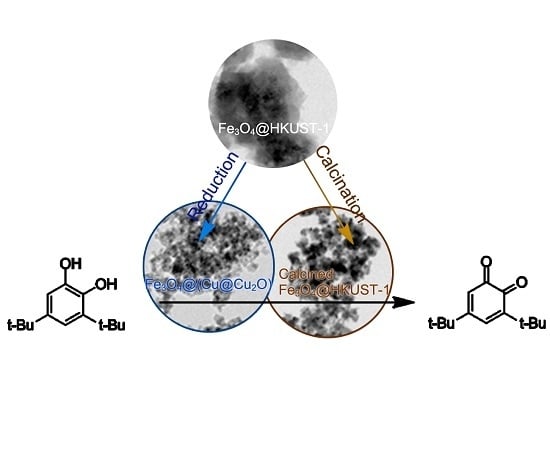
Preparation of Cu@Cu2O Nanocatalysts by Reduction of HKUST-1 for Oxidation Reaction of Catechol
Abstract
:1. Introduction
2. Results and Discussion
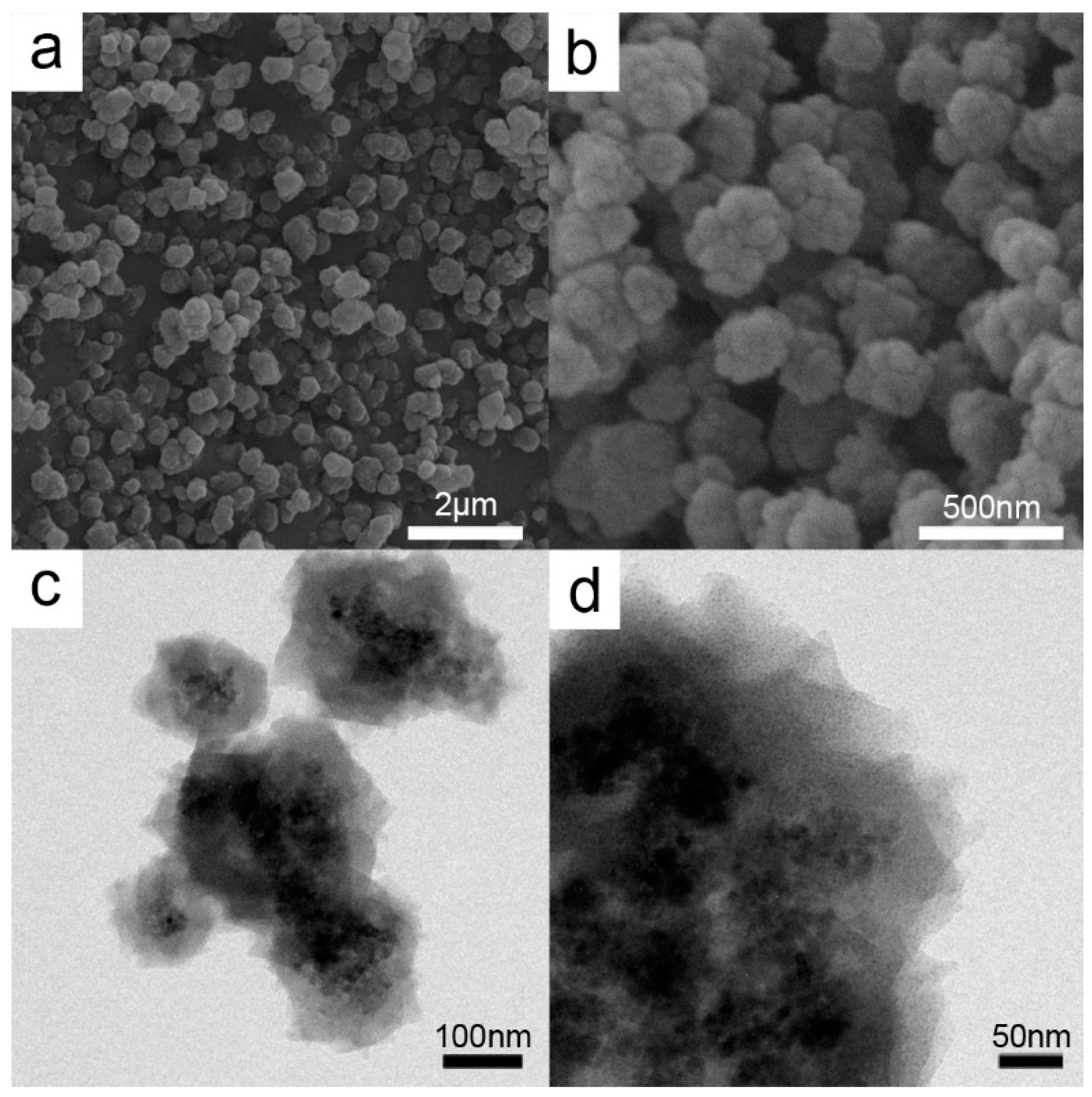
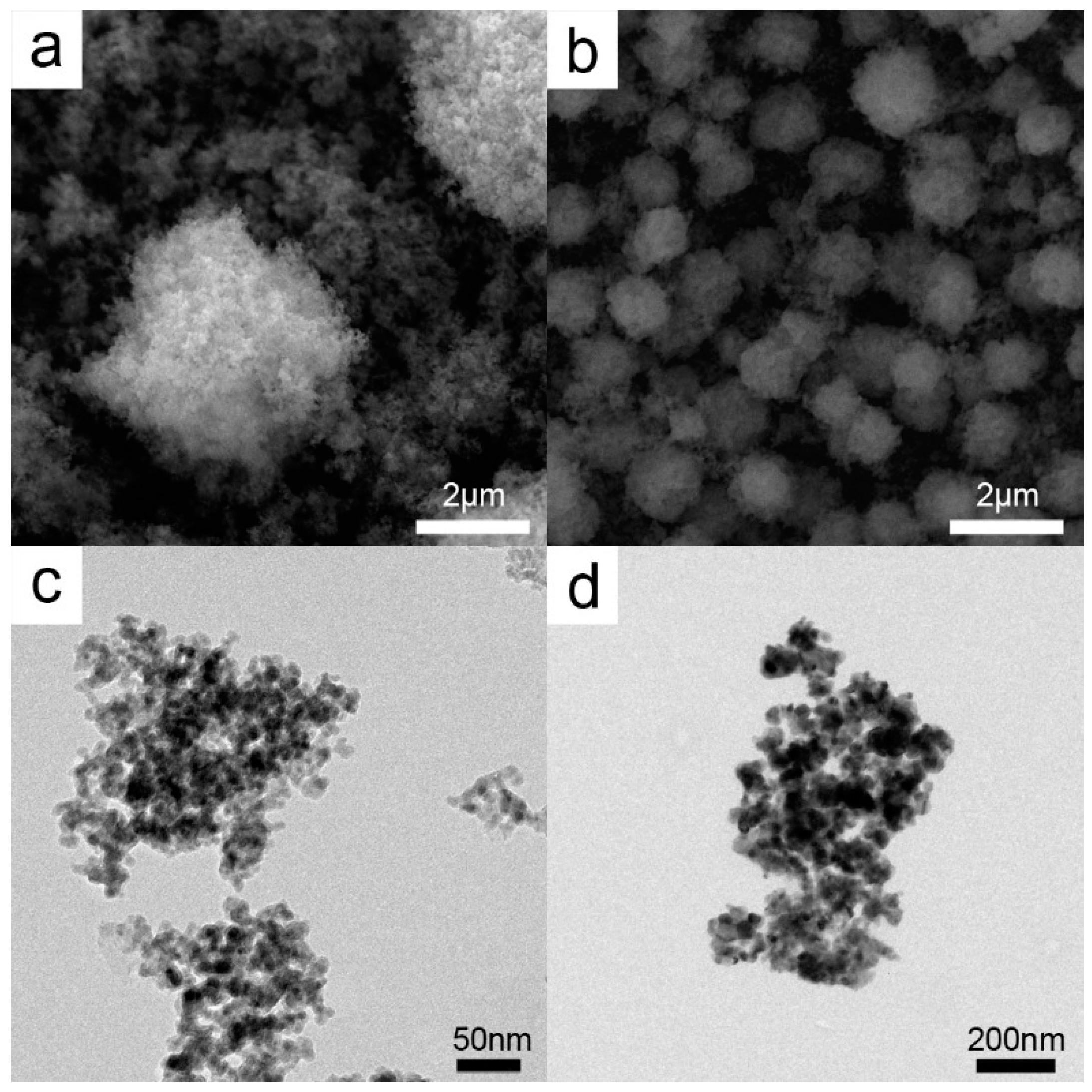
2.1. Catalyst Characterization
2.2. Oxidation of 3,5-di-tert-Butylcatechol Catalyzed by HKUST-1 Structure
3. Materials and Methods
3.1. General Remarks
3.2. Synthesis of Nanoparticles
3.2.1. Synthesis of Fe3O4 Nanospheres
3.2.2. Synthesis of Fe3O4@HKUST-1 Core-Shell Structure
3.2.3. Synthesis of Cu@Cu2O Core-Shell Structures
3.2.4. Synthesis of Calcined HKUST-1 Structures
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MOF | Metal organic framework |
References
- Reboul, J.; Furukawa, S.; Horike, N.; Tsotsalas, M.; Hirai, K.; Uehara, H.; Kondo, M.; Louvain, N.; Sakata, O.; Kitagawa, S. Mesoscopic architectures of porous coordination polymers fabricated by pseudomorphic replication. Nat. Mater. 2012, 11, 717–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.S.; Liang, F.Y.; Bux, H.; Feldhoff, A.; Yang, W.S.; Caro, J. Molecular Sieve Membrane: Supported Metal-Organic Framework with High Hydrogen Selectivity. Angew. Chem. Int. Ed. 2010, 49, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Stavila, V.; Talind, A.A.; Allendorf, M.D. MOF-based electronic and opto-electronic devices. Chem. Soc. Rev. 2014, 43, 5994–6010. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal-Organic Frameworks in Biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Hu, A.; Zhang, L.; Lin, W. A Homochiral Porous Metal-Organic Framework for Highly Enantioselective Heterogeneous Asymmetric Catalysis. J. Am. Chem. Soc. 2005, 127, 8940–8941. