardi, T.; Patil, B.S.; Taddeo, S.S.; Murphy, M.E.; Pike, L.M.; Chapkin, R.S.; Lupton, J.R.; Turner, N.D. Suppression of colon carcinogenesis by bioactive compounds in grapefruit. Carcinogenesis 2006, 27, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- El-Readi, M.Z.; Hamdan, D.; Farrag, N.; El-Shazly, A.; Wink, M. Inhibition of P-glycoprotein activity by limonin and other secondary metabolites from Citrus species in human colon and leukaemia cell lines. Eur. J. Pharmacol. 2010, 626, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Patil, J.R.; Jayaprakasha, G.K.; Chidambara Murthy, K.N.; Chetti, M.B.; Patil, B.S. Characterization of Citrus aurantifolia bioactive compounds and their inhibition of human pancreatic cancer cells through apoptosis. Microchem. J. 2010, 94, 108–117. [Google Scholar] [CrossRef]
- Han, Y.L.; Yu, H.L.; Li, D.; Meng, X.L.; Zhou, Z.Y.; Yu, Q.; Zhang, X.Y.; Wang, F.J.; Guo, C. Inhibitory effects of limonin on six human cytochrome P450 enzymes and P-glycoprotein in vitro. Toxicol. In Vitro 2011, 25, 1828–1833. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Miyamoto, S.; Fujii, G.; Nakanishi, R.; Onuma, W.; Ozaki, Y.; Fujimoto, K.; Yano, T.; Mutoh, M. Suppression of intestinal carcinogenesis in Apc-mutant mice by limonin. J. Clin. Biochem. Nutr. 2015, 57, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Langeswarana, K.; Kumar, S.G.; Perumal, S.; Revathy, R.; Balasubramaniam, M.P. Limonin—A citrus limonoid, establish anticancer potential by stabilizing lipid peroxidation and antioxidant status against N-nitrosodiethylamine induced experimental hepatocellular carcinoma. Biomed. Prev. Nutr. 2013, 3, 165–171. [Google Scholar] [CrossRef]
- Das, A.; Miller, R.; Lee, P.; Holden, C.A.; Lindhorst, S.M.; Jaboin, J.; Vandergrift, W.A.; Banik, N.L.; Giglio, P.; Varma, A.K.; et al. A novel component from citrus, ginger, and mushroom family exhibits antitumor activity on human meningioma cells through suppressing the Wnt/β-catenin signaling pathway. Tumor Biol. 2015, 36, 7027–7034. [Google Scholar] [CrossRef] [PubMed]
- Chidambara Murthy, K.N.; Jayaprakasha, G.K.; Patil, B.S. Citrus limonoids and curcumin additively inhibit human colon cancer cells. Food Funct. 2013, 4, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Poulose, S.M.; Harris, E.D.; Patil, B.S. Antiproliferative effects of citrus limonoids against human neuroblastoma and colonic adenocarcinoma cells. Nutr. Cancer 2006, 56, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Li, J.; Polson, M.; Mackie, K.; Quiroga, W.; Patil, B.S. Citrus limonoids and flavonoids: Enhancement of phase II detoxification enzymes and their potential in chemoprevention. ACS Symp. Ser. 2006, 936, 130–143. [Google Scholar]
- Mahmoud, M.F.; Hamdan, D.I.; Wink, M.; El-Shazly, A.M. Hepatoprotective effect of limonin, a natural limonoid from the seed of Citrus aurantium var. bigaradia, on d-galactosamine-induced liver injury in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2014, 387, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.F.; Gamal, S.; El-Fayoumi, H.M. Limonin attenuates hepatocellular injury following liver ischemia and reperfusion in rats via toll like receptor dependent pathway. Eur. J. Pharmacol. 2014, 740, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jayaprakasha, G.K.; Muthuchamy, M.; Patil, B.S. Structure-function relationships of citrus limonoids on p38 MAP kinase activity in human aortic smooth muscle cells. Eur. J. Pharmacol. 2011, 670, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Vikram, A.; Jayaprakasha, G.K.; Jesudhasan, P.R.; Pillai, S.D.; Patil, B.S. Limonin 7-methoxime interferes with Escherichia coli biofilm formation and attachment in type 1 pili and antigen 43 dependent manner. Food Control 2012, 26, 427–438. [Google Scholar] [CrossRef]
- Ribeiro, A.B.; Abdelnur, P.V.; Garcia, C.F.; Belini, A.; Severino, V.G.P.; da Silva, M.F.; Fernandes, J.B.; Vieira, P.C.; de Carvalho, S.A.; de Souza, A.A.; et al. Chemical characterization of Citrus sinensis grafted on C. limonia and the effect of some isolated compounds on the growth of Xylella fastidiosa. J. Agric. Food Chem. 2008, 56, 7815–7822. [Google Scholar] [CrossRef] [PubMed]
- Pichaiyongvongdee, S.; Haruenkit, R. Investigation of limonoids, flavanones, total polyphenol content and antioxidant activity in seven Thai pummelo cultivars. Kasetsart J. (Nat. Sci.) 2009, 43, 458–466. [Google Scholar]
- Mandadi, K.K.; Jayaprakasha, G.K.; Bhat, N.G.; Patil, B.S. Red Mexican grapefruit: A novel source for bioactive limonoids and their antioxidant activity. Z. Naturforsch. C 2007, 62, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Balestrieri, E.; Pizzimenti, F.; Ferlazzo, A.; Giofrè, S.V.; Iannazzo, D.; Piperno, A.; Romeo, R.; Chiacchio, M.A.; Mastino, A.; Macchi, B. Antiviral activity of seed extract from Citrus bergamia towards human retroviruses. Bioorg. Med. Chem. 2011, 19, 2084–2089. [Google Scholar] [CrossRef] [PubMed]
- Bilal, H.; Akram, W.; Ali-Hassan, S. Larvicidal activity of citrus limonoids against Aedes albopictus larvae. J. Arthropod Borne Dis. 2012, 6, 104–111. [Google Scholar] [PubMed]
- Hafeez, F.; Akram, W.; Shaalan, E.A. Mosquito larvicidal activity of citrus limonoids against Aedes albopictus. Parasitol. Res. 2011, 109, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.L.; Jayaprakasha, G.K.; Cadena, A.; Martinez, E.; Ahmad, H.; Patil, B.S. In vivo induction of phase II detoxifying enzymes, glutathione transferase and quinone reductase by citrus triterpenoids. BMC Complement. Altern. Med. 2010, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Ono, E.; Inoue, J.; Hashidume, T.; Shimizu, M.; Sato, R. Anti-obesity and anti-hyperglycemic effects of the dietary citrus limonoid nomilin in mice fed a high-fat diet. Biochem. Biophys. Res. Commun. 2011, 410, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Kimira, Y.; Taniuchi, Y.; Nakatani, S.; Sekiguchi, Y.; Kim, H.J.; Shimizu, J.; Ebata, M.; Wada, M.; Matsumoto, A.; Mano, H. Citrus limonoid nomilin inhibits osteoclastogenesis in vitro by suppression of NFATc1 and MAPK signaling pathways. Phytomedicine 2015, 22, 1120–1124. [Google Scholar] [CrossRef] [PubMed]
- Murthy, K.N.; Jayaprakasha, G.K.; Patil, B.S. Cytotoxicity of obacunone and obacunone glucoside in human prostate cancer cells involves Akt-mediated programmed cell death. Toxicology 2015, 329, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jayaprakasha, G.K.; Patil, B.S. Obacunone exhibits anti-proliferative and anti-aromatase activity in vitro by inhibiting the p38 MAPK signaling pathway in MCF-7 human breast adenocarcinoma cells. Biochimie 2014, 105, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Murthy, K.N.; Jayaprakasha, G.K.; Patil, B.S. Obacunone and obacunone glucoside inhibit human colon cancer (SW480) cells by the induction of apoptosis Food Chem. Toxicol. 2011, 49, 1616–1625. [Google Scholar]
- Vikram, A.; Jayaprakasha, G.K.; Jesudhasan, P.R.; Pillai, S.D.; Patil, B.S. Obacunone represses salmonella pathogenicity Islands 1 and 2 in an envz-dependent fashion. Appl. Environ. Microbiol. 2012, 78, 7012–7022. [Google Scholar] [CrossRef] [PubMed]
