Human Lysozyme Synergistically Enhances Bactericidal Dynamics and Lowers the Resistant Mutant Prevention Concentration for Metronidazole to Helicobacter pylori by Increasing Cell Permeability
Abstract
:1. Introduction
2. Results
2.1. H. pylori Was Insensitive to Lysozyme
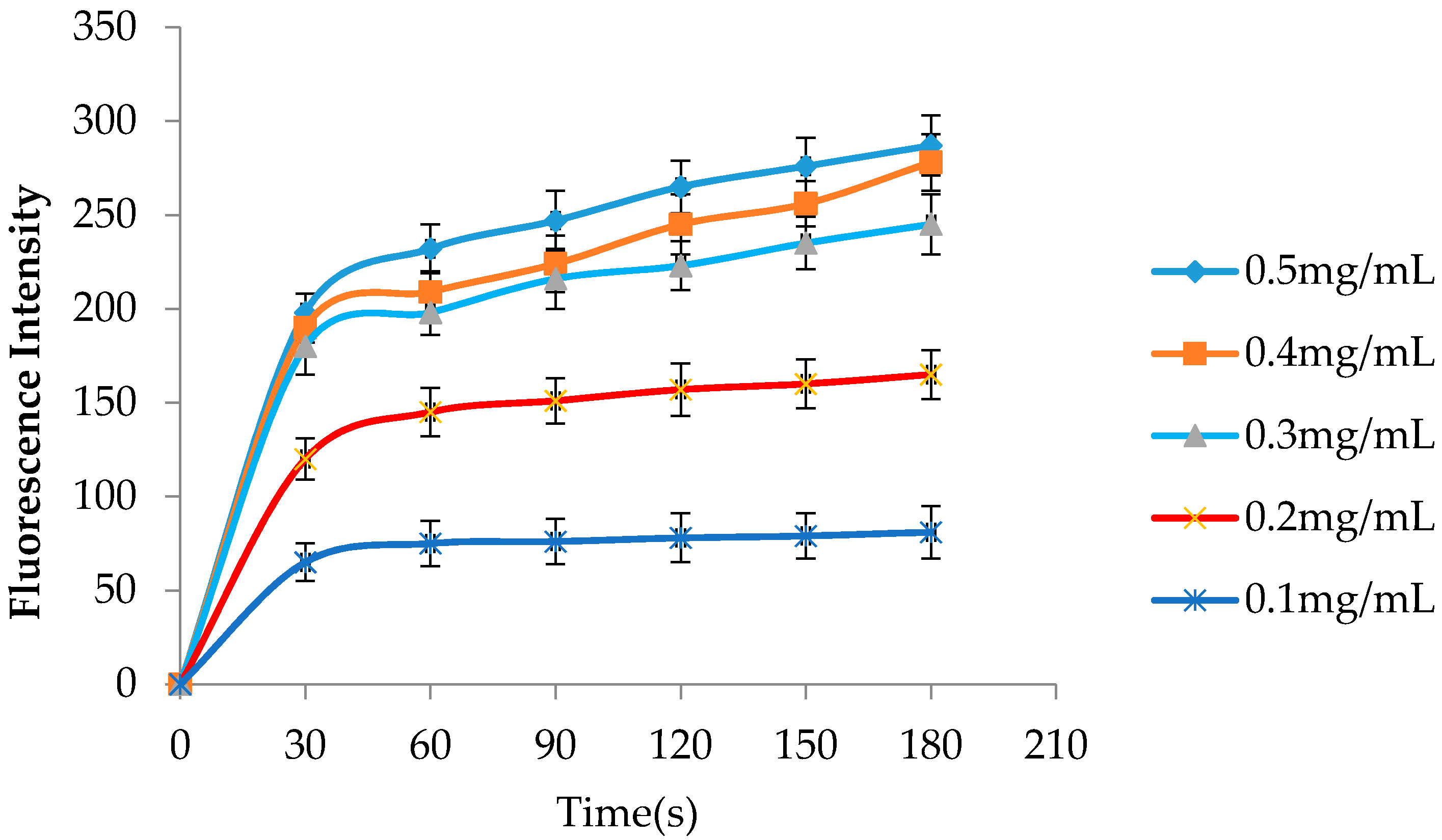
2.2. hLYS Promotes Increased Outer Membrane Penetration
2.3. hLYS Can Disrupt the Integrity of the Inner Membrane
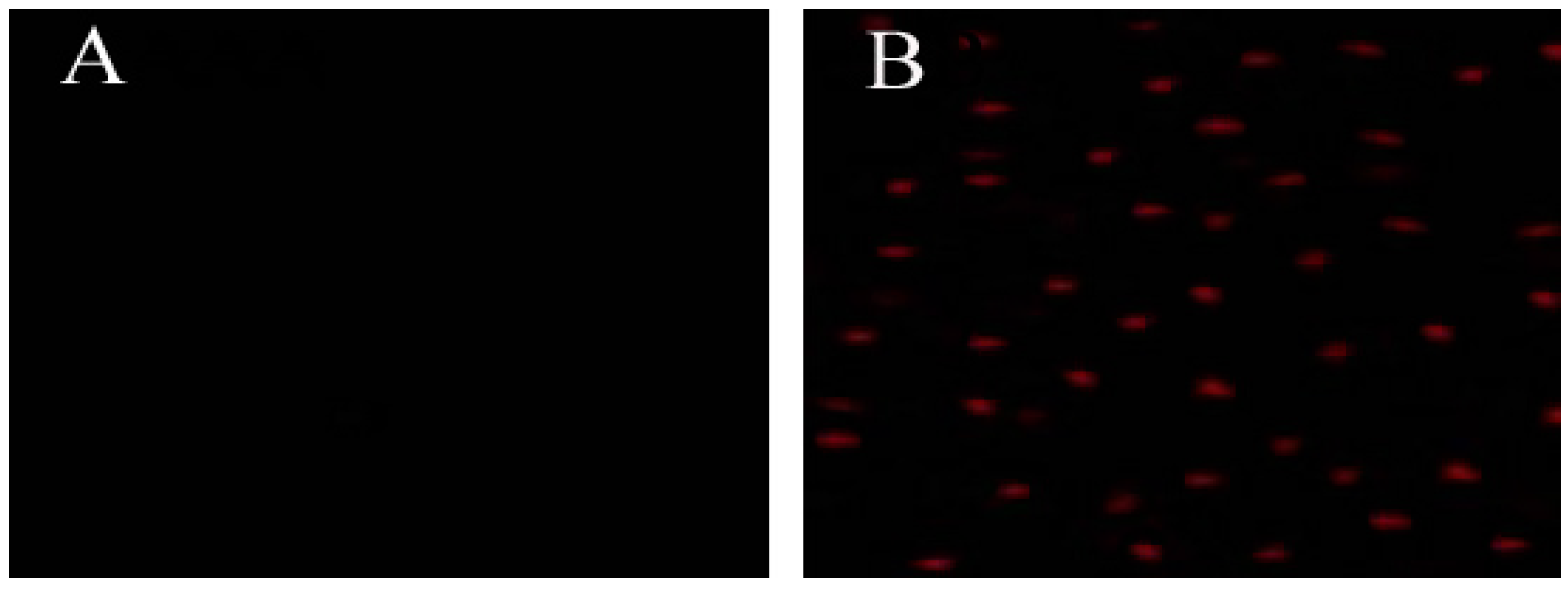
2.4. hLYS Could Promote PI Uptake by Cells
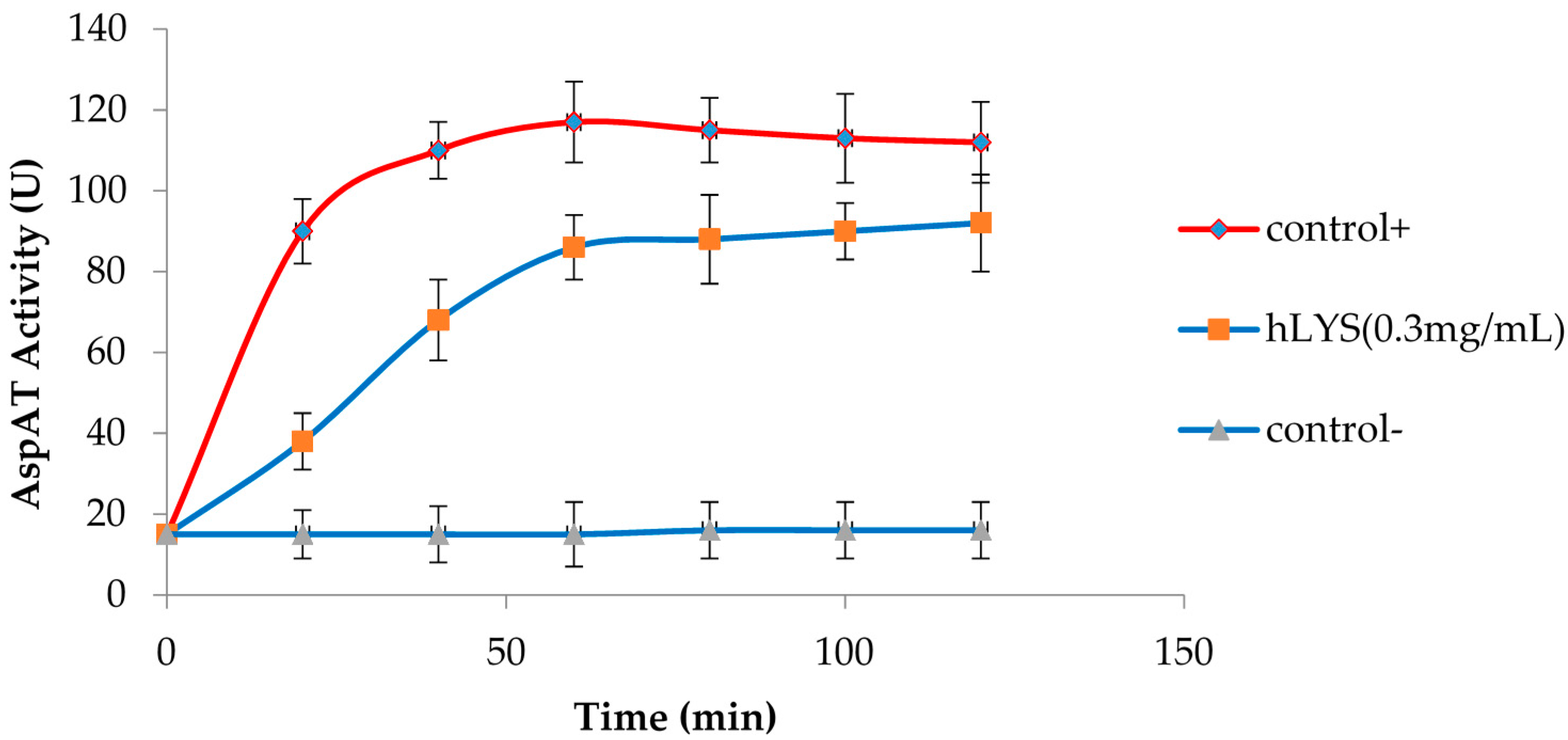
2.5. Bactericidal Kinetics of hLYS and Metronidazole Treatment
2.6. Effect of hLYS on the MPC and MIC of Metronidazole to H. pylori
3. Discussion
4. Materials and Methods
4.1. Bacterial Strain and Chemicals
4.2. Culture Conditions for H. pylori
4.3. Cell Survival Assays to Determine hLYS Sensitivity
4.4. Permeabilization Assay of the Outer Membrane (OM)
4.5. Integrity Assay of the Inner Membrane (IM)
4.6. Cell Uptake Assay
4.7. Bactericidal Kinetics Activity Based on hLYS and Metronidazole Synergy
4.8. Measurement of MPC
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moayyedi, P.; Hunt, R.H. Helicobacter pylori public health implications. Helicobacter 2004, 9, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Chan, F.K.; McColl, K.E. Peptic ulcer disease. Lancet 2009, 374, 1449–1461. [Google Scholar] [CrossRef]
- Kuipers, E.J. H. pylori and the risk and management of associated diseases: Gastritis, ulcer disease, atrophic gastritis and gastric cancer. Aliment. Pharmacol. Ther. 1997, 11, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, R.C.; Zheng, Y.X.; Zhao, S.S.; Li, N.; Zhou, R.R.; Huang, Y.; Huang, Z.B.; Fan, X.G. Helicobacter pylori infection may increase the risk of progression of chronic hepatitis B disease among the Chinese population: A meta-analysis. Int. J. Infect. Dis. 2016, 50, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhao, J.; Cheng, W.F.; Shi, W.J.; Liu, W.; Pan, X.L.; Zhang, G.X. Efficacy of H. pylori eradication therapy on functional dyspepsia: A meta-analysis of randomized controlled studies with 12-month follow-up. J. Clin. Gastroenterol. 