A Theoretical Study of the Relationship between the Electrophilicity ω Index and Hammett Constant σp in [3+2] Cycloaddition Reactions of Aryl Azide/Alkyne Derivatives
Abstract
:1. Introduction
2. Results and Discussion
3. Computational Details
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huisgen, R. 1,3-Dipolar Cycloaddition Chemsitry; Wiley: New York, NY, USA, 1984; Volume 1. [Google Scholar]
- Ostrovskii, V.A.; Koldobskii, G.I.; Trifonov, R.E. Tetrazoles. In Comprehensive Heterocyclic Chemistry III; Elsevier: Oxford, UK, 2008; Volume 6, p. 257. [Google Scholar]
- Jewett, J.C.; Bertozzi, C.R. Cu-free click cycloaddition reactions in chemical biology. Chem. Soc. Rev. 2010, 39, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. Engl. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective ligation of azides and terminal alkynes. Angew. Chem. Int. Ed. Engl. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Huisgen, R. 1,3-dipolar cycloadditions. Past and future. Angew. Chem. Int. Ed. Engl. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Huisgen, R. 1,3-dipolar cycloadditions. Concerted nature of 1,3-dipolar cycloadditions and the question of diradical intermediates. J. Org. Chem. 1976, 41, 403–419. [Google Scholar] [CrossRef]
- Domingo, L.R.; Sáez, J.A. Understanding the mechanism of polar Diels–Alder reactions. Org. Biomol. Chem. 2009, 7, 3576–3583. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Emamian, S.R. Understanding the mechanisms of [2+3] cycloaddition reactions. The pseudoradical versus the zwitterionic mechanism. Tetrahedron 2014, 70, 1267–1273. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. A new model for C–C bond formation processes derived from the Molecular Electron Density theory in the study of the mechanism of [2+3] cycloaddition reactions of carbenoid nitrile ylides with electron-deficient ethylenes. Tetrahedron 2016, 72, 1524–1532. [Google Scholar] [CrossRef]
- Domingo, L.R. Molecular electron density theory: A modern view of reactivity in organic chemistry. Molecules 2016, 21, 1319. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Aurell, M.J.; Pérez, P. A mechanistic study of the participation of azomethine ylides in [3+2] cycloaddition reactions. Tetrahedron 2015, 71, 1050–1057. [Google Scholar] [CrossRef]
- Domingo, L.R.; Aurell, M.J.; Pérez, P. A DFT analysis of the participation of TACs in zw-type [3+2] cycloaddition reactions. Tetrahedron 2014, 70, 4519–4525. [Google Scholar] [CrossRef]
- Molteni, G.; Ponti, A. Regioselectivity of aryl azide cycloaddition to methyl propiolate in aqueous media: Experimental evidence versus local DFT HSAB principle. Arkivoc 2006, 49–56. [Google Scholar]
- Siadati, S.A. A theoretical study on stepwise and concertedness of mechanism of 1,3-dipolar cycloaddition reaction between tetra amino ethylene and trifluoro methyl azide. Comb. Chem. High Throughput Screen. 2016, 19, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Klumpp, G.W. Reactivity in Organic Chemistry; Wiley: New York, NY, USA, 1982; p. 274. [Google Scholar]
- Hammett, L.P. Linear free energy relationships in rate and equilibrium phenomena. Trans. Faraday Soc. 1938, 34, 156–165. [Google Scholar] [CrossRef]
- Hansch, C.; Hoekman, D.; Gao, H. Comparative QSAR: Toward a deeper understanding of chemicobiological interactions. Chem. Rev. 1996, 96, 1045–1076. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Ríos-Gutérrez, M.; Pérez, P. Applications of the conceptual density functional theory indices to organic chemistry reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef] [PubMed]
- Hansch, C.; Leo, A.; Taft, R.W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 1991, 91, 165–195. [Google Scholar] [CrossRef]
- Chattaraj, P.K.; Sarkar, U.; Roy, D.R. Electrophilicity index. Chem. Rev. 2006, 106, 2065–2091. [Google Scholar] [CrossRef] [PubMed]
- Parr, R.G.; von Szentpaly, L.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Domingo, L.R.; Pérez, P.; Contreras, R. Electronic contributions to the σp parameter of the Hammett equation. J. Org. Chem. 2003, 68, 6060–6062. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Aurell, M.J.; Pérez, P.; Contreras, R. Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in Diels–Alder reactions. Tetrahedron 2002, 58, 4417–4423. [Google Scholar] [CrossRef]
- Mayr, H.; Kempf, B.; Ofial, A.R. Pi-nucleophilicity in carbon-carbon bond-forming reactions. Acc. Chem. Res. 2003, 36, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Mayr, H. Reference scales for the characterization of cationic electrophiles and neutral nucleophiles. J. Am. Chem. Soc. 2001, 123, 9500–9512. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision E.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Domingo, L.R.; Sáez, J.A.; Pérez, P. A comparative analysis of the electrophilicity of organic molecules between the computed IPs and EAs and the HOMO and LUMO energies. Chem. Phys. Lett. 2007, 438, 341–345. [Google Scholar] [CrossRef]
- Sample Availability: Not available.