- Vikram, A.; Jesudhasan, P.R.; Jayaprakasha, G.K.; Pillai, B.S.; Patil, B.S. Grapefruit bioactive limonoids modulate E. coli O157:H7 TTSS and biofilm. Int. J. Food Microbiol. 2010, 140, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Horiba, T.; Katsukawa, M.; Mita, M.; Sato, R. Dietary obacunone supplementation stimulates muscle hypertrophy, and suppresses hyperglycemia and obesity through the TGR5 and PPARγ pathway. Biochem. Biophys. Res. Commun. 2015, 463, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ding, G.; Zhi, X.; Xu, H. Insight into reduction of obacunone, and their ester derivatives as insecticidal agents against Mythimna separata Walker. Bioorg. Med. Chem. Lett. 2015, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Shi, D.; Zhi, X.; Li, Q.; Yao, X.; Xu, H. Synthesis and quantitative structure-activity relationship (QSAR) study of C7-oxime ester derivatives of obacunone as insecticidal agents. RSC Adv. 2015, 5, 31700–31707. [Google Scholar] [CrossRef]
- Kim, J.; Jayaprakasha, G.K.; Vikram, A.; Patil, B.S. Methyl nomilinate from citrus can modulate cell cycle regulators to induce cytotoxicity in human colon cancer (SW480) cells in vitro. Toxicol. In Vitro 2012, 26, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Yang, L.; Zhao, H.Y.; Jiang, J.G.; Xu, H.L. Protective effect of compounds from the flowers of Citrus aurantium L. var. amara Engl against carbon tetrachloride-induced hepatocyte injury. Food Chem. Toxicol. 2013, 62, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Vikram, A.; Jesudhasan, P.R.; Jayaprakasha, G.K.; Pillai, S.D.; Patil, B.S. Citrus limonoids interfere with Vibrio harveyi cell–cell signalling and biofilm formation by modulating the response regulator LuxO. Microbiology 2011, 157, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Vikram, A.; Jesudhasan, P.R.; Pillai, S.D.; Patil, B.S. Isolimonic acid interferes with Escherichia coli O157:H7 biofilm and TTSS in QseBC and QseA dependent fashion. BMC Microbiol. 2012, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Zunino, S.J.; Storms, D.H.; Freytag, T.L.; Adkins, Y.C.; Bonnel, E.L.; Woodhouse, L.R.; Breksa, A.P.; Manners, G.D.; Mackey, B.E.; Kelley, D.S. Dietary supplementation with purified citrus limonin glucoside does not alter ex vivo functions of circulating T lymphocytes or monocytes in overweight/obese human adults. Nutr. Res. 2016, 36, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jayaprakasha, G.K.; Uckoo, R.M.; Patil, B.S. Evaluation of chemopreventive and cytotoxic effect of lemon seed extracts on human breast cancer (MCF-7) cells. Food Chem. Toxicol. 2012, 50, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Jadegoud, Y.; Nagana, G.; Patil, B.S. Bioactive compounds from sour orange inhibit colon cancer cell proliferation and induce cell cycle arrest. J. Agric. Food Chem. 2010, 58, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.S.; Adkins, Y.C.; Zunino, S.J.; Woodhouse, L.R.; Bonnel, E.L.; Breksa, A.P.; Manners, G.D.; Mackey, B.E. Citrus limonin glucoside supplementation decreased biomarkers of liver disease and inflammation in overweight human adults. J. Funct. Foods 2015, 12, 271–281. [Google Scholar] [CrossRef]
- Poulose, S.M.; Harris, E.D.; Patil, B.S. Citrus limonoids induce apoptosis in human neuroblastoma cells and have radical scavenging activity. J. Nutr. 2005, 135, 870–877. [Google Scholar] [PubMed]
- Lv, M.; Xu, P.; Tian, Y.; Liang, J.; Gao, Y.; Xu, F.; Zhang, Z.; Sun, J. Medicinal uses, phytochemistry and pharmacology of the genus Dictamnus (Rutaceae). J. Ethnopharmacol. 2015, 171, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Okamura, H.; Yamauchi, K.; Miyawaki, K.; Iwagawa, T.; Nakatani, M. Synthesis and biological activities of degraded limonoids, (±)-fraxinellonone and its related compounds. Tetrahedron Lett. 1997, 38, 263–266. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Menichini, F. An oerview on chemical aspects and potential health benefits of limonoids and their derivatives. Crit. Rev. Food Sci. Nutr. 2014, 54, 225–250. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Di, Y.T.; Hao, X.J. The advances in the limonoid chemistry of the Meliaceae family. Curr. Org. Chem. 2011, 15, 1363–1391. [Google Scholar]
- Connolly, J.D.; Hill, R.A. Triterpenoids. Nat. Prod. Rep. 2005, 22, 487–503. [Google Scholar] [CrossRef] [PubMed]
- Connolly, J.D.; Hill, R.A. Triterpenoids. Nat. Prod. Rep. 2007, 24, 465–486. [Google Scholar] [CrossRef] [PubMed]
- Connolly, J.D.; Hill, R.A. Triterpenoids. Nat. Prod. Rep. 2008, 25, 794–830. [Google Scholar] [CrossRef] [PubMed]
- Connolly, J.D.; Hill, R.A. Triterpenoids. Nat. Prod. Rep. 2010, 27, 79–132. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2011, 28, 1087–1117. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2012, 29, 780–818. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2013, 30, 1028–1065. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2015, 32, 273–327. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.L.; Keserü, G.M.; Leeson, P.D.; Rees, D.C.; Reynolds, C.H. The role of ligand efficiency metrics in drug discovery. Nat. Rev. Drug Discov. 2014, 13, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Shultz, M.D. Setting expectations in molecular optimizations: Strengths and limitations of commonly used composite parameters. Bioorg. Med. Chem. Lett. 2013, 23, 5980–5991. [Google Scholar] [CrossRef] [PubMed]
- Abad-Zapatero, C.; Metz, J.T. Ligand efficiency indices as guideposts for drug discovery. Drug Discov. Today 2005, 10, 464–469. [Google Scholar] [CrossRef]
- Gualdani, R.; Cavalluzzi, M.M.; Lentini, G. Recent trends in the discovery of small molecule blockers of sodium channels. Curr. Med. Chem. 2016, 23, 2289–2332. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, T.J.; Macdonald, J.F. The impact of aromatic ring count on compound developability—Are too many aromatic rings a liability in drug design? Drug Discov. Today 2009, 14, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, T.J.; Macdonald, S.J.F.; Young, R.J.; Pickett, S.T. The impact of aromatic ring count on compound developability: Further insights by examining carbo- and hetero-aromatic and -aliphatic ring types. Drug Discov. Today 2011, 16, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Lovering, F.; Bikker, J.; Humblet, C. Escape from Flatland: Increasing saturation as an approach to improving clinical success. J. Med. Chem. 2009, 52, 6752–6756. [Google Scholar] [CrossRef] [PubMed]
- Kingwell, K. Medicinal chemistry: Exploring the third dimension. Nat. Rev. Drug Discov. 2009, 8, 931. [Google Scholar] [CrossRef] [PubMed]
- Hann, M.M. Molecular obesity, potency and other addictions in drug discovery. Med. Chem. Commun. 2011, 2, 349–355. [Google Scholar] [CrossRef]
- Ikegami, F. Phellodendri Cortex (Phellodendron Bark). Wakanyaku 2010, 689, 8–9. [Google Scholar]
- Schechter, M.S.; Haller, H.L. The identity of obaculactone, evodin and dictamnolactone with limonin. J. Am. Chem. Soc. 1940, 62, 1307–1309. [Google Scholar] [CrossRef]
- Online Etymology Dictionary. Available online: http://www.etymonline.com/index.php?search=lemon&searchmode=none (accessed on 1 September 2016).