2014, 48, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Guo, S.; Zhang, Y. Treatment of gastric MALT lymphoma with a focus on H. pylori eradication. Int. J. Hematol. 2013, 97, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Shiotani, A.; Cen, P.; Graham, D.Y. Eradication of gastric cancer is now both possible and practical. Semin. Cancer Biol. 2013, 23, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Atherton, J.; Axon, A.T.; Bazzoli, F.; Gensini, G.F.; Gisbert, J.P.; Graham, D.Y.; Rokkas, T.; et al. Management of H. pylori infection-the Maastricht IV/Florence Consensus Report. Gut 2012, 61, 646–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banić, M.; Franceschi, F.; Babić, Z.; Gasbarrini, A. Extragastric manifestations of H. pylori infection. Helicobacter 2012, 17, 49–55. [Google Scholar]
- Georgopoulos, S.D.; Polymeros, D.; Triantafyllou, K.; Spiliadi, C.; Mentis, A.; Karamanolis, D.G.; Ladas, S.D. Hypergastrinemia is associated with increased risk of distal colon adenomas. Digestion 2006, 74, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Houben, M.H.; van de Beek, D.; Hensen, E.F.; de Craen, A.J.; Rauws, E.A.; Tytgat, G.N. A systematic review of H. pylori eradication therapy-the impact of antimicrobial resistance on eradication rates. Aliment. Pharmacol. Ther. 1999, 13, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.Y.; Qureshi, W.A. Antibiotic-resistant H. pylori infection and its treatment. Curr. Pharm. Des. 2000, 6, 1537–1544. [Google Scholar] [PubMed]
- Zhao, X.; Drlica, K. Restricting the selection of antibiotic-resistant mutants: A general strategy derived from fluoroquinolone studies. Clin. Infect. Dis. 2001, 33, S147–S156. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, S.K.; Cars, O. Optimizing drug exposure to minimize selection of antibiotic resistance. Clin. Infect. Dis. 2007, 45, S129–S136. [Google Scholar] [CrossRef] [PubMed]