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Deactivation of Cu3(BTC)2 in the Synthesis of 2-Phenylquinoxaline. Catal. Lett. 2015, 145, 1600–1605. [Google Scholar] [CrossRef]
- Ameloot, R.; Gobechiya, E.; Uji-i, H.; Martens, J.A.; Hofkens, J.; Alaerts, L.; Sels, B.F.; de Vos, D.E. Direct Patterning of Oriented Metal-Organic Framework Crystals via Control over Crystallization Kinetics in Clear Precursor Solutions. Adv. Mater. 2010, 22, 2685–2688. [Google Scholar] [CrossRef] [PubMed]
- Arslan, H.K.; Shekhah, O.; Wohlgemuth, J.; Franzreb, M.; Fischer, R.A.; Woll, C. High-Throughput Fabrication of Uniform and Homogeneous MOF Coatings. Adv. Funct. Mater. 2011, 21, 4228–4231. [Google Scholar] [CrossRef]
- Keitz, B.K.; Yu, C.J.; Long, J.R.; Ameloot, R. Lithographic Deposition of Patterned Metal-Organic Framework Coatings Using a Photobase Generator. Angew. Chem. Int. Ed. 2014, 53, 5561–5565. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Li, S.Z.; Guo, Z.; Farha, O.K.; Hauser, B.G.; Qi, X.Y.; Wang, Y.; Wang, X.; Han, S.Y.; Liu, X.G.; et al. Imparting functionality to a metal-organic framework material by controlled nanoparticle encapsulation. Nat. Chem. 2012, 4, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Ke, F.; Qiu, L.-G.; Zhu, J. Fe3O4@MOF core-shell magnetic microspheres as excellent catalysts for the Claisen-Schmidt condensation reaction. Nanoscale 2014, 6, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Ke, F.; Wang, L.; Zhu, J. Multifunctional Au-Fe3O4@MOF core-shell nanocomposite catalysts with controllable reactivity and magnetic recyclability. Nanoscale 2015, 7, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Chui, S.S.Y.; Lo, S.M.F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3]n. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Schlichte, K.; Kratzke, T.; Kaskel, S. Improved synthesis, thermal stability and catalytic properties of the metal-organic framework compound Cu3(BTC)2. Microporous Mesoporous Mater. 2004, 73, 81–88. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Metal-Organic Frameworks as Efficient Heterogeneous Catalysts for the Regioselective Ring Opening of Epoxides. Chem. Eur. J. 2010, 16, 8530–8536. [Google Scholar] [CrossRef] [PubMed]
- Pascanu, V.; Yao, Q.X.; G´omez, A.B.; Gustafsson, M.; Yun, Y.F.; Wan, W.; Samain, L.; Zou, X.D.; Martín-Matute, B. Sustainable Catalysis: Rational Pd Loading on MIL-101Cr-NH2 for more efficient and recyclable Suzuki-Miyaura reactions. Chem. Eur. J. 2013, 19, 17483–17493. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, T.; Wu, W.; Jiang, S.; Zhang, H.; Li, B. Water-mediated promotion of direct oxidation of benzene over the metal-organic framework HKUST-1. RSC Adv. 2015, 5, 56020–56027. [Google Scholar] [CrossRef]
- Caillet, S.; Yu, H.L.; Lassard, S.; Lamoureux, G.; Ajdukovic, D.; Lacroix, M. Fenton reaction applied for screening natural antioxidants. Food Chem. 2007, 100, 542–552. [Google Scholar] [CrossRef]