- Bernays, A.J. Limonin. Justus Liebigs Ann. Chem. 1841, 40, 317–318. [Google Scholar]
- Curiously, most scientists do not report the initials of Bernays’ names and truncated its family name to “Bernay”. See Hartog, P.J. Bernays, Albert James (DNB01). In Dictionary of National Biography (1st Supplement) I; Lee, S., Ed.; Smith, Elder & Co.: London, UK, 1901; pp. 245–246. Available online: https://en.wikisource.org/wiki/Bernays,_Albert_James_(DNB01) (accessed on 1 September 2016).
- Phetkul, U.; Wanlaso, N.; Mahabusarakam, W.; Phongpaichit, S.; Carroll, A.R. New acridone from the wood of Citrus reticulata Blanco. Nat. Prod. Res. 2013, 27, 1922–1926. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.Y.; Wu, T.S.; Kuo, Y.H. Chemical constituents and cytotoxicity from the stem bark of Citrus medica. Heterocycles 2009, 78, 1309–1316. [Google Scholar]
- Panthong, K.; Srisud, Y.; Rukachaisirikul, V.; Hutadilok-Towatana, N.; Voravuthikunchai, S.P.; Tewtrakul, S. Benzene, coumarin and quinolinone derivatives from roots of Citrus hystrix. Phytochemistry 2013, 88, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Biavatti, M.W.; Vieira, P.C.; Silva, M.D.; Fernandes, J.B.; Albuquerque, S. Limonoids from the endemic Brazilian species Raulinoa echinata. Z. Naturforsch. 2001, 56, 570–574. [Google Scholar] [CrossRef]
- Chansriniyom, C.; Ruangrungsi, N.; Lipipun, V.; Kumamoto, T.; Ishikawa, T. Isolation of acridone alkaloids and N-[(4-monoterpenyloxy)phenylethyl]-substituted sulfur-containing propanamide derivatives from Glycosmis parva and their anti-herpes simplex virus activity. Chem. Pharm. Bull. 2009, 57, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Wang, R.R.; Cai, X.H.; Zheng, Y.T.; Luo, X.D. Anti-human immunodeficiency virus-1 constituents of the bark of Poncirus trifoliata. Chem. Pharm. Bull. 2010, 58, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.B.; Jiang, N.; Lv, M.Y.; Wang, P.; Xu, F.G.; Liang, J.Y.; Qu, W. Limonoids from the root bark of Dictamnus angustifolius: Potent neuroprotective agents with biometal chelation and halting copper redox cycling properties. RSC Adv. 2015, 5, 24750–24757. [Google Scholar] [CrossRef]
- Lv, M.; Tang, B.; Teng, J.; Liang, J.; Xu, F.; Zhang, Z.; Sun, J. Chemotaxonomic significance of limonoids and triterpenoids from Dictamnus angustifolius G. Don ex Sweet. Biochem. Syst. Ecol. 2015, 59, 311–313. [Google Scholar] [CrossRef]
- Emerson, O.H. Bitter Principles of Citrus. II. Relation of Nomilin and Obacunone. J. Am. Chem. Soc. 1951, 73, 2621–2623. [Google Scholar] [CrossRef]
- Min, Y.D.; Kwon, H.C.; Yang, M.C.; Lee, K.H.; Choi, S.U.; Lee, K.R. Isolation of limonoids and alkaloids from Phellodendron amurense and their multidrug resistance (MDR) reversal activity. Arch. Pharm. Res. 2007, 30, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Sang, H.S.; Young, C.K. Neuroprotective limonoids of root bark of Dictamnus dasycarpus. J. Nat. Prod. 2008, 71, 208–211. [Google Scholar]
- Yang, J.L.; Liu, L.L.; Shi, Y.P. Limonoids and quinoline alkaloids from Dictamnus dasycarpus. Planta Med. 2011, 77, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Choodej, S.; Sommit, D.; Pudhom, K. Rearranged limonoids and chromones from Harrisonia perforata and their anti-inflammatory activity. Bioorg. Med. Chem. Lett. 2013, 23, 3896–3900. [Google Scholar]
- Garnier-Suillerot, A. Impaired accumulation of drug in multidrug resistant cells. What are the respective contributions of the kinetics of uptake and of P-glycoprotein-mediated efflux of drug? Curr. Pharm. Des. 1995, 1, 69–82. [Google Scholar]
- Koller, G.; Czerny, H. Über das limonin, den bitterstoff der orangenkerne den bitterstoff der orangenkerne. Chem. Mon. 1936, 67, 248–268. [Google Scholar] [CrossRef]
- Emerson, O.H. The bitter principles of citrus fruit. I. Isolation of nomilin, a new bitter principle from the seeds of oranges and lemons. J. Am. Chem. Soc. 1948, 70, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Poulose, S.M.; Harris, E.D.; Patil, B.S. Cytotoxic and antineoplastic effects of citrus limonoids against human neuroblastoma and colonic adenocarcinoma cells. FASEB J. 2006, 20, A11–A12. [Google Scholar]