- Drlica, K. The mutant selection window and antimicrobial resistance. J. Antimicrob. Chemother. 2003, 52, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Drlica, K.; Zhao, X. Mutant selection window hypothesis updated. Clin. Infect. Dis. 2007, 44, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Akinbi, H.T.; Epaud, R.; Bhatt, H.; Weaver, T.E. Bacterial killing is enhanced by expression of lysozyme in the lungs of transgenic mice. J. Immunol. 2000, 165, 5760–5766. [Google Scholar] [CrossRef] [PubMed]
- Markart, P.; Korfhagen, T.R.; Weaver, T.E.; Akinbi, H.T. Mouse lysozyme M is important in pulmonary host defense against Klebsiella pneumoniae infection. Am. J. Respir. Crit. Care Med. 2004, 169, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.G.; Waldon, M.; Huang, Q.; Alvarez, S.; Oren, A.; Sandoval, N.; Du, M.; Zhou, F.; Zenz, A.; Lohner, K.; et al. Membrane-targeted synergistic activity of docosahexaenoic acid and lysozyme against Pseudomonas aeruginosa. Biochem. J. 2009, 419, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.L. Bacterial wall as target for attack: Past, present, and future research. Clin. Microbiol. Rev. 2003, 16, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.R.; Matsuzaki, T.; Aoki, T. Genetic evidence that antibacterial activity of lysozyme is independent of its catalytic function. FEBS Lett. 2001, 506, 27–32. [Google Scholar] [CrossRef]
- Nash, J.A.; Ballard, T.N.; Weaver, T.E.; Akinbi, H.T. The peptidoglycan degrading property of lysozyme is not required for bactericidal activity in vivo. J. Immunol. 2006, 177, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Lo, L.F.; Forsberg, L.S.; Maier, R.J. Helicobacter pylori peptidoglycan modifications confer lysozyme resistance and contribute to survival in the host. MBio 2012, 3, e00409-12. [Google Scholar] [CrossRef] [PubMed]