- Bukowska, B.; Kowalska, S. Phenol and Catechol induce prehaemolytic and haemolytic changes in human erythrocytes. Toxicol. Lett. 2004, 152, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, S.; Hirosawa, I.; Hirakawa, K.; Kawanishi, S. Site specificity and mechanism of oxidative DNA damage induced by carcino carcinogenic catechol. Carcinogenesis 2001, 22, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Kei, F.; Qui, L.-G.; Yuan, Y.-P.; Jiang, X.; Zhu, J.-F. Fe3O4@MOF core-shell magnetic microspheres with a designable metal-organic framework shell. J. Mater. Chem. 2012, 22, 9497–9500. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.

| Entry | Catalyst | Temperature (°C) | Time (h) | Yield (%) 2 |
|---|---|---|---|---|
| 1 | - | 30 | 2.5 | 5 |
| 2 | 5.0 mol % Cu@Cu2O | 30 | 2.5 | 100 |
| 3 | 2.5 mol % Cu@Cu2O | 30 | 0.75 | 100 |
| 4 | 2.5 mol % Cu@Cu2O | 30 | 0.5 | 71 |
| 5 | 2.5 mol % HKUST-1 | 30 | 0.75 | 0 |
| 6 | 2.5 mol % Calcined HKUST-1 | 50 | 2.5 | 68 |
| 7 | 5.0 mol % Calcined HKUST-1 | 60 | 3.0 | 92 |
| 8 | 5.0 mol % Calcined HKUST-1 | 70 | 3.0 | 94 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.; Yoon, C.; Lee, J.M.; Park, S.; Park, K.H. Preparation of Cu@Cu2O Nanocatalysts by Reduction of HKUST-1 for Oxidation Reaction of Catechol. Molecules 2016, 21, 1467. https://doi.org/10.3390/molecules21111467
Jang S, Yoon C, Lee JM, Park S, Park KH. Preparation of Cu@Cu2O Nanocatalysts by Reduction of HKUST-1 for Oxidation Reaction of Catechol. Molecules. 2016; 21(11):1467. https://doi.org/10.3390/molecules21111467
Chicago/Turabian StyleJang, Seongwan, Chohye Yoon, Jae Myung Lee, Sungkyun Park, and Kang Hyun Park. 2016. "Preparation of Cu@Cu2O Nanocatalysts by Reduction of HKUST-1 for Oxidation Reaction of Catechol" Molecules 21, no. 11: 1467. https://doi.org/10.3390/molecules21111467