- Breksa, A.P.; Hidalgo, M.B.; Wong, R.Y. Stability of limonin glucoside in beverage matrices. J. Sci. Food Agric. 2008, 88, 2194–2200. [Google Scholar] [CrossRef]
- Ng, K.M.; Gray, A.I.; Waterman, P.G.; But, P.P.H.; Kong, Y.C. Limonoids, alkaloids, and a coumarin from the root and stem barks of Tetradium glabrifolium. J. Nat. Prod. 1987, 50, 1160–1163. [Google Scholar] [CrossRef]
- Gai, L.; Rao, G.; Song, C.; Hu, Z. Studies on the chemical constituents of Evodia rutaecarpa (Juss.) Benth. var. officinalis (Dode) Huang. Acta Pharm. Sin. 2001, 36, 743–745. [Google Scholar]
- Melera, A.; Schaffner, K.; Arigoni, D.; Jeger, O. Zur konstitution des limonins I. Über den verlauf der alkalischen hydrolyse von limonin und limonol. Helv. Chim. Acta 1957, 40, 1420–1437. [Google Scholar] [CrossRef]
- Dreyer, D.L. Citrus bitter principles—II: Application of NMR to structural and stereochemical problems. Tetrahedron 1965, 21, 75–87. [Google Scholar] [CrossRef]
- Kondo, Y.; Suzuki, H.; Nozoe, S. Two γ-hydroxybutenolides from the bark of Phellodendron amurense and photooxidation of limonoids. Yakugaku Zasshi J. Pharm. Soc. Jpn. 1985, 105, 742–746. [Google Scholar]
- Biavatti, M.W.; Westerlon, R.; Burger, C.; Mora, T.C.; De Souza, M.M. Antinociceptive action of limonexic acid obtained from Raulinoa echinata. J. Pharm. Pharmacol. 2007, 59, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Biavatti, M.W.; Westerlon, R.; Vieira, P.C.; Silva, M.F.; Fernandes, J.B.; Penaflor, M.F.; Bueno, O.C.; Ellena, J. Leaf-cutting ants toxicity of limonexic acid and degraded limonoids from Raulinoa echinata. X-ray structure of epoxy-fraxinellone. J. Braz. Chem. Soc. 2005, 16, 1443–1447. [Google Scholar] [CrossRef]
- Coy Barrera, C.A.; Coy Barrera, E.D.; Granados Falla, D.S.; Delgado Murcia, G.; Cuca Suarez, L.E. Seco-limonoids and quinoline alkaloids from Raputia heptaphylla and their antileishmanial activity. Chem. Pharm. Bull. 2011, 59, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Breksa, A.P.; Dragull, K.; Wong, R.Y. Isolation and identification of the first C-17 limonin epimer, epilimonin. J. Agric. Food Chem. 2008, 56, 5595–5598. [Google Scholar] [CrossRef] [PubMed]
- Manners, G.; Jacob, R.B.; Breksa, A.P.; Schoch, T.K.; Hasegawa, S. Bioavailability of citrus limonoids in humans. J. Agric. Food Chem. 2003, 51, 4156–4161. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Mandadi, K.K.; Poulose, S.M.; Jadegoud, Y.; Nagana Gowda, G.A.; Patil, B.S. Novel triterpenoid from Citrus aurantium L. possesses chemopreventive properties against human colon cancer cells. Bioorg. Med. Chem. 2008, 16, 5939–5951. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.D.; Hasegawa, S.; Herman, Z. Glucosides of acidic limonoids in Citrus. Phytochemistry 1989, 28, 2777–2781. [Google Scholar] [CrossRef]
- Dreyer, D.L.; Pickering, M.V.; Cohan, P. Distribution of limonoids in the rutaceae. Phytochemistry 1972, 11, 705–713. [Google Scholar] [CrossRef]
- Herman, Z.; Fong, C.H.; Hasegawa, S. Analysis of limonoids in citrus seeds. In Modern Methods of Plant Analysis: Seed Analysis; Linskens, H.F., Jackson, J.F., Eds.; Springer: Berlin, Germany, 1992; Volume 14, pp. 361–375. [Google Scholar]
- Hasegawa, S.; Berhow, M.A.; Fong, C.H. Analysis of bitter principles in Citrus. In Modern Methods of Plant Analysis: Fruit Analysis; Linskens, H.F., Jackson, J.F., Eds.; Springer: Berlin, Germany, 1996; Volume 18, pp. 59–80. [Google Scholar]
- Jayaprakasha, G.K.; Brodbelt, J.S.; Bhat, N.G.; Patil, B.S. Methods for the separation of limonoids from Citrus. In Potential Health Benefits of Citrus; ACS Symposium Series; Patil, B.S., Turner, N.D., Miller, E., Brodbelt, J., Eds.; Oxford University Press: Oxford, MS, USA, 2006; Volume 936, Chapter 3; pp. 34–51. [Google Scholar]
- Phetkul, U.; Phongpaichit, S.; Watanapokasin, R.; Mahabusarakam, W. New depside from Citrus reticulata Blanco. Nat. Prod. Res. 2014, 28, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Zandi, P.; Mirbagheri, E. Quantitation of limonin in Iranian orange juice concentrates using high-performance liquid chromatography and spectrophotometric methods. Eur. Food Res. Technol. 2005, 221, 202–207. [Google Scholar] [CrossRef]