- Helander, I.M.; Mattila-Sandholm, T. Fluorometric assessment of gram-negative bacterial permeabilization. J. Appl. Microbiol. 2000, 88, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Loh, B.; Grant, C.; Hancock, R.E. Use of the fluorescent probe 1-N-Phenylnaphthyl-amine to study the interactions of aminoglycoside antibiotics with the outer membrane of pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1984, 26, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, I.; Migliorati, G.; Pagliacci, M.C.; Grignani, F.; Riccardi, C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 1991, 139, 271–279. [Google Scholar] [CrossRef]
- Zhang, X.L.; Jiang, A.M.; Ma, Z.Y.; Li, X.B.; Xiong, Y.Y.; Dou, J.F.; Wang, J.F. The synthetic antimicrobial peptide Pexiganan and its nanoparticles (PNPs) exhibit the anti-H. pylori activity in vitro and in vivo. Molecules 2015, 20, 3972–3985. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, V.; Giorgio, F.; Hassan, C.; Manes, G.; Vannella, L.; Panella, C.; Ierardi, E.; Zullo, A. Worldwide H. pylori antibiotic resistance: A systematic review. J. Gastrointest. Liver Dis. 2010, 19, 409–414. [Google Scholar]
- Mégraud, F. H. pylori antibiotic resistance: Prevalence, importance, and advances in testing. Gut 2004, 53, 1374–1384. [Google Scholar]
- Gao, W.; Cheng, H.; Hu, F.; Li, J.; Wang, L.; Yang, G.; Xu, L.; Zheng, X. The evolution of H. pylori antibiotics resistance over 10 years in Beijing, China. Helicobacter 2010, 15, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Borody, T.J.; Carrick, J.; Hazell, S.L. Symptoms improve after the eradication of gastric Campylobacter pyloridis. Med. J. Aust. 1987, 146, 450–451. [Google Scholar] [PubMed]