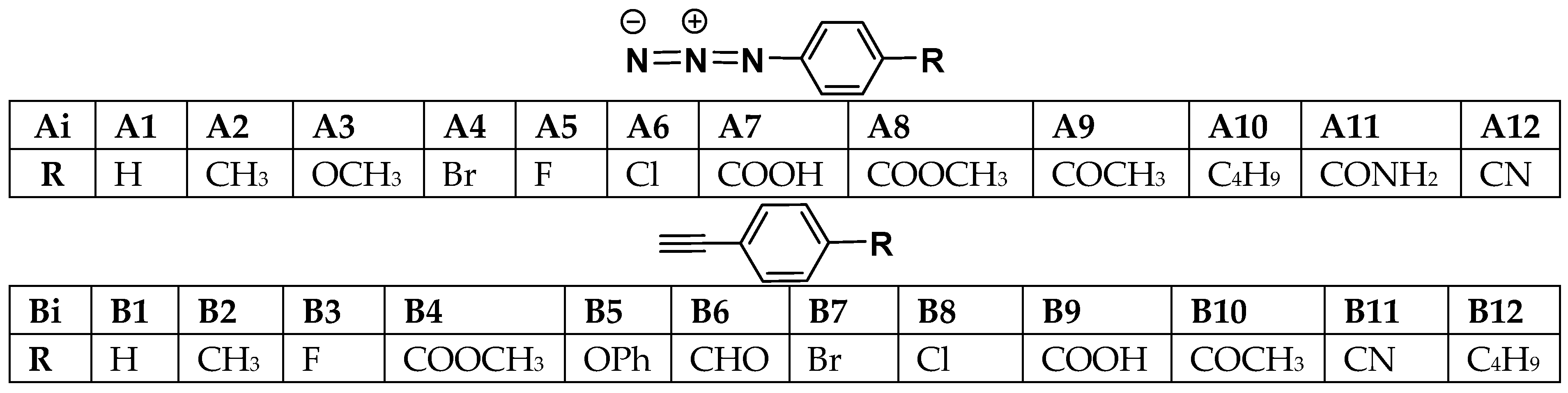



| Compound | R | μ | η | ω | σp a | Log(ω/ωH) |
|---|---|---|---|---|---|---|
| A1 | H | −3.62 | 5.14 | 1.27 | 0.00 | 0.000 |
| A2 | Me | −3.48 | 5.03 | 1.21 | −0.17 | −0.019 |
| A3 | MeO | −3.29 | 4.76 | 1.13 | −0.27 | −0.046 |
| A4 | Br | −3.75 | 5.01 | 1.42 | 0.23 | 0.048 |
| A5 | F | −3.67 | 5.03 | 1.34 | 0.06 | 0.025 |
| A6 | Cl | −3.78 | 5.03 | 1.42 | 0.23 | 0.052 |
| A7 | COOH | −4.82 | 4.79 | 1.79 | 0.45 | 0.152 |
| A8 | COOMe | −4.05 | 4.82 | 1.71 | 0.45 | 0.130 |
| A9 | COMe | −4.16 | 4.65 | 1.86 | 0.50 | 0.169 |
| A10 | tert-butyl | −3.46 | 5.03 | 1.19 | −0.20 | −0.020 |
| A11 | CONH2 | −3.97 | 4.93 | 1.60 | 0.36 | 0.103 |
| A12 | CN | −4.33 | 4.82 | 1.94 | 0.66 | 0.187 |
| B1 | H | −3.51 | 5.52 | 1.13 | 0.00 | 0.000 |
| B2 | Me | −3.37 | 5.39 | 1.06 | −0.17 | −0.028 |
| B3 | F | −3.54 | 5.47 | 1.14 | 0.06 | 0.003 |
| B4 | COOMe | −4.11 | 4.84 | 1.74 | 0.45 | 0.187 |
| B5 | PhO | −3.24 | 5.12 | 1.03 | −0.03 | −0.042 |
| B6 | CHO | −4.41 | 4.63 | 2.10 | 0.42 | 0.269 |
| B7 | Br | −3.73 | 5.22 | 1.33 | 0.23 | 0.070 |
| B8 | Cl | −3.75 | 5.28 | 1.32 | 0.23 | 0.067 |
| B9 | COOH | −4.22 | 4.87 | 1.82 | 0.45 | 0.206 |
| B10 | COMe | −4.22 | 4.71 | 1.90 | 0.50 | 0.225 |
| B11 | CN | −4.38 | 4.90 | 1.97 | 0.66 | 0.241 |
| B12 | tert-butyl | −3.35 | 6.07 | 0.92 | −0.20 | −0.089 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben El Ayouchia, H.; Anane, H.; El Idrissi Moubtassim, M.L.; Domingo, L.R.; Julve, M.; Stiriba, S.-E. A Theoretical Study of the Relationship between the Electrophilicity ω Index and Hammett Constant σp in [3+2] Cycloaddition Reactions of Aryl Azide/Alkyne Derivatives. Molecules 2016, 21, 1434. https://doi.org/10.3390/molecules21111434
Ben El Ayouchia H, Anane H, El Idrissi Moubtassim ML, Domingo LR, Julve M, Stiriba S-E. A Theoretical Study of the Relationship between the Electrophilicity ω Index and Hammett Constant σp in [3+2] Cycloaddition Reactions of Aryl Azide/Alkyne Derivatives. Molecules. 2016; 21(11):1434. https://doi.org/10.3390/molecules21111434
Chicago/Turabian StyleBen El Ayouchia, Hicham, Hafid Anane, Moulay Lahcen El Idrissi Moubtassim, Luis R. Domingo, Miguel Julve, and Salah-Eddine Stiriba. 2016. "A Theoretical Study of the Relationship between the Electrophilicity ω Index and Hammett Constant σp in [3+2] Cycloaddition Reactions of Aryl Azide/Alkyne Derivatives" Molecules 21, no. 11: 1434. https://doi.org/10.3390/molecules21111434