- Zhao, P.; Duan, L.; Guo, L.; Dou, L.L.; Dong, X.; Zhou, P.; Li, P.; Liu, E.H. Chemical and biological comparison of the fruit extracts of Citrus wilsonii Tanaka and Citrus medica L. Food Chem. 2015, 173, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Li, S.L.; Yin, Z.Q.; Ye, W.C.; Zhang, Q.W. Simultaneous quantification of coumarins, flavonoids and limonoids in Fructus Citri Sarcodactylis by high performance liquid chromatography coupled with diode array detector. J. Pharm. Biomed. Anal. 2012, 66, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Bilal, H.; Akram, W.; Hassan, S.A.; Sahar, S.; Iqbal, M.M. Determination of limonin and nomilin contents in different citrus cultivar using high performance liquid chromatography. Pak. J. Sci. Ind. Res. Ser. B Biol. Sci. 2013, 56, 36–40. [Google Scholar]
- Liu, C.; Yan, F.; Gao, H.; He, M.; Wang, Z.; Cheng, Y.; Deng, X.; Xu, J. Features of citrus terpenoid production as revealed by carotenoid, limonoid and aroma profiles of two pummelos (Citrus maxima) with different flesh color. J. Sci. Food Agric. 2015, 95, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.D.; Chen, K.S.; Chen, Y.; Chen, Q.J. Contents and antioxidant capacity of limonin and nomilin in different tissues of citrus fruit of four cultivars during fruit growth and maturation. Food Chem. 2005, 93, 599–605. [Google Scholar] [CrossRef]
- McIntosh, C.A. Quantification of limonin and limonoate A-ring monolactone during growth and development of citrus fruit and vegetative tissues by radioimmunoassay. In Citrus Limonoids. Functional Chemicals in Agriculture and Foods; ACS Symposium Series 758; Berhow, M.A., Hasegawa, S., Manners, G.D., Eds.; American Chemical Society: Washington, DC, USA, 2000; Chapter 6; pp. 73–95. [Google Scholar]
- Hasegawa, S.; Ou, P.; Fong, C.H.; Herman, Z.; Coggins, C.W.; Atkin, D.R. Changes in the limonoate A-ring lactone and limonin 17-β-d-glucopyranoside content of navel oranges during fruit growth and maturation. J. Agric. Food Chem. 1991, 39, 262–265. [Google Scholar] [CrossRef]
- Fong, C.H.; Hasegawa, S.; Coggins, C.W.; Atkin, D.R.; Miyake, M. Contents of limonoids and limonin 17-β-d-glucopyranoside in fruit tissue of Valencia orange during fruit growth and maturation. J. Agric. Food Chem. 1992, 40, 1178–1181. [Google Scholar] [CrossRef]
- Breksa, A.P.; Zukas, A.A.; Manners, G.D. Determination of limonoate and nomilinoate A-ring lactones in citrus juices by liquid chromatography–electrospray ionization mass spectrometry. J. Chromatogr. A 2005, 1064, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Raman, G.; Cho, M.; Brodbelt, J.S.; Patil, B.S. Isolation and purification of closely related Citrus limonoid glucosides by flash chromatography. Phytochem. Anal. 2005, 16, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Breksa, A.P.; Hidalgo, M.B.; Yuen, M.L. Liquid chromatography–electrospray ionisation mass spectrometry method for the rapid identification of citrus limonoid glucosides in citrus juices and extracts. Food Chem. 2009, 117, 739–744. [Google Scholar] [CrossRef]
- Breksa, A.P.; Dragull, K. Development and validation of a decigram-scale method for the separation of limonin from limonin glucoside by C18 flash chromatography. Food Chem. 2009, 113, 1308–1313. [Google Scholar] [CrossRef]
- Vikram, A.; Jayaprakasha, G.K.; Patil, B.S. Simultaneous determination of citrus limonoid aglycones and glucosides by high performance liquid chromatography. Anal. Chim. Acta 2007, 590, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Dandekar, D.V.; Tichy, S.E.; Patil, B.S. Simultaneous separation and identification of limonoids from citrus using liquid chromatography-collision-induced dissociation mass spectra. J. Sep. Sci. 2011, 34, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Breksa, A.P.; Ibarra, P. Colorimetric method for the estimation of total limonoid aglycones and glucoside contents in citrus juices. J. Agric. Food Chem. 2007, 55, 5013–5017. [Google Scholar] [CrossRef] [PubMed]