- Kuo, C.H.; Hsu, P.I.; Kuo, F.C.; Wang, S.S.; Hu, H.M.; Liu, C.J.; Chuah, S.K.; Chen, Y.H.; Hsieh, M.C.; Wu, D.C.; et al. Comparison of 10 day bismuth quadruple therapy with high-dose metronidazole or levofloxacin for second-line H. pylori therapy: A randomized controlled trial. J. Antimicrob. Chemother. 2013, 68, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Xu, X.; Zheng, Q.; Zhang, W.; Sun, Q.; Liu, W.; Xiao, S.; Lu, H. Efficacy of bismuth-containing quadruple therapies for clarithromycin-, metronidazole-, and fluoroquinolone-resistant H. pylori infections in a prospective study. Clin. Gastroenterol. Hepatol. 2013, 11, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.Y.; Mukhopadhyay, A.K.; Dailidiene, D.; Wang, Y.; Velapatiño, B.; Gilman, R.H.; Parkinson, A.J.; Nair, G.B.; Wong, B.C.; Lam, S.K.; et al. Sequential inactivation of rdxA (HP0954) and frxA (HP0642) nitroreductase genes causes moderate and high-level metronidazole resistance in Helicobacter pylori. J. Bacteriol. 2000, 182, 5082–5090. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, A.; Kersulyte, D.; Sisson, G.; Veldhuyzen van Zanten, S.J.O.; Berg, D.E.; Hoffman, P.S. Metronidazole resistance in Helicobacter pylori is due to null mutations in a gene (rdxA) that encodes an oxygen-insensitive NADPH nitroreductase. Mol. Microbiol. 1998, 28, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Binh, T.T.; Suzuki, R.; Trang, T.T.; Kwon, D.H.; Yamaoka, Y. Search for novel candidate mutations for metronidazole resistance in Helicobacter pylori using next-generation sequencing. Antimicrob. Agents Chemother. 2015, 59, 2343–2348. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.E.; de Vos, W.M.; van der Oost, J. Molecular analysis of the role of two aromatic aminotrans-ferases and a broad-specificity aspartate aminotransferase in the aromatic amino acid metabolism of Pyrococcus furiosus. Archaea 2002, 1, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Drlica, K. Restricting the selection of antibiotic-resistant mutant bacteria: Measurement and potential use of the mutant selection window. J. Infect. Dis. 2002, 185, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, J.M.; Zhao, X.; Hansen, G.; Drlica, K. Mutant prevention concentration of fluoroquinolones for clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 2001, 45, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds metronidazole, human lysozyme, 1-N-phenylnaphthylamine (NPN), propidium iodide(PI), N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), malate dehy-drogenase (MDH), pyridoxal-5′-phosphate l-aspartate, NADH, α-ketoglutarate are available from the authors.


| Time (h) | Cell Survival | |||
|---|---|---|---|---|
| 0.3 mg/mL rhLYS | 30 mg/mL hLYS | |||
| CFU/mL | % Survival | CFU/mL | % Survival | |
| 0 | 7.5 × 107 | 100% | 7.5 × 107 | 100% |
| 2 | 5.3 × 107 | 70.6% | 1.6 × 107 | 21.3% |
| 4 | 1.2 × 107 | 16% | 4.8 × 106 | 6.4% |
| 6 | 8.7 × 106 | 11.5% | 5.3 × 104 | 0.07% |
| Formulation | MIC | MPC | MSW |
|---|---|---|---|
| MNZ | 8 μg/mL | 153.6 μg/mL | 145.6 μg/mL |
| MNZ + hLYS | 1 μg/mL * | 25.6 μg/mL ** | 24.6 μg/mL ** |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Jiang, A.; Yu, H.; Xiong, Y.; Zhou, G.; Qin, M.; Dou, J.; Wang, J. Human Lysozyme Synergistically Enhances Bactericidal Dynamics and Lowers the Resistant Mutant Prevention Concentration for Metronidazole to Helicobacter pylori by Increasing Cell Permeability. Molecules 2016, 21, 1435. https://doi.org/10.3390/molecules21111435
Zhang X, Jiang A, Yu H, Xiong Y, Zhou G, Qin M, Dou J, Wang J. Human Lysozyme Synergistically Enhances Bactericidal Dynamics and Lowers the Resistant Mutant Prevention Concentration for Metronidazole to Helicobacter pylori by Increasing Cell Permeability. Molecules. 2016; 21(11):1435. https://doi.org/10.3390/molecules21111435
Chicago/Turabian StyleZhang, Xiaolin, Anmin Jiang, Hao Yu, Youyi Xiong, Guoliang Zhou, Meisong Qin, Jinfeng Dou, and Jianfei Wang. 2016. "Human Lysozyme Synergistically Enhances Bactericidal Dynamics and Lowers the Resistant Mutant Prevention Concentration for Metronidazole to Helicobacter pylori by Increasing Cell Permeability" Molecules 21, no. 11: 1435. https://doi.org/10.3390/molecules21111435