- Breksa, A.P.; Kahn, T.; Zukas, A.A.; Hidalgo, M.B.; Lee Yuen, M. Limonoid content of sour orange varieties. J. Sci. Food Agric. 2011, 91, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Minamisawa, M.; Yoshida, S.; Uzawa, A. The functional evaluation of waste yuzu (Citrus junos) seeds. Food Funct. 2014, 5, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Kuroyanagi, M.; Ishii, H.; Kawahara, N.; Sugimoto, H.; Yamada, H.; Okihara, K.; Shirota, O. Flavonoid glycosides and limonoids from Citrus molasses. J. Nat. Med. 2008, 62, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Arbona, V.; Iglesias, D.J.; Gómez-Cadenas, A. Non-targeted metabolite profiling of citrus juices as a tool for variety discrimination and metabolite flow analysis. BMC Plant Biol. 2015, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Vaclavik, L.; Schreiber, A.; Lacina, O.; Cajka, T.; Hajslova, J. Liquid chromatography–mass spectrometry-based metabolomics for authenticity assessment of fruit juices. Metabolomics 2012, 8, 793–803. [Google Scholar] [CrossRef]
- Pan, Z.; Li, Y.; Deng, X.; Xiao, S. Non-targeted metabolomic analysis of orange (Citrus sinensis [L.] Osbeck) wild type and bud mutant fruits by direct analysis in real-time and HPLC-electrospray mass spectrometry. Metabolomics 2014, 10, 508–523. [Google Scholar] [CrossRef]
- Ledesma-Escobar, C.A.; Priego-Capote, F.; Luque de Castro, M.D. Characterization of lemon (Citrus limon) polar extract by liquid chromatography–tandem mass spectrometry in high resolution mode. J. Mass Spectrom. 2015, 50, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Li, Y.; Zuo, R.; Wang, H.J.; Si, N.; Zhao, H.Y.; Han, L.Y.; Yang, J.; Bian, B.L. Species-related difference between limonin and obacunone among five liver microsomes and zebrafish using ultra-high-performance liquid chromatography coupled with a LTQ-Orbitrap mass spectrometer. Rapid Commun. Mass Spectrom. 2014, 28, 2292–2300. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Xin, S.K.; Han, L.Y.; Zuo, R.; Li, Y.; Gong, M.X.; Wei, X.L.; Zhou, Y.Y.; He, J.; Wang, H.J.; et al. Comparative metabolism of four limonoids in human liver microsomes using ultra-high-performance liquid chromatography coupled with high-resolution LTQ-Orbitrap mass spectrometry. Rapid Commun. Mass Spectrom. 2015, 29, 2045–2056. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Miller, E.G.; Jayaprakasha, G.K.; Patil, B.S. An improved HPLC method for the analysis of citrus limonoids in culture media. J. Chromatogr. B 2007, 846, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Activity-guided isolation and identification of antioxidant components from ethanolic extract of Peltiphyllum peltatum (Torr.) Engl. Nat. Prod. Commun. 2008, 3, 1321–1324. [Google Scholar]
- Habtemariam, S. Activity-guided isolation and identification of free Radical-scavenging components from ethanolic extract of Boneset (Leaves of Eupatorium perfoliatum). Nat. Prod. Commun. 2008, 3, 1317–1320. [Google Scholar]
- Habtemariam, S. Methyl-3-O-Methyl Gallate and Gallic Acid from the Leaves of Peltiphyllum peltatum: Isolation and Comparative Antioxidant, Prooxidant, and Cytotoxic Effects in Neuronal Cells. J. Med. Food 2011, 14, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S.; Cowley, R.A. Antioxidant and anti-α-glucosidase compounds from the rhizome of Peltiphyllum peltatum (Torr.) Engl. Phytother. Res. 2012, 26, 1656–1660. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S.; Dagne, E. Comparative antioxidant, prooxidant and cytotoxic activity of sigmoidin A and eriodictyol. Planta Med. 2010, 76, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Investigation into the antioxidant and antidiabetic potential of Moringa stenopetala: Identification of the active principles. Nat. Prod. Commun. 2015, 10, 475–478. [Google Scholar] [PubMed]
- Habtemariam, S.; Varghese, G.K. A Novel Diterpene Skeleton: Identification of a highly aromatic, cytotoxic and antioxidant 5-methyl-10-demethyl-abietane-type diterpene from Premna serratifolia. Phytother. Res. 2015, 29, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Juan-Badaturugea, M.; Habtemariam, S.; Jackson, C.; Thomas, M.J.K. Antioxidant principles of Tanacetum vulgare L. aerial part. Nat. Prod. Commun. 2009, 4, 1561–1564. [Google Scholar]
- Juan-Badaturuge, M.; Habtemariam, S.; Thomas, M.J.K. Antioxidant compounds from a South Asian beverage and medicinal plant, Cassia auriculata. Food Chem. 2011, 125, 221–225. [Google Scholar] [CrossRef]
- Roselli, M.; Lentini, G.; Habtemariam, S. Phytochemical, antioxidant and anti-alpha-glucosidase activity evaluations of Bergenia cordifolia. Phytother. Res. 2012, 26, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S.; Dagne, E. Differential cytotoxic and prooxidnant activity of knipholone and knipholone anthrone. Planta Med. 2009, 75, 885–885. [Google Scholar] [CrossRef]
- Habtemariam, S. Cytotoxicity and immunosuppressive activity of withanolides from Discopodium penninervium. Planta Med. 1997, 63, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Cytotoxicity of diterpenes from Premna schimperi and Premna oligotricha. Planta Med. 1995, 61, 368–369. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Cytotoxic and cytostatic activity of erlangerins from Commiphora erlangeriana. Toxicon 2003, 41, 723–727. [Google Scholar] [CrossRef]
- Mireku, E.A.; Mensah, A.Y.; Mensah, M.L.K.; Tocher, D.A.; Habtemariam, S. Anti-inflammatory properties of the stem-bark of Anopyxis kalineana and its major constituent, methyl angolensate. Phytother. Res. 2014, 28, 1855–1860. [Google Scholar] [CrossRef] [PubMed]
- Cavalluzzi, M.M.; Viale, M.; Bruno, C.; Carocci, A.; Catalano, A.; Carrieri, A.; Franchini, C.; Lentini, G. A convenient synthesis of lubeluzole and its enantiomer: Evaluation as chemosensitizing agents on human ovarian adenocarcinoma and lung carcinoma cells. Bioorg. Med. Chem. Lett. 2013, 23, 4820–4823. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Naruko, A.; Nakazawa, Y.; Zhao, L.; Hayashi, Y.; Hirama, M. Total synthesis of limonin. Angew. Chem. Int. Ed. 2015, 54, 8538–8541. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Cao, J.; Luo, F.; Wang, D.; Chen, W.; Li, X.; Sun, C.; Chen, K. Simultaneous purification of limonin, nomilin and isoobacunoic acid from Pomelo Fruit (Citrus grandis) segment membrane. J. Food Sci. 2014, 79, C1956–C1963. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, R.L.D.; Jiménez, R.A.A.; Rueda, L.E.A.; Méndez, A.J.J.; Murillo, A.W. Relationship between content of limonin in citrus waste and antifeedant activity against Spodoptera frugiperda. Rev. Colomb. Entomol. 2014, 40, 164–169. [Google Scholar]
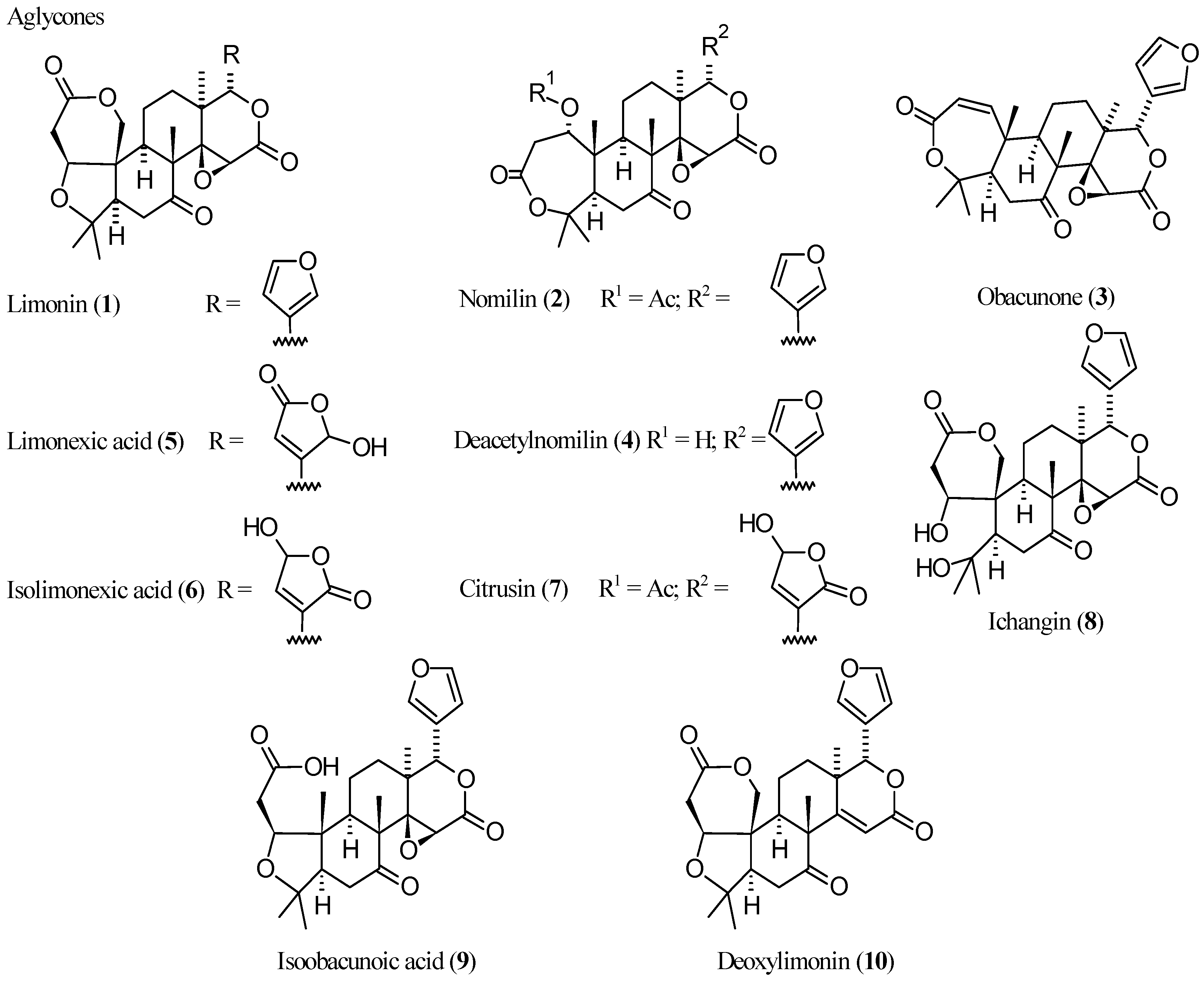
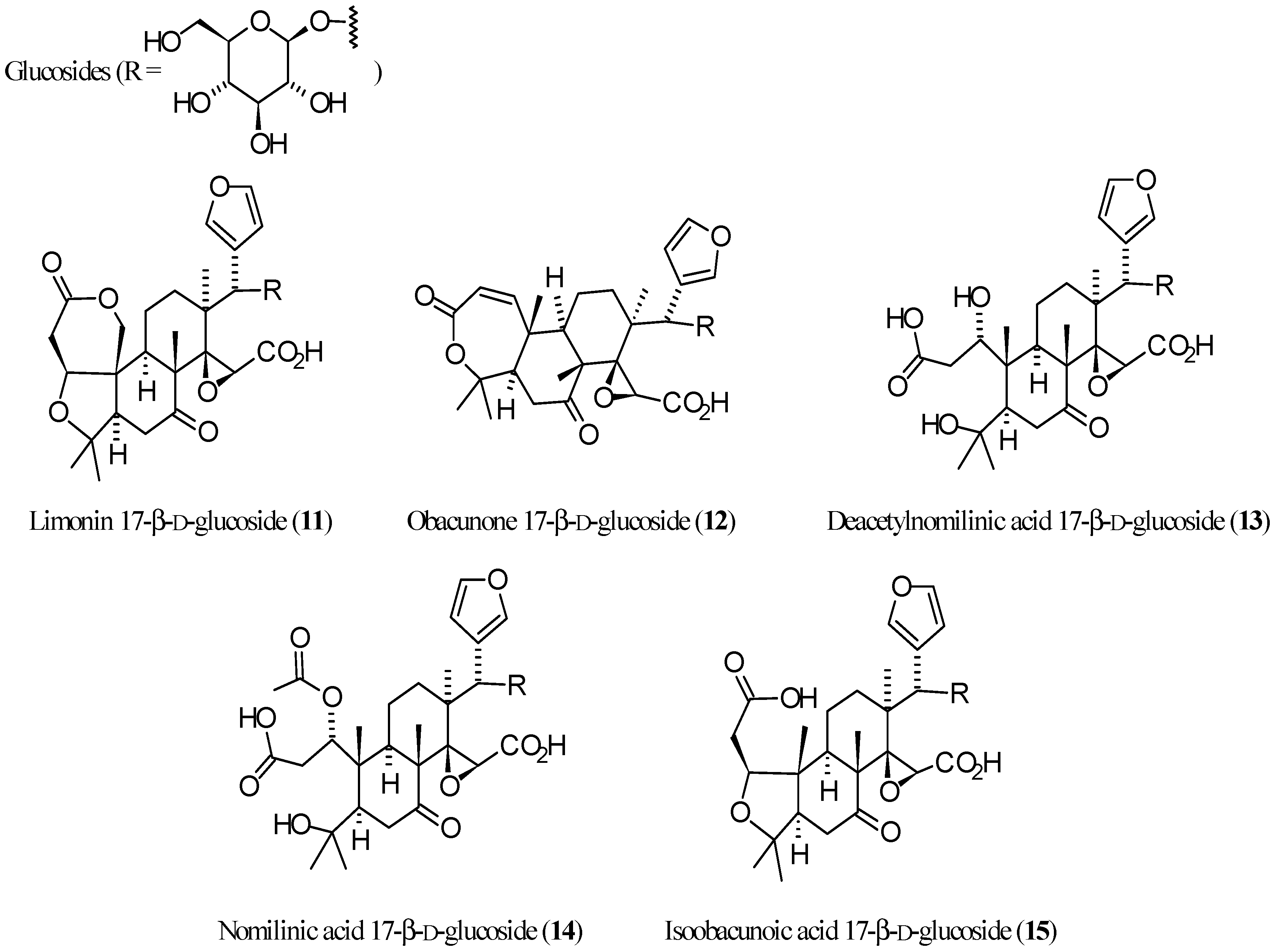
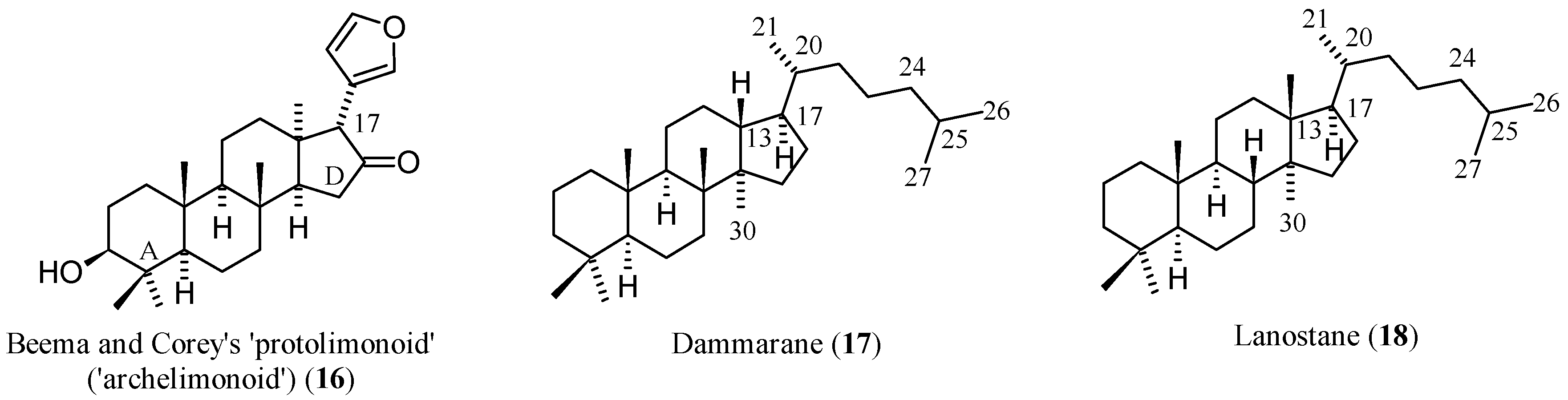
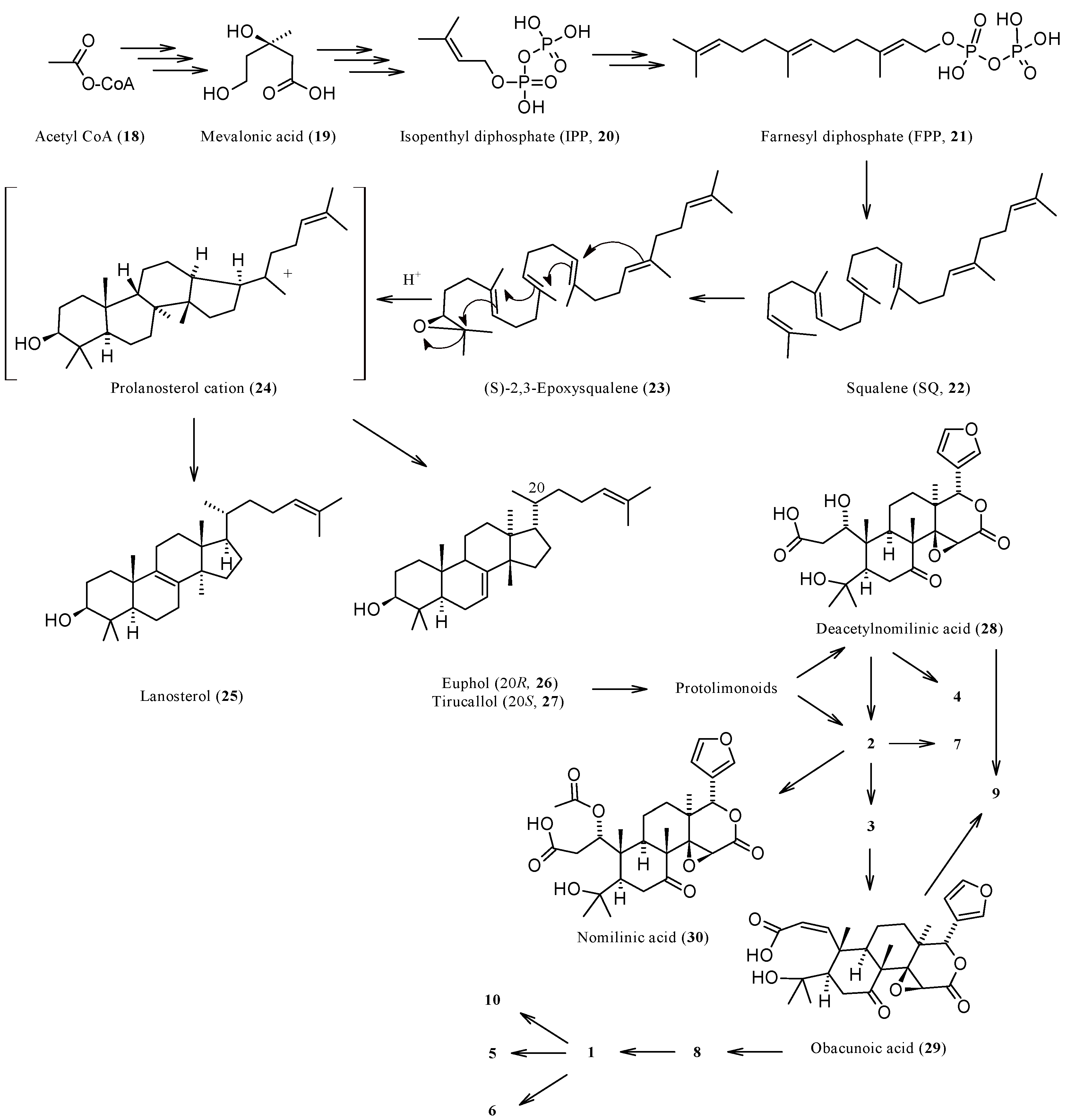










| Compound | Name | Molecular Formula | n′ | n′′ | R1 | R2 | R3 | R4 | R5 | R6 | X | Y | Z | Main Functional Groups | Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Limonin | C26H30O8 | 2 | 1 | O | Cycle bond | Cycle bond | O–CH=CH | Dilactone | - | |||||
| 2 | Nomilin | C28H34O9 | 2 | 1 | Cycle bond | OAc | Cycle bond | H | Cycle bond | O–CH=CH | Dilactone, acetic ester | - | |||
| 3 | Obacunone | C26H30O7 | 1 | 1 | Cycle bond | - | Cycle bond | H | Cycle bond | O–CH=CH | Dilactone | Dehydrated analog of 4 | |||
| 4 | Deacetylnomilin | C26H32O8 | 2 | 1 | Cycle bond | OH | Cycle bond | H | Cycle bond | O–CH=CH | Dilactone | Deacetyl-derivative of 2 | |||
| 5 | Limonexic acid | C26H30O10 | 2 | 1 | O | Cycle bond | Cycle bond | CO–O–CHOH | Dilactone, pseudoacid | Hydroxybutenolide analog of 1 | |||||
| 6 | Isolimonexic acid | C26H30O10 | 2 | 1 | O | Cycle bond | Cycle bond | CHOH–O–CO | Dilactone, pseudoacid | Constitutional isomer of 5 | |||||
| 7 | Citrusin | C28H34O11 | 2 | 1 | Cycle bond | OAc | Cycle bond | H | Cycle bond | CHOH–O–CO | Dilactone, acetic ester, pseudoacid | Hydroxybutenolide analog of 2 | |||
| 8 | Ichangin | C26H32O9 | 2 | 1 | OH | OH | Cycle bond | Cycle bond | O–CH=CH | Dilactone | Spiro analog of 1 | ||||
| 9 | Isoobacunoic acid | C26H32O8 | 2 | 1 | O | H | H | Cycle bond | O–CH=CH | Lactone, carboxylic acid | Product of formal reductive cleavage of 1 | ||||
| 10 | Deoxylimonin | C26H30O7 | 2 | 0 | O | Cycle bond | Cycle bond | O–CH=CH | Dilactone | Deoxidized analog of 1 | |||||
| 11 | Limonin 17-β-d-glucoside | C32H42O14 | 2 | 1 | O | Cycle bond | Oglc 1 | H | O–CH=CH | Lactone, carboxylic acid | 17-β-d-Glucopyranoside of 1 | ||||
| 12 | Obacunone 17-β-d-glucoside | C32H42O13 | 1 | 1 | Cycle bond | - | Cycle bond | H | Oglc 1 | H | O–CH=CH | Lactone, carboxylic acid | 17-β-d-Glucopyranoside of 3 | ||
| 13 | Deacetylnomilinic acid 17-β-d-glucoside | C32H46O15 | 2 | 1 | OH | OH | H | H | Oglc 1 | H | O–CH=CH | Dicarboxylic acid | 17-β-d-Glucopyranoside of hydrolyzed 4 | ||
| 14 | Nomilinic acid 17-β-d-glucoside | C34H48O16 | 2 | 1 | OH | OAc | H | H | Oglc 1 | H | O–CH=CH | Dicarboxylic acid, acetic ester | Ac-derivative of 13 | ||
| 15 | Isoobacunoic acid 17-β-d-glucoside | C32H44O14 | 2 | 1 | O | H | H | Oglc 1 | H | O–CH=CH | Dicarboxylic acid | 17-β-d-Glucopyranoside of 9 | |||
| Compound | Name (Other Trivial Names) | CAS Number | MW | Acidity 1 | Log P 1 | Log D 1 (pH 7.4) | Most Studied Biological Activities |
|---|---|---|---|---|---|---|---|
| 1 | Limonin (citrolimonin, dictamnolactone, evodin, obaculactone) | 1180-71-8 | 470 | pKa > 8 | 1.66 | 1.66 | Analgesic [45], anticancer [46,47,48,49,50,51,52,53,54,55,56,57,58,59], anti-inflammatory [45,60,61,62], antibacterial [63,64], antioxidant [4,65,66], antiviral [67], larvicidal [68,69] |
| 2 | Nomilin | 1063-77-0 | 514 | pKa > 8 | 2.47 | 2.47 | Anticancer [46,47,58,59,70], anti-hyperglycemic [71], anti-inflammatory [25,62], antioxidant [4,65,66], antiviral [67], larvicidal [68,69], osteoclastogenesis inhibition [72] |
| 3 | Obacunone (casimirolide, obacunon, tricoccin S3) | 753-03-1 | 454 | pKa > 8 | 2.91 | 2.91 | Anticancer [46,47,58,73,74,75], antibacterial [76,77] anti-hyperglycemic [78], antioxidant [66], larvicidal [79,80] |
| 4 | Deacetylnomilin (isolimonin, deacetylnomilinate) | 3264-90-2 | 472 | pKa > 8 | 1.57 | 1.57 | Anticancer [58,70], antioxidant [66] |
| 5 | Limonexic acid (limonexin, shihulimonin A, substance X) 2 | 99026-99-0 | 502 | 6 ≤ pKa ≤ 8 | −0.93 | −0.7 | Anticancer [28,48,52,81], antioxidant [28], hepatoprotection [82] |
| 6 | Isolimonexic acid 3 | 113164-90-2 | 502 | 6 ≤ pKa ≤ 8 | −0.93 | −0.7 | Anticancer [48,52] |
| 7 | Citrusin 3 | 108943-57-3 | 546 | 6 ≤ pKa ≤ 8 | −0.04 | 0.24 | Anti-inflammatory [25] |
| 8 | Ichangin | 10171-61-6 | 488 | pKa > 8 | 0.13 | 0.13 | Antibacterial [83,84] |
| 9 | Isoobacunoic acid (iso-obacunoic acid) | 751-28-0 | 472 | 4 ≤ pKa < 6 | 2.33 | −1.03 | Antibacterial [83] |
| 10 | Deoxylimonin (desoxylimonin) | 989-23-1 | 454 | pKa > 8 | 2.34 | 2.34 | Analgesic [45], anti-inflammatory [45] |
| 11 | Limonin 17-β-d-glucoside (limonin 17-β-d-glucopyranoside, glucosyl-limonin) | 123564-61-4 | 650 | 4 ≤ pKa < 6 | −0.46 | −3.66 | Anticancer [52,85,86,87], antioxidant [66,86], hepatoprotection [88] |
| 12 | Obacunone 17-β-d-glucoside (obacunone 17-β-d-glucopyranoside) 4 | 123564-64-7 | 635 | 4 ≤ pKa < 6 | 0.79 | −2.61 | Anticancer [75,76,86,89], antioxidant [86,89] |
| 13 | Deacetylnomilinic acid 17-β-d-glucoside (deacetylnomilinic acid 17-β-d-glucopyranoside) 4 | 125107-16-6 | 671 | pKa < 4 | −2.2 | −5.9 | Anticancer [89], antioxidant [89] |
| 14 | Nomilinic acid 17-β-d-glucoside (nomilinic acid 17-β-d-glucopyranoside) 4 | 125107-15-5 | 713 | pKa < 4 | 0.04 | −4.42 | Anticancer [46,89], antioxidant [89] |
| 15 | Isoobacunoic acid 17-β-d-glucoside (isoobacunoic acid 17-β-d-glucopyranoside) 4 | 125225-95-8 | 653 | pKa < 4 | 0.21 | −3.49 | Antibacterial [83] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gualdani, R.; Cavalluzzi, M.M.; Lentini, G.; Habtemariam, S. The Chemistry and Pharmacology of Citrus Limonoids. Molecules 2016, 21, 1530. https://doi.org/10.3390/molecules21111530
Gualdani R, Cavalluzzi MM, Lentini G, Habtemariam S. The Chemistry and Pharmacology of Citrus Limonoids. Molecules. 2016; 21(11):1530. https://doi.org/10.3390/molecules21111530
Chicago/Turabian StyleGualdani, Roberta, Maria Maddalena Cavalluzzi, Giovanni Lentini, and Solomon Habtemariam. 2016. "The Chemistry and Pharmacology of Citrus Limonoids" Molecules 21, no. 11: 1530. https://doi.org/10.3390/molecules21111530
APA StyleGualdani, R., Cavalluzzi, M. M., Lentini, G., & Habtemariam, S. (2016). The Chemistry and Pharmacology of Citrus Limonoids. Molecules, 21(11), 1530. https://doi.org/10.3390/molecules21111530









